Severe liver injury and clinical characteristics of occupational exposure to 2-amino-5-chloro-N,3-dimethylbenzamide: A case series
Meng-Xiao Feng ,Hua Zou ,Yuan-Qiang Lu ,*
a Department of Emergency Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China
b Key Laboratory for Diagnosis and Treatment of Aging and Physic-chemical Injury Diseases of Zhejiang Province, Hangzhou 310003, China
c Occupational Health and Radiation Protection Institute, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou 310051, China
Keywords: 2-amino-5-chloro-N,3-dimethylbenzamide Poisoning Clinical characteristics Liver injury
ABSTRACT Background: The 2-amino-5-chloro-N,3-dimethylbenzamide is a key intermediate in the synthesis of pesticides and pharmaceuticals.However,no literature currently exists on 2-amino-5-chloro-N,3-dimethylbenzamide poisoning in humans.This study aimed to reveal the health hazard of this chemical for humans and summarize the clinical characteristics of patients with occupational 2-amino-5-chloro-N,3-dimethylbenzamide poisoning.Methods: This observational study included four patients with 2-amino-5-chloro-N,3-dimethylbenzamide poisoning from June 2022 to July 2022.The entire course of the incidents was described in detail.Blood 2-amino-5-chloro-N,3-dimethylbenzamide concentrations were detected by a mass spectrometer.Hematoxylin and eosin staining was performed to assess liver injury,and immunofluorescence was used to evaluate hepatic mitophagy.Results: The 2-amino-5-chloro-N,3-dimethylbenzamide powder (99% purity) entered the human body mainly via the skin and respiratory tract due to poor personal protective measures.The typical course of 2-amino-5-chloro-N,3-dimethylbenzamide poisoning was divided into latency,rash,fever,organic damage,and recovery phases in accordance with the clinical evolution.Rash and fever may be the important premonitory symptoms for further organ injuries.The chemical was detected in the blood of all patients and caused multiple organ injuries,predominantly liver injury,including kidney,myocardium,and microcirculation.Three patients recovered smoothly after comprehensive treatments,including artificial liver therapy,continuous renal replacement therapy,glucocorticoids,and other symptomatic and supportive treatments.One patient survived by liver transplantation.The postoperative pathological findings of the removed liver showed acute liver failure,and immunofluorescence staining confirmed the abundance of mitophagy in residual hepatocytes.Conclusions: This study is the first to elaborate the clinical characteristics of patients with 2-amino-5-chloro-N,3-dimethylbenzamide poisoning.The chemical enters the body through the respiratory tract and skin during industrial production.The 2-amino-5-chloro-N,3-dimethylbenzamide poisoning causes multiple-organ dysfunction with a predominance of liver injury.Liver transplantation may be an effective option for patients with severe liver failure.The mechanisms of liver injury induced by 2-amino-5-chloro-N,3-dimethylbenzamide might involve abnormal mitochondrial function and mitophagy.
Introduction
The 2-amino-5-chloro-N,3-dimethylbenzamide (C9H11ClN2O) is a key intermediate in the production of pharmaceuticals and the pesticide chlorantraniliprole.It is also known as K amine in many Chinese industries.The 2-amino-5-chloro-N,3-dimethylbenzamide appears as odorless powder at 20 °C with beige color.To date,nearly all studies about this chemical have focused on its synthetic procedure,while there is no literature reported on 2-amino-5-chloro-N,3-dimethylbenzamide poisoning in humans,and the underlying toxicological mechanisms are unclear for clinicians.Chlorantraniliprole is the end product of 2-amino-5-chloro-N,3-dimethylbenzamide,which was developed by DuPont Company in the year of 2000 and became commercially available in 2008.It belongs to broad-spectrum,high-effectivity insecticides with low toxicity for mammals and humans.The insecticidal mechanism involves the activation of ryanodine receptors [1,2].However,it is unknown whether 2-amino-5-chloro-N,3-dimethylbenzamide has an analogous mechanism of toxicity.
