Amebic liver abscess: An update
Ramesh Kumar, Rishabh Patel, Rajeev Nayan Priyadarshi, Ruchika Narayan, Tanmoy Maji, Utpal Anand, Jinit R Soni
Abstract Amebic liver abscess (ALA) is still a common problem in the tropical world, where it affects over three-quarters of patients with liver abscess. It is caused by an anaerobic protozoan Entamoeba hystolytica, which primarily colonises the cecum. It is a non-suppurative infection of the liver consisting primarily of dead hepatocytes and cellular debris. People of the male gender, during their reproductive years, are most prone to ALA, and this appears to be due to a poorly mounted immune response linked to serum testosterone levels. ALA is more common in the right lobe of the liver, is strongly associated with alcohol consumption, and can heal without the need for drainage. While majority of ALA patients have an uncomplicated course, a number of complications have been described, including rupture into abdomino-thoracic structures, biliary fistula, vascular thrombosis, bilio-vascular compression, and secondary bacterial infection. Based on clinico-radiological findings, a classification system for ALA has emerged recently, which can assist clinicians in making treatment decisions. Recent research has revealed the role of venous thrombosis-related ischemia in the severity of ALA. Recent years have seen the development and refinement of newer molecular diagnostic techniques that can greatly aid in overcoming the diagnostic challenge in endemic area where serology-based tests have limited accuracy. Metronidazole has been the drug of choice for ALA patients for many years. However, concerns over the resistance and adverse effects necessitate the creation of new, safe, and potent antiamebic medications. Although the indication of the drainage of uncomplicated ALA has become more clear, high-quality randomised trials are still necessary for robust conclusions. Percutaneous drainage appears to be a viable option for patients with ruptured ALA and diffuse peritonitis, for whom surgery represents a significant risk of mortality. With regard to all of the aforementioned issues, this article intends to present an updated review of ALA.
Key Words: Amebic liver abscess; Amebiasis; Ruptured liver abscess; Percutaneous drainage; Metronidazole
INTRODUCTION
Even 150 years after Friedrick Lösch made the initial discovery ofEntamoeba histolytica(EH), this ancient protozoan still continues to pose a threat to public health in developing nations. There has not been a recent global assessment on the epidemiology of amebiasis, nevertheless, previous estimates suggest that amebiasis affects approximately 40 million people worldwide and kills up to 0.1 million people annually[1-3]. It is the second leading cause of parasite diseaserelated deaths. The Indian subcontinent, Africa, Mexico, Central and South America, and Africa are among the regions with high rates of amebiasis[3-5]. In developed countries, immigrants from endemic areas account for the majority of cases[6].
The major source of infection is water or food contaminated with quadrinucleate cysts of EH. The trophozoites released in the small intestine lumen colonize the cecum. Due to some unclear mechanisms, the parasite turns into a pathogen in around 10% of infected subjects, invades the intestine, and enters the liver through portal venous circulation. The virulent amebic trophozoites in the liver cause inflammation and necrosis leading to the formation of abscess[7]. An amoebic liver abscess (ALA) is the most common extraintestinal manifestation of invasive amebiasis and the commonest form of liver abscess in tropical areas[8]. It is not clear why ALA develops in a small proportion of patients with intestinal amebiasis. The complex interactions between the genetic polymorphism in EH influencing the virulence, the host immune system, and the surrounding environment, particularly gut flora, appear to play an important role in imparting such susceptibility[1,9-11]. While EH is thought to be the only cause of ALA, a recent study from South America has found DNA sequences ofE. disparfrom the liver abscess aspirate, raising doubts about its causal involvement[12]. Recent years have seen the emergence of many new insights into the clinic-epidemiological characteristics, diagnostic techniques, and changing management paradigms for patients with ALA. The purpose of this article is to provide an updated review of ALA, taking into account all the relevant issues. The data source for this review article included PubMed, Google Scholar, the Cochrane Library, and the cross-references from the searched publications. The relevant articles published between January 1980 and October 2023 were searched using appropriate keywords.
CLlNlCO-EPlDEMlOLOGlCAL CHARACTERlSTlCS
In tropical areas, ALA is the most prevalent type of liver abscess. In the largest series of liver abscess patients reported from India, 81% of 1630 patients had ALA diagnosed by serological testing[8]. In another study that employed nested multiplex polymerase chain reaction (PCR) to identify specific DNA sequences of EH, 87% of patients with liver abscesses were found to have ALA[13]. On the other hand, ALA constitutes < 10% of all liver abscess patients in non-endemic regions[14].
Peculiar characteristics
Although an ALA is clinically indistinguishable from pyogenic liver abscess (PLA), certain peculiar characteristics make ALA stand out from other causes of liver abscess.
Lack of typical characteristics of an abscess:EH causes hepatic apoptosis and trogocytosis rather than a suppurative infection of the liver. Thus, the aspirate of ALA does not have the typical characteristics of pus. It is a thick brown acellular debris that is odourless and almost always sterile[15,16]. Unlike PLA, which contains leukocytes and appears hot on nuclear scanning, ALA lacks leukocytes and appears as a cold lesion on technetium-99m liver scan[17]. The absence of neutrophils in ALA is mainly due to lysis of the protozoan. The majority of ALA heal without the need for a drainage procedure. Therefore, it seems inaccurate to refer to ALA as an "abscess."
