A pilot study of the differentiated landscape of peripheral blood mononuclear cells from children with incomplete versus complete Kawasaki disease
Shu-Na Sun·Yan Zhou·Xing Fu·Yuan-Zheng Zheng·Cao Xie·Guo-You Qin·Fang Liu·Chen Chu·Feng Wang·Cheng-Long Liu·Qing-Tong Zhou·De-Hua Yang·Di Zhu·Ming-Wei Wang,8,9·Yong-Hao Gui
Kawasaki disease (KD) is a disorder of immune responses.The prevalence of KD among 100,000 children aged 0—4 years was 71.9—110.0 in China,170.9—194.9 in Korea,and 18.1—21.3 in the USA [1].The most severe complication of KD is coronary artery lesions (CALs),including coronary dilatation and coronary aneurysms,which may even lead to myocardial ischemia,myocardial infarction,and sudden death.The assessment of CALs is based primarily on maximal coronary artery luminal dimensions (normalized asZscores [2].The case fatality rate of KD in Japan was 0.015%from 2011 to 2012 [3].Among adults <40 years of age and children with KD,coronary artery aneurysms accounted for 5% of acute coronary syndromes [4].The incidence of KD is highest among the Asian population,and it is now the most common cause of CALs among children in developed countries [5].The recent primary healthcare reforms in lowand middle-income countries were expected to change this situation [6,7].The efficacy of intravenous immunoglobulin(IVIG) administered in the acute phase of KD is well established and reduces the incidence of CALs [8— 10].
The diagnosis of complete Kawasaki disease is made utilizing clinical criteria and excluding other similar clinical diseases.Patients who do not have sufficient principal clinical f indings may be considered as incomplete KD.These patients are still at risk for coronary artery abnormalities.The American Heart Association (AHA) created an algorithm to aid in the evaluation of incomplete KD [2].These patients may experience delayed diagnosis or be misdiagnosed,and delayed treatment leads to an increased risk of coronary artery damage.Due to difficulties in diagnosing incomplete KD solely based on its atypical clinical symptoms,it is of great signif icance to explore the immunological characteristics and differentially expressed genes (DEGs)associated with incomplete KD.
Although the etiology of KD remains elusive,most studies suggest that KD is a disorder of the immune-mediated inf lammatory cascade in both innate and adaptive immunity with the release of several pro-inf lammatory cytokines[11— 14].Recent studies have demonstrated that regulatory T(Treg) cells and monocyte subsets are biomarkers in determining the severity and susceptibility of KD [15— 19].The revolution in single-cell RNA sequencing (scRNA-seq)and gene ontology (GO) analysis has enabled an unbiased quantif ication of gene expression in thousands of individual cells [20— 23] and is capable of revealing common biological pathways [24,25],thereby providing more efficient tools to decipher immune responses involved in human diseases,including KD [26,27].This study aimed to elucidate the cellular landscape of KD using peripheral blood mononuclear cells (PBMCs) for scRNA-seq analysis,with emphasis on the differences in immune responses between incomplete and complete KD.In this investigation,we tried to explore the percentage of each immune cell type,the differentially expressed genes,the GO enrichment,and the T-cell receptor (TCR)/B-cell receptor (BCR) clone proportions.These immune parameters were detected by routine scRNA-seq analysis,which includes the following steps.All computational analyses were performed using Seurat,SingleR,scRepertoire,and other well-known scRNA-seq analysis software.
(1) FASTQ reads alignment and gene-barcode matrix generation;
(2) Quality control,data normalization,and dimension reduction;
(3) Clustering analysis and cell type annotation;
(4) Cell-type percentage calculation;
(5) Differentially expressed gene and GO enrichment analysis;
(6) TCR and BCR clonotype annotations;
(7) Proportion of expanded TCR/BCR clones (clone size >1) and expanded T and B cells.