Poisonous substances can enter the body through various pathways including the digestive tract,respiratory tract,or skin.Their toxicity is affected by many factors,such as chemical characteristics,dose,exposure route and duration,age,weight and health status of the patient.The liver is a vital metabolic and detoxifying organ that is susceptible to injury from multiple potential poisonous substances,such as hazardous biochemicals,pesticides,metals,medicines,and plants [3-8].Severe poisoning can lead to acute liver failure or even death.Mitochondria function to provide energy for the cell.Hepatic mitochondrial dysfunction is often associated with various liver diseases,including hepatitis,steatosis,fibrosis,and hepatocellular carcinoma.Evidence indicates that mitochondrial dysfunction is involved in hepatotoxicity induced by various substances,such as carbon tetrachloride,acetaminophen and so on [9-11].Hepatic mitophagy can remove impaired or nonfunctional mitochondria to maintain hepatic function and protect the liver from tissue damage,while inappropriate regulation of mitophagy plays an important role in the pathogenesis of multiple liver injuries [12,13].Currently,the toxicity mechanisms of 2-amino-5-chloro-N,3-dimethylbenzamide remains unknown and needs further exploration.
This study reports four patients with 2-amino-5-chloro-N,3-dimethylbenzamide poisoning for the first time.The course of severe liver injury and clinical features of occupational 2-amino-5-chloro-N,3-dimethylbenzamide poisoning are also elaborated.
Methods
Patients
This observational,single-center study enrolled four patients with acute 2-amino-5-chloro-N,3-dimethylbenzamide poisoning admitted to the First Affiliated Hospital,Zhejiang University School of Medicine from June 2022 to July 2022.All of them were diagnosed based on the history of exposure,clinical characteristics,laboratory detection of 2-amino-5-chloro-N,3-dimethylbenzamide concentration,while other toxic exposure was excluded.The clinical data were collected in detail.
Field investigation
The patients enrolled in this study were occupationally exposed to 2-amino-5-chloro-N,3-dimethylbenzamide powder (99%purity) in the same manufacturing plant during the drying process of 2-amino-5-chloro-N,3-dimethylbenzamide without the presence of other potential toxicants.The working time was 8 h per day for these four patients.The working environment was damp and muggy without proper ventilation.Notably,the body regions of neck,face and forearms were exposed to 2-amino-5-chloro-N,3-dimethylbenzamide powder due to non-standard donning of personal protective equipment.The powder entered the human body mainlyviathe skin and the respiratory tract.
Detection of 2-amino-5-chloro-N,3-dimethylbenzamide concentration
The blood samples from each patient were collected in ethylenediaminetetraacetic acid tubes upon admission.The 2-amino-5-chloro-N,3-dimethylbenzamide concentration was detected by a Waters Xevo TQ-S triple quad mass spectrometer(Chromatographic column C18,Milford,MA,USA).The molecular structure is shown in Fig.1.The samples were mixed with acetonitrile solution at 1:4 (volume/volume) ratio.The mixture was centrifuged for 10 min at 3500 r/min.A total of 5 μL supernatant was injected for analysis by liquid chromatography(LC)-mass spectrometry (MS) chromatograms.In the mobile phase,0.1%formic acid and 0.1% formic acid/acetonitrile were injected by an equal gradient.The retention time of 2-amino-5-chloro-N,3-dimethylbenzamide was 1.31 min.
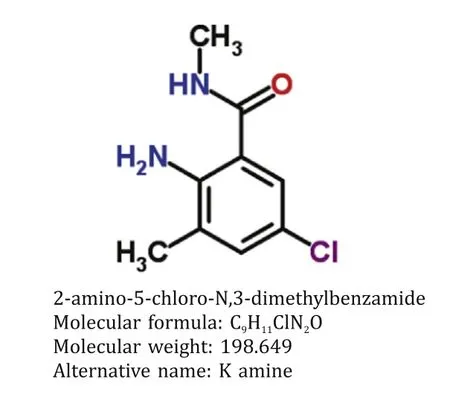
Fig.1. The molecular structure of 2-amino-5-chloro-N,3-dimethylbenzamide.
Histology
Human liver tissue was obtained from patient 4 who underwent liver transplantation.The liver tissue was fixed in 4%formaldehyde and embedded in paraffin.The sections were cut to 4μm thickness.The slices were stained with hematoxylin and eosin (HE),then observed and photographed under a light microscope.