Strong gender and age predilection:Despite a similar distribution of asymptomatic EH infection between the genders, the incidence of invasive amebiasis is much higher in males[18]. Males are 4-9 times more likely to have ALA than females[1,8,19]. This phenomenon has been observed even in travellers from non-endemic countries who acquire the disease[7]. Notably, such a marked gender difference does not exist among children. Males become more susceptible to ALA following puberty, with a peak incidence occurring around 40 years of age[20]. In a large study including over 2000 ALA patients, adult males between ages 30 and 50 years had the highest incidence of ALA[21]. This pronounced predilection for gender and age is thought to be due to the effect of testosterone and alcohol consumption[1,2,22,23]. Early cytokines production by natural killer T (NKT) cells, particularly Interferon (IFN) γ, plays a major role in EH invasion[24]. In response to hepatic samebiasis, gender-specific differences in cytokine production have been found in an animal study that revealed a lack of early IFN-γ response in male mice[23]. Furthermore, it was discovered that testosterone renders male mice more vulnerable to ALA by preventing NKT cells from secreting IFNγ[10]. In a study, immunoglobulin-G levels against EH were significantly higher in female asymptomatic carriers of EH than in corresponding male subjects. This could be one of the additional factors contributing to the lower incidence of ALA in the female population[25].
Predominant right lobar involvement:The EH trophozoites invade the cecum and travel to the liverviaportal circulation. Because the right lobe of the liver receives portal blood mostly through the superior mesenteric vein, which drains the cecum, ALA more frequently affects this part of the liver. Such lobar predilection is not observed in the case of PLA where bacterial invasion can occur through venous, arterial, or biliary systems.
Strong association with alcohol consumption:Alcohol consumption increases the risk of developing ALA. A history of significant alcohol consumption is present in up to 85% of patients with ALA[1,26,27]. A number of studies from the Indian subcontinent have found a strong correlation between the incidence of ALA and the intake of local alcoholic beverages such as toddy[1,20,26-28]. Moreover, alcoholics have been found to have larger ALA, more complications, and a longer recovery period[27]. The precise mechanism of how alcohol confers the susceptibility to ALA is yet to be defined. Numerous indirect mechanisms, including changes in the microbiota of the gut, elevated gut permeability, elevated expression of alcohol dehydrogenase-2 on EH, decreased immunity, and elevated hepatic iron concentration, have been hypothesised[27,29]. The possibility of direct oral transmission has been ruled out by studies that failed to demonstrate EH cysts in alcoholic beverages[1,30].
Clinical presentation
Uncomplicated cases of ALA usually present with a short history of fever and abdominal pain in the right upper quadrant. Most patients experience symptoms two to four weeks after the exposure; however, in travellers, lag times have been recorded to range from 23 to 563 d[31,32]. At the time of diagnosis, less than one-third of the patients have diarrhea, only about 10% of patients have jaundice, and 5%-14% of cases have pulmonary symptoms like chest pain and shortness of breath. Recently, three distinct clinical presentations of ALA have been described according to the severity and duration of symptoms[22,32]. These include: (1) Acute aggressive ALA - characterized by acute onset of severe symptoms, systemic toxicity, markedly deranged laboratory parameters, and high risk of rupture requiring a drainage procedure; (2) subacute mild ALA - characterized by mild-to-moderate symptoms with onset within 2-4 wk, usually responding to the medical therapy; and (3) chronic indolent ALA - characterized by late presentation (> 4 wk) with mild persistent symptoms including pain in abdomen. Such patients have a well-formed wall with only a negligible risk of rupture; yet, drainage may be necessary to reduce pain. Patients with complicated ALA have variable presentations depending upon the nature and severity of complications. Most complications of ALA are related to its rupture into the adjacent structures, vascular thrombosis or compression, and secondary bacterial infection[22].
COMPLlCATlONS OF ALA
Various complications resulting from ALA has been described in the literature (Figure 1). These complications can be broadly divided into two groups: those associated with ALA rupture and those unrelated to it.

Figure 1 Complications of amebic liver abscess. The complications can be broadly divided into two groups: Those related to amebic liver abscess rupture and those that are not. The common complications included rupture into the adjacent structures, vascular thrombosis, and secondary bacterial infection. ALA: Amebic liver abscess; SIRS: Systemic inflammatory response syndrome.
Complications due to rupture of ALA
The incidence of ALA rupture varies from 6% to 40%[22]. In terms of the sites of rupture, the intraperitoneal site currently predominates over pleuropulmonary rupture (10%-24%vs4.2%-7%)[33,34]. In a study by Priyadarshiet al[19], intraperitoneal rupture accounted for 83% of all 117 patients with ruptured ALA. A larger proportion (80%) of thoracic complications of ALA include sterile reactive pleural effusion rather than amebic empyema secondary to rupture[22,34]. Cardiac tamponade resulting from the rupture of the left lobe of ALA into the pericardium is one of the most serious complications of ALA[35,36]. Rupture of biliary ducts into the abscess cavity can result in biliary fistula, which is more common in large and centrally located ALA[22]. Although rupture of ALA into the hollow viscus leading to result in hepatic fistulas (hepatobronchial, hepatogastric, hepatoenteric and hepatocolonic fistula) is rare, such complications are often innocuous because of the spontaneous drainage of the abscess through the fistula[37-39].
Despite the fact that the incidence of rupture has not decreased much over the years, mortality rates have improved dramatically. In 1994, Menget al[40] reported a 22% incidence of rupture in 503 consecutive ALA patients with a mortality rate of 17%. In contrast, the incidence of rupture and mortality rate was 16% and 1.1%, respectively, in a large series of 1321 ALA patients published by Jindalet al[8] in 2021. Similarly, the mortality rate for 117 patients with ruptured ALA was merely 0.85% in a study published by Priyadarshiet al[19] in 2019.