We collected 11 fresh peripheral blood samples derived from three healthy children,two incomplete KD patients,and two complete KD patients (Fig.1).For each patient,the f irst blood sample was taken on the f ifth day after the onset of fever before IVIG therapy.The second sample was obtained at 48 hours after completion of IVIG therapy and subsidence of fever.In addition,they did not develop CALs and were not IVIG resistant.The diagnosis of complete and incomplete KD was made using the criteria proposed by the AHA [2].The diagnosis of complete KD includes fever and the presence of ≥ 4 of the f ive principal clinical features:extremity changes,rash,conjunctivitis,oral changes and cervical lymphadenopathy.Patients who lacked these full clinical features were considered to have incomplete KD.All patients and controls presented no recent history of fever,infection or immunization.

Fig.1 tSNE map and presentation of the proportion of immune cell types.a tSNE analysis performed with scRNA-seq analysis(H: healthy children,P: KD patient).b Cell clustering analysis performed with scRNA-seq analysis.c Total cell numbers of CD4+Th cells,CD4+naïve T cells,CD8+naïve T cells,CD8+Teffcells,NK cells,plasma cells,memory B cells,naïve B cells,monocytes,and neutrophils in all samples.d Cell number analysis in healthy,incom-plete KD (before and after treatment) and complete KD (before and after treatment) groups.e Cell fraction analysis in different samples.f The analysis of the percentage of immune cell number changes in different samples.tSNE t-distributed stochastic neighbor embedding,scRNA-seq single-cell RNA sequencing,KD Kawasaki disease,CD cluster of differentiation,Th helper T,Teffeffector T,NK natural killer
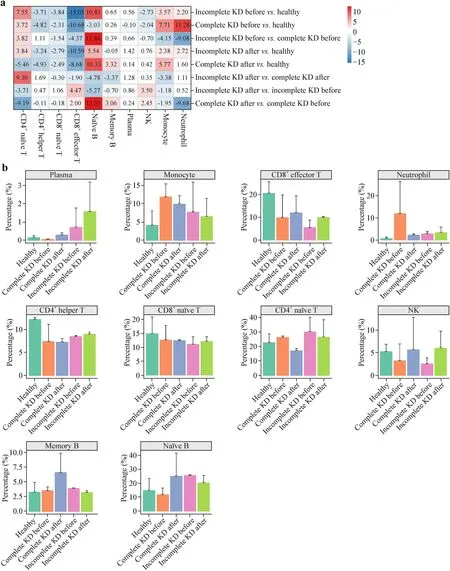
Fig.2 Percentage of each immune cell types in different groups.a Heatmap analysis of cell number changes of CD4+Th cells,CD4+naïve T cells,CD8+naïve T cells,CD8+Teffcells,NK cells,plasma cells,memory B cells,naïve B cells,monocytes,and neutrophils in healthy,incomplete,and complete KD patients before and after IVIG therapy.b Cell fraction analysis of each cell types in different groups.The Wilcox test was performed.Although there were no statistically signif icant differences between groups,there were differences in averages between groups.CD cluster of differentiation,KD Kawasaki disease,IVIG intravenous immunoglobulin,Th helper T,Teffeffector T,NK natural killer
PBMCs were isolated from blood samples.The cell types identif ied by scRNA-seq with two-dimensional representation are shown in Fig.1.The percentage of each immune cell type for incomplete and complete KD patients before and after IVIG therapy was analyzed using the Wilcox test.Figure 2 and Supplementary Table 1 show that incomplete KD patients had a decreased percentage of monocytes,neutrophils and NK cells compared to complete KD patients,which suggesting that innate immunity is more dominant in complete KD than incomplete KD.The increased percentage of cluster of differentiation (CD)4+naïve T,CD4+Th,naïve B,memory B cells,and the decreased percentage of CD8 +naïve T,CD8+Teffcells were found in incomplete KD compared with complete KD.The percentage of plasma cells was increased in incomplete KD patients compared to both complete KD and control subjects.Compared with the controls,both incomplete and complete KD patients displayed an increased percentage of CD4+naïve T cells,memory B cells,monocytes,and neutrophils,and a decreased percentage of CD4+helper T (Th),CD8+naïve T,CD8+effector T (Teff),and natural killer (NK) cells,in line with a previous report demonstrating an increased percentage of B cells and a lower percentage of CD8+Teffand NK cells in KD patients [26].These decreased cell types were all increased in incomplete KD after treatment.