Immunofluorescence
The liver tissues from patient 4 were fixed in 4% formaldehyde and embedded in paraffin.The sections were cut to 4μm thickness,deparaffinized,subjected to antigen retrieve and circles drawing,and then blocked.Anti-TOMM 20 antibodies (1:3000,GB111481,Servicebio,Wuhan,China) were incubated overnight at 4 °C and the secondary antibodies were incubated after the washing steps.Then,the sections were incubated with fluorescent brightener,followed by anti-LC3 A/B antibody (1:200,ab128025,Abcam,Cambridge,MA,USA) and the corresponding secondary antibodies.Subsequently,the slides were incubated with 4’,6-diamidino-2-phenylindole (DAPI) dye solution.After washing,the autofluorescence was quenched and the slides were sealed.Finally,the slices were observed and photographed under a fluorescence microscope.
Results
Patient 1
This 42-year-old male patient sustained liver injury on day 12 after exposure to 2-amino-5-chloro-N,3-dimethylbenzamide powder.He first presented with fatigue,followed by recurrent hyperthermia and other nonspecific symptoms of abdominal pain and distension,nausea,and vomiting.He carried hepatitis B virus but exhibited no liver dysfunction before the poisoning.Notably,the patient took a shower immediately after work every time and did not experience obvious rash during the disease course.The details of disease course for all patients were summarized in Fig.2.The 2-amino-5-chloro-N,3-dimethylbenzamide concentration was detected in the blood although the patient had been out of exposure for 3 days (Fig.3).He developed a distinct acute liver injury,exhibiting sharply increasing liver function indices of alanine transaminase (ALT),aspartate transaminase (AST),total bilirubin (TBIL),prothrombin time (PT) and model for end-stage liver disease (MELD) score.Notably,the serum level of AST was transiently higher than ALT (Fig.4 A).Besides,he appeared to have obvious acute kidney injury and slight myocardial injury during hospitalization,displaying increased serum level of creatinine and creatine kinase-MB (CK-MB) (Fig.5 A).He received comprehensive treatment,including artificial liver therapy,continuous renal replacement therapy (CRRT),methylprednisolone glucocorticoid,and other symptomatic and supportive measures,such as liver protection,improving jaundice,infection control,albumin infusion,stomach protection,etc.Finally,he recovered and was discharged successfully (Table 1).