Non-rupture complications
Thrombosis of major vessels, such as the portal vein, hepatic vein, and inferior vena cava, has been reported in patients with ALA[41,42]. In an autopsy series, Krishnanet al[42] have reported IVC thrombosis in 8% of the 95 ALA patients, and all but one also had concomitant hepatic vein thrombosis. Nevertheless, new information obtained with a sensitive multidetector CT scan suggests that thrombosis of smaller branches of the portal and hepatic veins is quite prevalent in ALA patients. When the segmental and subsegmental branches of the hepatic and portal veins were combined, a recent study found that 69% of ALA patients had venous thrombosis. In addition, 53% patients, who had severe clinical course or ruptured ALA, showed a zone of perilesional ischemia[43]. An intracavitary hepatic artery pseudoaneurysm has also been reported in patients with ALA[44]. Moreover, ALA can present as reversible portal hypertension due to the compression of the portal vein and as Budd-Chiari syndrome due to the obstruction of the hepatic veins[45,46]. A large ALA located in the caudate lobe can cause compression of the vena cava, resulting in pedal edema. Obstructive jaundice may occur from an ALA close to the porta hepatis[47]. Jaundice in ALA can also result from a biliovascular fistula created due to the simultaneous injury to the bile ducts and hepatic veins. Interestingly, jaundice in such patients improves after biliary diversion with nasobiliary drainage[48]. Even though ALA is usually considered to be bacteriologically sterile, up to 20% of patients may develop a secondary bacterial infection, which could complicate the disease course[22]. In a recent study, the aerobic bacterial culture positivity rate of ALA aspirate was only about 5%, however, 37% of aspirate revealed molecular evidence of various anaerobes of gut microbiota, such as Fusobacterium, Peptococcus, and Prevotella[13]. These anaerobes are likely to be translocated from the gut and could be crucial in granting virulence to EH. The other rare complications of ALA include hepatic encephalopathy and acute respiratory distress syndrome[49,50].
Factors associated with rupture
The risk of ruptures is high for ALAs in the subcapsular location, left lobe, or caudate lobe[51,52]. Due to the lesser bulk and larger area beneath the left hemidiaphragm, the left lobe ALA is at a higher risk of rupture[51]. Thin, immature, incomplete, and ragged ALA walls are also important risk factors for its rupture[22,32]. Jhaet al[33] reported that older age, chronic alcoholism, hyperbilirubinemia, hypoalbuminemia, leucocytosis, and hyponatremia were the important predictors of ALA complications, which largely (88%) included rupture. Other factors, such as strain virulence and host immunity may contribute to the risk of rupture, however, there is currently little evidence to support this assumption. A recent study found an increased incidence (53%) of intraperitoneal rupture of ALA in COVID-19-recovered patients. This was presumed to be due to alteration in the immune state of these patients[53]. ALA with concomitant ileocolonic ulceration is associated with a high risk of rupture, which might be due to an infection with a more virulent strain of EH[54].
DlAGNOSTlC lSSUES AND ADVANCES
For liver abscessper se, ultrasonography is the preferable initial test. It is a rapid, widely available, inexpensive and sensitive test for detection of an abscess. Computed tomography (CT) scan is usually required for complicated ALA patients (Figure 2). Recently, Priyadarshiet al[32] have described three distinct morphological patterns of ALA on CT scans with clinical correlation. Type I ALA, the clinically aggressive type, had absent or incomplete wall and ragged edges with irregular enhancement, Type II ALA had a complete rim enhancement and peripheral hypodense halo and was clinically less aggressive, and Type III ALA, the least common type, demonstrated a non-enhancing wall and a chronic indolent course (Figure 3). Patients who have bleeding symptoms from ileocolonic ulcerations or ruptured ALA into the gastrointestinal tract may require endoscopic evaluation (Figure 4).
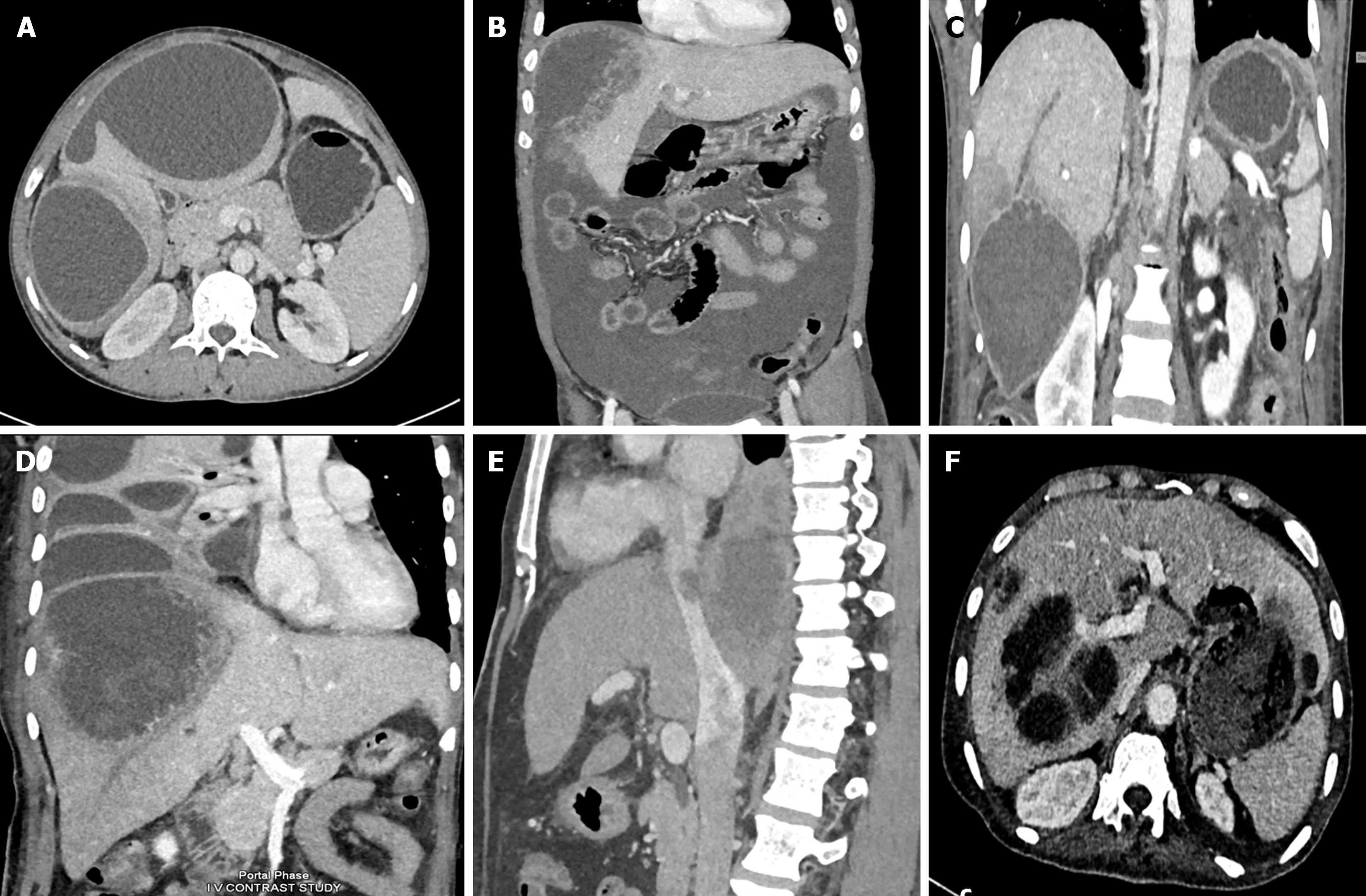
Figure 2 Computed tomographic scans showing various spectrum of complicated amebic liver abscess. A: Axial computed tomography (CT) scan showing a contained rupture of an abscess with localized fluid collection in the perihepatic area. There is a thin enhancing rim around the abscess, characteristic of a type II amebic liver abscess (ALA); B: Coronal CT scan of a 54-year-old male showing rupture of the abscess with intraperitoneal fluid collection diffusely spreading throughout the entire peritoneal cavity, indicative of free abscess rupture; C: Coronal contrast-enhanced CT image showing an amebic abscess located in segment VI with long-segment thrombosis in the peripheral branch of the right hepatic vein. Note the triangular hypodense area surrounding the abscess, indicating hypoperfused parenchyma; D: Coronal contrast-enhanced CT image showing a large amebic abscess that has ruptured into the thoracic cavity with loculated pleural fluid collections. Note that the abscess demonstrates ragged edges with indistinct enhancement, characteristic of a type I abscess; E: Sagittal contrast-enhanced venous phase CT image of a patient showing thrombus extending from the ALA into the inferior vena cava; F: Axial CT scan showing a communication of left lobe ALA with the gastric lumen forming a hepatogastric fistula.
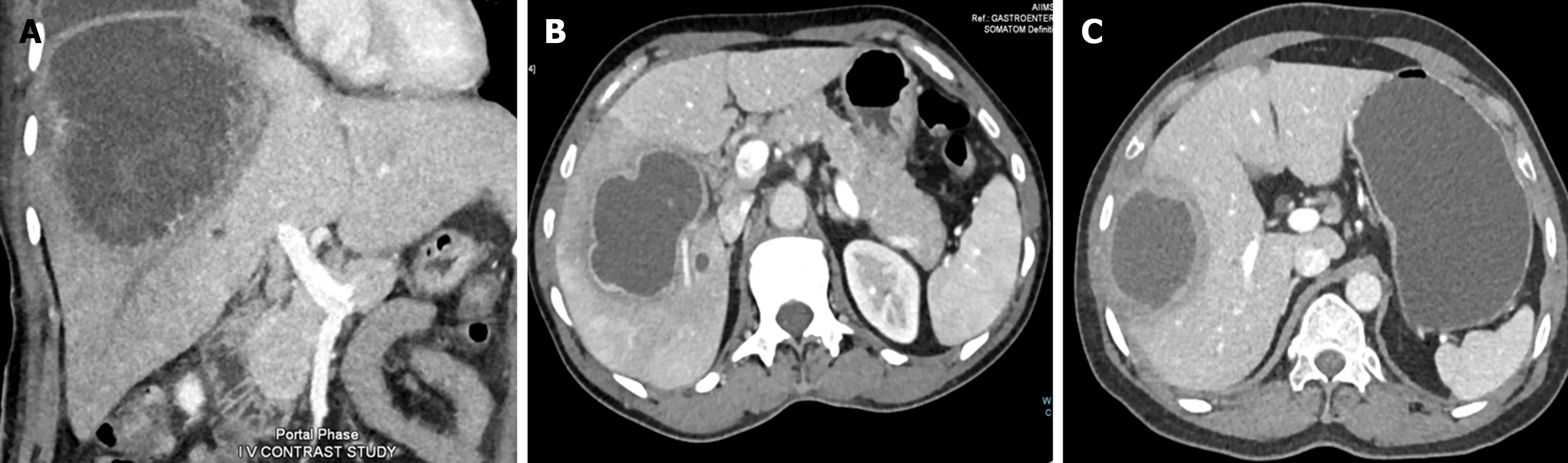
Figure 3 Computed tomographic classification of amebic liver abscess. A: Type I amebic liver abscess (ALA) shows incomplete wall and ragged edges with irregular enhancement; B: Type II ALA showing a complete rim enhancement with peripheral hypodense halo; C: Type III ALA showing a chronic non-enhancing wall.
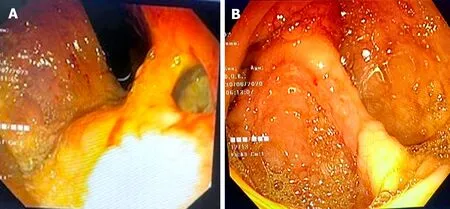
Figure 4 Endoscopic images of complicated amebic liver abscess patients. A: Shows an upper endoscopic view of hepatogastric fistula resulting from rupture of a left lobe amebic liver abscess (ALA) into the stomach near high lesser curve; B: Shows colonoscopic view of a complicated ALA patient presenting with lower gastrointestinal bleeding from multiple small ulcers from the cecum.