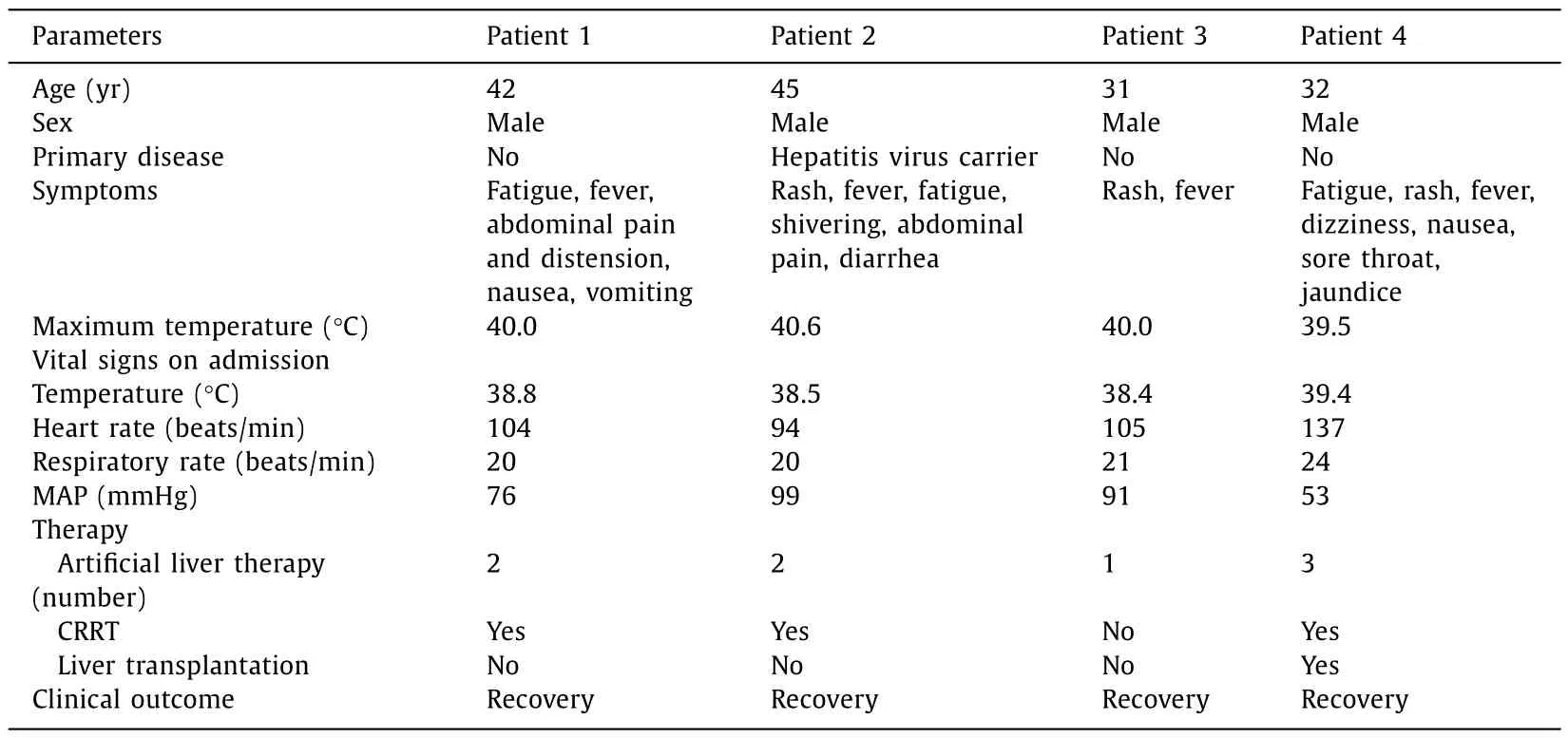
Table 1Baseline and clinical characteristics of four patients with 2-amino-5-chloro-N,3-dimethylbenzamide poisoning.

Fig.2. The specific disease courses of each patient.Red rectangle: artificial liver therapy.
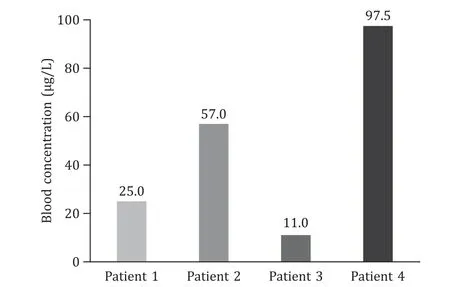
Fig.3. Blood concentrations of 2-amino-5-chloro-N,3-dimethylbenzamide on admission.
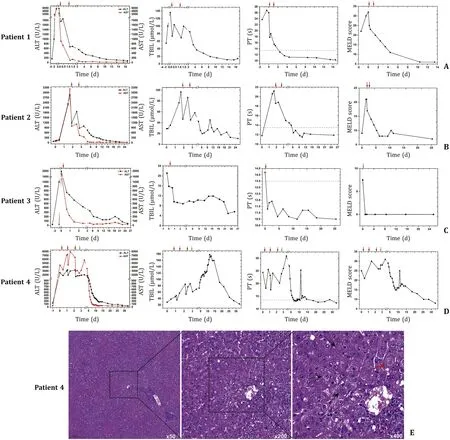
Fig.4. The 2-amino-5-chloro-N,3-dimethylbenzamide induced significant liver injury in humans.A -D: The trends of liver function indices for these four patients.Day 0 was defined as the day when the patients were admitted to our hospital.Red arrow: time of artificial liver therapy;Green arrow: time of liver transplantation.E :Histopathological findings of liver tissue from patient 4.Black arrow: necrotic hepatocytes;Red arrow: swollen hepatocytes;Blue arrow: inflammatory cell infiltration.ALT:alanine aminotransferase;AST: aspartate aminotransferase;TBIL: total bilirubin;PT: prothrombin time;MELD: model for end-stage liver disease.

Fig.5. The 2-amino-5-chloro-N,3-dimethylbenzamide induced other organ injuries in humans.Day 0 was defined as the day when the patients were admitted to our hospital.Red arrow: time of artificial liver therapy;Green arrow: time of liver transplantation.Black arrow: time of CRRT.CK-MB: creatine kinase myocardial isoenzyme;CRRT: continuous renal replacement therapy.
Patient 2
This 45-year-old male patient showed the onset symptom of rash on the forearms and neck after 14 days exposure to 2-amino-5-chloro-N,3-dimethylbenzamide powder.The rash was red and fused with an obvious itch.Five days later,hyperthermia and liver injury both emerged with other nonspecific symptoms of shivering,abdominal pain,and diarrhea (Fig.2).This chemical was detected in the blood at the end of the day of exposure (Fig.3).The liver function got worse within 1 day after admission.Transient ALT/AST of less than 1 appeared on day 7 after onset (Fig.4 B).This patient also manifested transient kidney and myocardial injury,as well as consistently elevated level of lactate during hospitalization(Fig.5 B).Comprehensive therapies similar to those adopted for patient 1 were timely conducted.He finally showed satisfactory clinical improvement (Table 1).