More importantly, there is no clinical or imaging characteristic that can accurately diagnose ALA or distinguish it from a PLA. Such differentiation is crucial in order to prevent a delay in the implementation of appropriate therapy. Determination of an accurate diagnosis of ALA relies mainly on laboratory tests that use immunological, molecular, and parasitological methods to confirm EH in different specimens[55].
Stool examination
The utility of microscopic stool testing for cysts is limited because only 10%-40% of ALA patients have concurrent intestinal amebiasis, and EH cysts are morphologically identical to nonpathogenic strains of Entamoeba such asE. disparandE. Moshkovskii[56]. Stool antigen assays based on enzyme immunoassay or PCR are highly sensitive in detecting EH and distinguishing it from nonpathogenic strains; however, they are neither widely available nor well standardised[57,58]. Moreover, as EH trophozoites are only viable for a few minutes, fresh stool specimens must be examined for both stool microscopy and antigen testing.
Serum-based tests:The enzyme-linked immuno sorbent assay (ELISA) and hemagglutination assay are commonly used in conjunction with clinico-radiological to diagnose ALA[59-61]. Anti-amebic antibodies can be detected in about 95% of cases of ALA. Recently, ELISA, targeting the IgG1 subclass antibody to EH exhibited 100% sensitivity and 99.1% specificity in patients with ALA[62]. However, anti-amebic antibodies become detectable in serum only 5-7 d after infection and continue to exist for 6-12 months after the infection has been eradicated. As a result, they might be false negatives during the first week of illness and might not be useful for those living in highly endemic areas. Therefore, the current applications of serological testing are mostly limited to sero-epidemiological research and the diagnosis of ALA in travellers from endemic locations. Detection of circulating antigen or DNA of EH can be helpful tools for the diagnosis of acute infection and follow-up after therapy in endemic regions. Recently, circulating Gal/GalNAc lectin of EH was detected in the serum of ALA patients with high sensitivity (96%) prior to anti-amebic therapy[63]. Also, PCR can detect circulating DNA of EH in the serum of ALA patients with 89.5% sensitivity and 100% specificity[64]. However, the expense is a barrier to their regular usage in the poorly resourced endemic nations.
ALA aspirate-based tests:ALA aspirate appears as a thick, brown, odourless fluid that contains acellular proteinaceous debris and resembles an anchovy sauce. ALA is typically regarded as sterile lesion unless there is a secondary bacterial infection. Nonetheless, various anaerobes of gut microbiota, such asFusobacterium,PeptococcusandPrevotella, have been found in the 37% of aspirate[13]. Demonstration of amebic trophozoites in the pus can be confirmatory for diagnosis, however, this is only observed in a small proportion of aspirates (7.2%-25%) and only when the cyst wall is sampled[65,66]. In a study, amebic antigen was detected by ELISA in 97.6% of pus specimens from ALA patients[67]. However, the sample must be obtained before the beginning of the anti-amebic treatment, which often causes rapid loss of antigen.
Recently, a simple and efficient technique for DNA extraction has been developed. Consequently, molecular diagnostic assays based on PCR have emerged as the diagnostic gold standard[68,69]. Numerous commercial PCR assays, including conventional PCR, real-time PCR, nested PCR, and multiplex PCR have been designed to identify amebic DNA from both pus and stool samples. It has been shown in numerous studies that PCR has very high sensitivity (84%-100%) and specificity (100%) for detecting EH-DNA from ALA aspirate[70,71]. In a comparison study, the sensitivity of real-time PCR in detecting EH in ALA aspirate was significantly higher than that of an ELISA-based antigen detection test (97%vs40%)[72]. It should be noted that the sensitivity of PCR for detecting EH-DNA reduces after initiation of anti-amebic treatment; hence, the test sample should be aspirated before initiation of therapy[66]. Currently, no commercial rapid diagnostic test is available to diagnose ALA. Nonetheless, a preliminary investigation has found that the IgG4-based rapid dipstick test, which detects anti-EH pyruvate phosphate dikinase antibody, has an excellent diagnostic performance for the rapid identification of ALA[73].
To summarize, serological testing in association with clinico-radiological features is still relied upon to diagnose ALA in most of the endemic regions due to the high cost and unavailability of molecular diagnostic tests. Since serological tests can be fallacious in early stages of disease and in endemic locations, efforts should be undertaken to develop a molecular diagnostic test that is rapid, sensitive, specific, and inexpensive so that it may be used in low-resource countries.
EVlDENCE-BASED MANAGEMENT AND CHANGlNG TREATMENT PARADlGM
Tissue amebicides form the mainstay of management of all patients with ALA. The need for interventional treatment is determined by a number of variables, including the abscess characteristics, persistence of symptoms, and existence of complications. Figure 5 shows the proposed treatment algorithm for ALA.

Figure 5 Proposed treatment algorithm for patient with amebic liver abscess. For uncomplicated amebic liver abscess (ALA): Upfront percutaneous drainage (PD) should be considered only in the presence of high risk signs; PD doesn’t provide added benefit when ALA size is < 5 cm, and ALA with size > 5 should be treated initially with medical therapy (MT) consisting of anti-amebic drug for 3-5 d before considering PD in case of non-response. For PD, a percutaneous catheter drainage (PCD) is preferred over needle aspiration, particularly for larger and incompletely liquified ALA. For complicated ALA patients, some form of drainage procedure is always required. A large majority of such patients can be treated with PCD along with MT. ALA with biliary fistula can be treated with prolonged PCD, and only on rare occasion, an endoscopic retrograde cholangiopancreatography will be required. Finally, ALA with rupture into a hollow viscus can be treated with MT alone. MT: Medical therapy; PD: Percutaneous drainage; ALA: Amebic liver abscess; PCD: Percutaneous catheter drainage; ERCP: Endoscopic retrograde cholangiopancreatography.
Management of uncomplicated ALA
Two prospective studies that assessed the outcome of conservative therapy in uncomplicated ALA found that the majority of patients could be managed with an antiamebic drug alone[74,75]. After receiving pharmacotherapy for 72 h, only 13% of patients in one study and 18% of patients in another study needed drainage. Size of ALA (> 7.7 cm and 10.7 cm, respectively) was the most significant factor determining radiological intervention in both studies. Thus, a conservative strategy should be adopted for most patients with uncomplicated ALA.
Drug of choice
Metronidazole (MTZ), the most widely used nitroimidazole, is the cornerstone of treatment for uncomplicated ALA for more than four decades. MTZ has a good hepatic penetration, and when used at a dose of 500 mg to 750 mg three times a day for seven to ten days, it resolves symptoms within 72 h of treatment[76]. Since the parasites can linger in the colon, MTZ treatment should be followed with a luminal agent, such as paromomycin (500 mg 3 times a day for 7 d) or diloxanide furoate (500 mg three times a day for 20 d). The failure to take luminal medicines can result in relapse of infection in about 10% patients[77].
Emerging concerns with MTZ
Even though resistance to MTZ is uncommon,in vitrostudies and sporadic reports of treatment failures and relapse indicate emergence of resistance to this drug[78,79]. The inhibitory concentration of nitroimidazoles against EH was found to be rising in a recent study from India[80]. The nim gene-encoded nitroimidazole reductase enzyme is commonly associated with resistance to MTZ[81]. In a recent study, 22.2% of recurrent ALA patients revealed the presence ofnimEgene[77]. Other issues related to MTZ include adverse symptoms such nausea, vomiting, disorientation, metallic taste, and peripheral neuropathy. Concerns have also been raised about its carcinogenic potential in animals and mutagenic potential in bacteria such asH. pyloriandE. coli[82,83].Moreover, MTZ is not very effective in treating asymptomatic intestinal amebiasis.
Alternative anti-amebic drugs
Although there is limited clinical experience, other nitroimidazole compounds that have been used in the treatment of ALA include tinidazole, ornidazole, and nitazoxanide. Both ornidazole and tinidazole have better tolerance and longer half-lives, making them suitable for a shorter course of treatment (3-5 d)[84]. In a small study, 2 g tinidazole once daily for 2 d resulted in completed recovery of ALA in all (n= 10) subjects[85]. Nitazoxanide has emerged as an effective agent against a variety of parasite infections. An in-vitro study has found it to be more active than metronidazole against amebiasis[86]. It also has the advantage of being a tissue as well as luminal amebicide. In a recent randomized controlled trial (RCT), nitazoxanide, at 500 mg twice daily for 10 d, was found more tolerable and as effective as MTZ in uncomplicated ALA patients[87]. However, clinical response was faster with MTZ compared to nitazoxanide.
Emerging anti-amebic molecules
Apart from nitroimidazole compounds, there has not been much progress made in the creation of alternative anti-amebic drugs thus far. Riluzole, a benzothiazole derivative, and andrographolide, a repurposing drug, have recently demonstrated strong anti-amoebic action against EH[80,88]. Apocynin, an NADPH oxidase enzyme inhibitor, was found to prevent ALA in animals[89]. Proton pump inhibitor, which has a benzimidazole nucleus resembling MTZ, was recently found to inhibit thioredoxin reductase of EH, an enzyme essential to the pathogen's virulence and survival[90,91]. Moreover, the inhibitory concentration of Rabeprazole and Pantoprazole was found to be much lesser than MTZ[90]. However, the utility of these newer and repurposing medications needs to be investigated in ALA patients in further studies.
Drainage of uncomplicated ALA
The role of upfront drainage of uncomplicated ALA is controversial. The results of multiple RCTs have produced mixed conclusions (Table 1). While some RCTs have reported no added benefits of percutaneous needle aspiration (PNA)[92,93], others found minor to significant improvements in certain outcome parameters[94-100]. In a recent systematic review and meta-analysis of 570 ALA patients, the addition of PNA to MTZ therapy produced additional benefits in terms of early resolution of abdominal pain and tenderness in patients with ALA of size > 5 cm. However, there was no discernible effect on the fever, abscess size, and length of hospital stay[101]. Additionally, patients with small ALAs (< 5 cm) did not show any benefit after draining. It is noteworthy that most of the included studies employed PNA as a drainage technique, rather than the more effective percutaneous catheter drainage (PCD), making it difficult to draw firm conclusions on the efficacy of adjunct drainage.