Patient 3
This 31-year-old male patient first displayed similar rash in the forearms and neck after 12 days exposure to 2-amino-5-chloro-N,3-dimethylbenzamide powder.Hyperthermia and liver injury emerged successively (Fig.2).Slight blood 2-amino-5-chloro-N,3-dimethylbenzamide was detected on day 6 out of exposure (Fig.3).He exhibited rapidly increasing liver enzymes and transiently prolonged PT,while TBIL levels were normal during the disease course (Fig.4 C).Besides,this patient showed no kidney and myocardial injury,with mildly and transiently elevated lactate level (Fig.5 C).He presented a significant improvement after comprehensive treatments except CRRT(Table 1).
Patient 4
This 32-year-old male patient presented a rapid onset of fatigue on day 5 after exposure to 2-amino-5-chloro-N,3-dimethylbenzamide powder,followed by small rashes in the neck and face,and hyperpyrexia,and other symptoms of dizziness,nausea,sore throat and jaundice (Fig.2).The highest blood concentration was detected in this patient on day 3 out of exposure compared with the others (Fig.3).He developed the most rapid and severe liver failure among the four patients after 12 days of exposure.The ratios of ALT/AST were maintained at less than 1 for about 5 days in the early stage during hospitalization (Fig.4 D).Unfortunately,this patient indicated persistently progressing liver failure despite comprehensive treatments.Therefore,he had to undergo liver transplantation to save his life on day 10 after disease onset and obtained a good clinical outcome (Table 1).The histopathological presentations of the removed liver tissue confirmed the existence of acute liver failure.The gross view exhibited gray-yellow and gray-green liver cut surface without an obvious mass.HE staining showed a clearly destroyed liver lobular structure and hepatocyte swelling with patchy necrosis.The liver confluence zone and interstitium indicated a large infiltration of acute and chronic inflammatory cells (Fig.4 E).In addition,kidney and myocardial injury were apparent (Fig.5 D).He also showed refractory elevation of lactate levels with an ischemic change of toes,revealing that 2-amino-5-chloro-N,3-dimethylbenzamide could induce severe microcirculatory disturbance.This patient ended up with liver transplantation.
To provide further insights into the underlying mechanisms of 2-amino-5-chloro-N,3-dimethylbenzamide toxicity,we applied immunofluorescence staining to evaluate mitophagy in the removed liver tissues.Significant colocalization of TOMM20 and LC3A/B was observed in the liver section,which confirmed the abundance of mitophagy in 2-amino-5-chloro-N,3-dimethylbenzamideinduced liver failure (Fig.6).
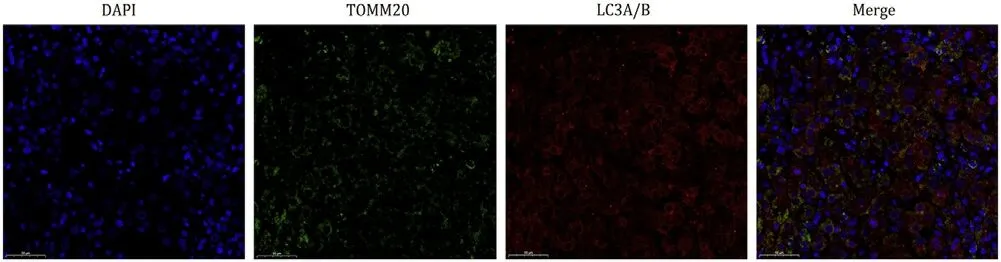
Fig.6. Mitophagy-related immunofluorescent staining of the liver tissue from patient 4.Mitochondria were labeled with TOMM20 (green fluorescence).Autophagy was labeled with LC3A/B (red fluorescence).The slices were observed and photographed under a fluorescence microscopy at original magnification × 400.