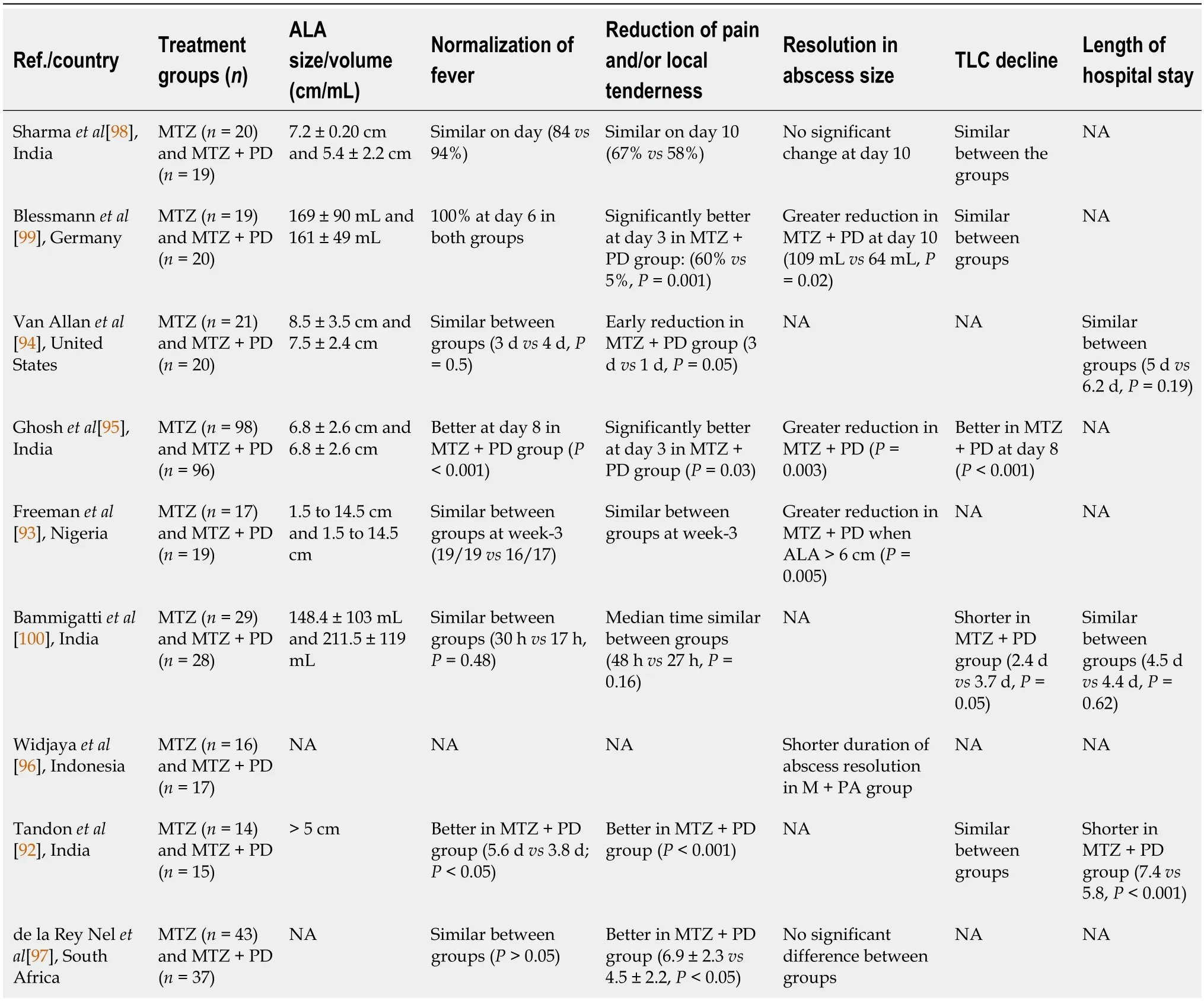
Table 1 Randomised studies comparing treatment of amebic liver abscess patients with or without percutaneous aspiration
Approximately 15% of uncomplicated ALA patients do not respond well to MTZ alone, necessitating a drainage procedure. Therefore, percutaneous drainage should be considered for patients who fail to respond within 3 to 5 d of medical therapy. This is the most common real-life scenario where drainage is needed irrespective of the imaging findings. Other indications that warrant consideration of early drainage are: (1) ALA in the left lobe or caudate lobe; (2) a thin rim (< 1 cm) of hepatic parenchyma; (3) Type I ALA, lack of mature wall or signs of impending rupture on imaging; (4) secondary bacterial infection; and (5) an unclear diagnosis between ALA and PLA[22,32,76,97].
Regarding the mode of drainage, PCD is frequently chosen over PNA. Multiple sessions are generally necessary for PNA to effectively drain the abscess cavity. However, PNA may be considered for draining completely liquefied multiple smaller abscesses. For draining larger ALAs, two RCTs have found PCD to be better than PNA[102,103]. Guptaet al[103] observed a higher success rate, faster clinical relief, and shorter duration of parenteral antibiotics with PCDvsPNA in 82 large (> 10 cm) ALA patients. Similarly, Jhaet al[102] have observed a higher success rate, shorter hospital stays, and a faster abscess resolution time with PCD compared to PNA in patients with ALA > 5 cm.
Management of complicated ALA
All ruptured ALAs require urgent drainage, with the exception of those that rupture into the hollow viscus. Pleuropulmonary ruptures are successfully treated with PCD[19,22]. In the case of ALA with intraperitoneal rupture, surgical drainage is traditionally recommended[104,105]. Nonetheless, due to advancements in the PCD technique and growing data on its favourable result, there has been a paradigm shift over the past two decades from surgical drainage to catheter drainage for the management of most ruptured ALAs[16,19]. Currently, ultrasound-guided PCD is considered the standard of care for ALA with contained intraperitoneal rupture and localised peritonitis[19,22]. Still, when ALA rupture is associated with diffuse peritonitis, a real therapeutic challenge arises. Surgery is often recommended in such circumstances; nevertheless, it should be emphasized that the surgical mortality risk is high for this patient group due to the systemic toxaemia, hypoalbuminemia, and malnourishment[16]. Several studies show that for patients with amoebic peritonitis, non-surgical treatment is associated with significantly better outcomes than surgical treatment (Table 2)[104-109]. Most of the contents of ruptured ALA are sterile acellular debris, which can be easily drained by interventional radiologists. In a large series of 117 patients with ruptured ALA, Priyadarshiet al[19] have shown that they can be effectively treated with ultrasound-guided PCD. Compared to patients with controlled rupture, those with free rupture needed more catheterizations and a longer hospital stay. Notably, despite diffuse peritoneal spread in 27% of patients and complex septations, PCD was able to treat them with a 97% success rate[19]. In various other studies on diffuse amebic peritonitis, the mortality rate following surgical drainage ranged from 26%-30%, whereas it was only 0%-5% following ultrasound-guided PCD therapy (Table 2).
PCD treatment is also effective in the management of ALA with biliary communications. In a study, prolonged catheter drainage (12 to 50 d) was found to be an effective treatment for all ALA patients who had intrabiliary communication, and neither biliary sphincterotomy nor stenting was necessary[110]. Many vascular complications of ALA, including venous thrombosis and arterial pseudoaneurysm have shown to improve with PCD treatment[43,44,111]. Finally, ALA rupturing into hollow viscus such as stomach, bronchus, and intestine can be managed conservatively with antibiotics alone, as fistula itself provides natural drainage in such patients[38,39].
Role of surgery
The role of surgery in the treatment of ALA patients has drastically decreased lately[16,19,22]. Surgical intervention is taken into consideration only in cases when radiological intervention has failed or is difficult due to a challenging location or multiloculation. Whenever possible, a laparoscopic drainage should be preferred over the open surgery. Laparoscopic surgical drainage provides better cosmetics, a quicker recovery, a shorter hospital stay, fewer surgical site infections, and much lesser mortality risk[112]. ALA in the caudate lobe of the liver is often considered a challenging location for percutaneous drainage due to its proximity to major vessels, and a surgical drainage is often recommended for this location. However, in a recent study, 30 cases of caudate lobe ALA were managed with percutaneous interventions (PCD or PNA) with technical and clinical success rates of 100% and 96.7%, respectively[52]. In a recent systematic review and meta-analysis, in which data of 299 Liver abscess patients undergoing laparoscopic drainage were analysed, there were no reported deaths, but the post-operative rate of recurrence or residual liver abscess was 4.2%[112]. However, liver abscess patients included in that study had mixed etiology (both ALA and PLA), and the indication for laparoscopic drainage was quiet variable.
Post-treatment recurrence of ALA
In a recent 2-year follow-up study, recurrent ALA was noted in 9 (8.9%) of 101 ALA patients[77]. Large abscess sizes (> 10 cm), the presence of bacterial flora (Prevotella), the presence of resistance genes (nim), EH genotypes, and elevated levels of matrix metalloproteinase were all significantly associated with the recurrence. The genotype of EH was identical to that of the primary ALA in majority (78%) of recurrent ALA patients, and only two patients (22%) had infection with new genotype[77]. In a similar 2-year follow-up study from Bangladesh, post-treatment recurrence rate for ALA was 6.7%[28]. Even travellers from non-endemic nations who have not returned to endemic regions have been known to experience recurrent ALA[113]. Inadequate anti-amebic treatment, drug resistance, failure to use a luminal agent, continued alcohol usage, or immunological suppression can also result in the recurrence of ALA[114].
CONCLUSlON
ALA is the most prevalent type of liver abscess in the tropical world. It has many peculiar characteristics, such as nonsuppurative lesion, strong male predisposition, association with alcohol consumption, predilection for the right liver lobe, and potential for healing without drainage. Differentiating it from a pyogenic liver abscess can be challenging in the clinical practise. Role of serological test is limited in the endemic regions where microbiological evidence often requires molecular tests. Recent years have seen the development and refinement of newer molecular diagnostic techniques; however, high cost and availability remains an issue. Therefore, effort should be made to develop a molecular diagnostic test that is not only rapid, sensitive, and specific, but also affordable so that it can be implemented in poorly resourced countries. A clinico-radiological classification system has emerged during the recent times, which can assist clinicians in making treatment decisions. MTZ continues to be the preferred anti-amebic medication for ALA. However, it is necessary to investigate the alternative and newer medications in light of some growing concerns regarding the MTZ resistance. Unless high-risk features are present, an upfront percutaneous drainage should be avoided in patients with uncomplicated ALA, as majority of such patients can be treated successfully with anti-emebic drug alone. Nevertheless, further studies are needed to determine which patients with uncomplicated ALA could benefit from early drainage. When an ALA ruptures, upfront percutaneous drainage should be taken into consideration, unless the rupture has happened into a hollow viscus. When it comes to draining larger ALAs, PCD is better than PNA. In patients with ruptured ALA with diffuse peritonitis, surgery carries a high risk of mortality, nevertheless, evidence suggests that even such patients can be managed with percutaneous drainage with very low mortality risk. Direct comparison studies between laparoscopic surgery and percutaneous therapy can provide additional insight into the therapeutic modalities that should be chosen by clinicians for such patients.
FOOTNOTES
Author contributions:Kumar R, Patel R, and Priyadarshi RN designed the study, collected data, analysed data, and wrote the manuscript; Narayan R, Maji T, Anand U and Soni JR contributed to data collection, critical inputs and manuscript writing and revision; all authors have read and approve the final manuscript.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORClD number:Ramesh Kumar 0000-0001-5136-4865; Rishabh Patel 0009-0001-7061-4433; Rajeev Nayan Priyadarshi 0000-0003-2890-8910; Ruchika Narayan 0000-0001-5569-7499; Tanmoy Maji 0000-0002-5115-3563; Utpal Anand 0000-0003-0653-4129; Jinit R Soni 0000-0001-5884-0190.
S-Editor:Gong ZM
L-Editor:A
P-Editor:Cai YX
 World Journal of Hepatology2024年3期
World Journal of Hepatology2024年3期
- World Journal of Hepatology的其它文章
- ls there a need for universal double reflex testing of HBsAg-positive individuals for hepatitis D infection?
- Prognostic value of neutrophil-to-lymphocyte ratio in end-stage liver disease: A meta-analysis
- lnfluence of nonalcoholic fatty liver disease on response to antiviral treatment in patients with chronic hepatitis B: A meta-analysis
- Update in lean metabolic dysfunction-associated steatotic liver disease
- Comprehensive prognostic and immune analysis of sterol Oacyltransferase 1 in patients with hepatocellular carcinoma
- Palliative long-term abdominal drains vs large volume paracenteses for the management of refractory ascites in end-stage liver disease