Discussion
The 2-amino-5-chloro-N,3-dimethylbenzamide is generally used as an intermediate in the synthesis of pharmaceuticals and chlorantraniliprole,thus has been popular worldwide including many Chinese industries.To the best of our knowledge,there is no study on 2-amino-5-chloro-N,3-dimethylbenzamide poisoning in humans.Our study indicated that 2-amino-5-chloro-N,3-dimethylbenzamide could cause the occurrence of poisoning event,even severe adverse event of organ failure requiring organ transplantation.We found that the whole disease course was divided into latency,rash,fever,organic damage,and recovery phase in accordance with the clinical symptoms.The rash was mainly distributed in the neck,face and forearms that were exposed for 2-amino-5-chloro-N,3-dimethylbenzamide powder in the lack of appropriate protection.Rash was not observed on the body parts that were not exposed to the powder.That is to say,the rash site was on exposed skin.Besides,the rashes gradually evolved to pigmentation with the improved condition.The 2-amino-5-chloro-N,3-dimethylbenzamide could induce multiple organ injuries,predominantly liver injury.Each phase was shown to potentially occur together and be cross-presented.Rash and fever may be the important premonitory symptoms for further organ injuries,which implies the importance of early prevention from exposure to the toxic environment and timely medical treatment.Overall,the poisoned patients made gradual recovery after comprehensive treatments or a progression to liver failure necessitated liver transplantation (Fig.7).In addition,we found that the blood 2-amino-5-chloro-N,3-dimethylbenzamide concentration of patient 4,the most severe one,was significantly higher than that of patient 3.But we could not determine whether early detection or exposure caused the higher concentration in patient 4 due to lacking dynamic monitoring of blood concentration during their working.Therefore,the dosage-effect relationship between 2-amino-5-chloro-N,3-dimethylbenzamide concentrations and disease severity needs further exploration.
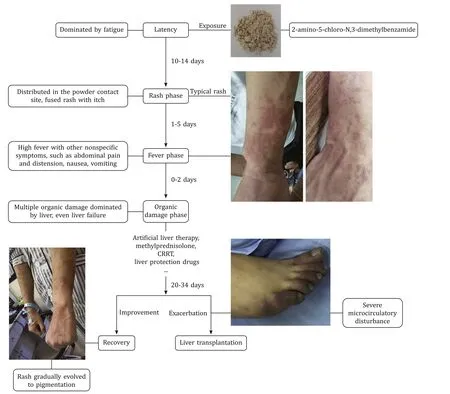
Fig.7. Summary of clinical course of 2-amino-5-chloro-N,3-dimethylbenzamide poisoning.CRRT: continuous renal replacement therapy.
In this study,the four patients mainly presented with liver injury and even life-threatening liver failure,revealing the liver as a likely target organ.Diverse chemical substances can be absorbed to produce occupational organs dysfunction when effective protective measures are not enough.Liver is an important metabolic and detoxifying organ where many poisons are absorbed,accumulated or metabolized.Chronic occupational exposure to organic compounds,such as n-hexane,may damage the liver in humans [14].Repeated exposure to various heavy metals and metalloids has a chronic and long-term poisonous effect on the liverviaingestion,inhalation,and skin contact,such as aluminum,chromium,cobalt,copper,cadmium,selenium,and arsenic [15-18].Severe patients may develop liver failure,multi-organ failure and death.Therefore,early identification,timely isolation from exposure and effective therapies are critical to treat poisoning patients.Liver transplantation is the only curative treatment for end-stage chronic liver disease,when standard conservative measures fail.Liver transplantation has been applied in the toxicological field.It is sometimes the only way to save patients’ life.Multiple poisons and certain drugs,including mushroom,yellow phosphoruscontaining rodenticide,chromated copper arsenate,and acetaminophen [19-22]cause liver failure.In the present study,liver transplantation was also suggested as an effective therapy to save the life of patient 4 with liver failure induced by 2-amino-5-chloro-N,3-dimethylbenzamide.
The underlying mechanisms of 2-amino-5-chloro-N,3-dimethylbenzamide toxicity are still unclear.Chlorantraniliprole,the end product of 2-amino-5-chloro-N,3-dimethylbenzamide,is the first insecticide with the structure of anthranilic diamides.It has been attributed low toxicity in humans albeit with undefined toxicology.Only one study [23]reported a 26-year-old female patient presented with symptomatic Mobitz type I atrioventricular block after ingesting 10 mL chlorantraniliprole and without other organ injury.This clinical manifestation was potentially related to the activation of ryanodine receptors,although further exploration was lacking [1,2,23].In contrast,the entering paths in our study were the skin and respiratory tract.Furthermore,the patients in our study presented multi-organ injuries with a predominance of liver.No patient manifested obvious atrioventricular block on the electrocardiogram;therefore,we speculated the presence of other toxic mechanisms of 2-amino-5-chloro-N,3-dimethylbenzamide,which were distinct from the mechanisms of chlorantraniliprole toxicity.
Hepatotoxicity induced by various substances occurs in multiple forms,including direct hepatotoxicity,immune-mediated liver injury,and mitochondrial dysfunction [24,25].Hepatocytes are rich in mitochondria that contribute to maintaining the energy hemostasis and regulating the biological function of liver tissues.Increasing evidence suggests that mitochondrial dysfunction and membrane damage participate in the toxic mechanisms of hepatic injuries,and the establishment and progression of hepatoma,cholangiocarcinoma and chronic hepatitis [26-28].Therefore,it is necessary to maintain mitochondrial homeostasis,with mitophagy playing an essential role: it helps to remove dysfunctional and damaged mitochondria.However,excessive mitophagy can deplete mitochondria and cause metabolic disorders,which may accelerate the progression of liver disease [29-32].In the present study,histopathological presentations of removed liver tissue obviously indicated liver failure.All patients represented hyperpyrexia,implying the possibility of energy metabolism disturbance.Ratios of ALT/AST less than 1 appeared during the disease course in three patients,revealing that mitochondrial dysfunction might participate in hepatotoxicity induced by 2-amino-5-chloro-N,3-dimethylbenzamide.The immunofluorescence of removed liver tissues indicated the existence of a high level of mitophagy.Therefore,we speculated that the mechanisms of 2-amino-5-chloro-N,3-dimethylbenzamide-induced liver injury may involve abnormal mitochondrial function and mitophagy.
Events of 2-amino-5-chloro-N,3-dimethylbenzamide poisoning are extremely rare.This study is the first to elaborate the clinical characteristics of 2-amino-5-chloro-N,3-dimethylbenzamide poisoning to remind clinicians of its significance and to help guide treatments.The 2-amino-5-chloro-N,3-dimethylbenzamide can enter the human body through the skin and the airway,which emphasizes the importance of personal protective measures during industrial production.In addition,this study provides a certain level of assistance for the intensive investigation of specific toxic mechanisms.
Inevitably,this study still has some limitations: first,the small sample size may limit the robustness of the conclusions,while 2-amino-5-chloro-N,3-dimethylbenzamide poisoning is especially rare.Second,liver biopsy is an invasive examination,so our pathological and immunofluorescence staining results after liver removal in patient 4 was performed without control.Third,our study could not explore the toxic mechanisms in depth,and the specific molecular processes require further elucidation.The injury mechanisms of other organs also need to be further studied.
In conclusion,2-amino-5-chloro-N,3-dimethylbenzamide can cause multiple-organ injury with a predominance of liver injury when entering through the respiratory tract and skin during industrial production.Liver transplantation is an effective treatment option for patients with severe liver failure because of this type of poisoning.The mechanisms of 2-amino-5-chloro-N,3-dimethylbenzamide-induced liver injury might involve abnormal mitochondrial function and mitophagy.
Acknowledgments
None.
CRediT authorship contribution statement
Meng-Xiao Feng:Data curation,Formal analysis,Funding acquisition,Writing -original draft.Hua Zou:Investigation,Methodology.Yuan-Qiang Lu:Conceptualization,Formal analysis,Funding acquisition,Investigation,Supervision,Writing -review &editing.
Funding
This work was supported by grants from the Key Research and Development Program of Zhejiang Province (2019C03076) and the Fundamental Research Funds for the Central Universities (226-2022-00088).
Ethical approval
This study was approved by the Ethical Committee of the First Affiliated Hospital,Zhejiang University School of Medicine(2022803).The treatment protocols were carried out by the principles of theDeclaration of Helsinki.Informed consent was obtained from all cases included in the study.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2024年2期
Hepatobiliary & Pancreatic Diseases International2024年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Editors
- Information for Readers
- Meetings and Courses
- Liver transplantation and liver resection as alternative treatments for primary hepatobiliary and secondary liver tumors: Competitors or allies?
- Laparoscopic anatomical liver resection of segment 7 using a sandwich approach to the right hepatic vein (with video)
- Propensity score matching analysis for clinical impact of braided-type versus laser-cut-type covered self-expandable metal stents for endoscopic ultrasound-guided hepaticogastrostomy