TCR/BCR analysis and the clonal dynamics of T/B-cell cell detection (data not shown) were performed,and there were differences in averages between groups.T cells with clonal TCRs (clone size >1) were more enriched,and the number of expanded T cells and expanded different TCR clones were more remarkable for CD8+Teffcells than other T-cell types before and after therapy,supporting the proposition that CD8+Teffcells are directly involved in the pathogenesis of KD.Compared with complete KD patients,clonal expansions of CD4+naïve T,CD4+Th,and CD8+naïve T cells were increased,while that of CD8+Teffcells was decreased in incomplete KD patients.The clonal expansions of CD4+naïve T,CD4+Th cells,CD8+naïve T,and CD8 +Teffcells were all increased following IVIG treatment in the incomplete KD group (Fig.3 a,b and Supplementary Table 1).Likewise,B cells with clonal BCRs (clone size >1)were enriched for naïve B and memory B cells among KD patients,an observation in line with a previous study [26]and supporting a role of acquired immunity in the development of KD.In comparison with complete KD,the clonal expansion of naïve B and memory B cells was elevated in incomplete KD.The clonal expansion of memory B cells was remarkable in incomplete KD.After therapy,the clonal expansion of memory B cells was decreased in incomplete KD but increased in complete KD patients (Fig.3 c,d and Supplementary Table 1).
To identify genes involved in immune responses,we performed differential expression analysis separately for each cell type (Fig.4 and Supplementary Fig.2).GO and pathway analyses revealed that compared with complete KD,monocyte and granulocyte chemotaxis,neutrophil chemotaxis,and activation and regulation of leukocyte apoptotic processes were upregulated in incomplete KD patients,consistent with a markedly reduced percentage of monocytes and neutrophils before treatment.The expression of DEGs enriched for functions such as differentiation of mature B cells,regulation of antigen receptor-mediated signaling pathways,and p38 mitogen-activated protein kinase (p38 MAPK) activity important for initiation of B-cell signal transduction [27,28] was upregulated in incomplete vs.complete KD patients.This resulted in higher numbers of memory B and plasma cells in incomplete KD patients before treatment.Downregulated DEGs responsible for antigen processing and presentation of peptide antigen via major histocompatibility complex (MHC) class I and upregulated DEGs involved in T-cell differentiation coincide with a reduced percentage of CD8+Teffcells and an elevated percentage of CD4+naïve T cells in incomplete KD vs.complete KD.Upregulated DEGs among incomplete KD patients also cover functionalities related to T-cell activation,positive regulation of the T-cell receptor signaling pathway,regulation of interleukin (IL)-12 production which is important for T-cell activation [29],and nuclear factor kappa B (NFκB)-mediated T-cell signaling [30].In addition,the expression of human leukocyte antigen (HLA)-DRB5 encoding MHC class II [31] was upregulated before therapy in incomplete KD.These data were supported by a high percentage of CD4+Th cells in incomplete KD vs.complete KD prior to therapy.After treatment with IVIG,the percentage of most immune cell types tended to be normal in both incomplete and complete KD patients.The decreased percentage of CD4+Th and CD8+Teffcells observed before therapy did not return to normal after treatment,suggesting that def iciency in CD4+Th and CD8+Teffcells may be a risk factor for KD.

Fig.4 Gene ontology enrichment analysis of DEGs.a The number of DEGs in different immune cells between incomplete and complete KD groups,before or after treatment.b–f GO pathway analysis of monocyte,CD8+Teff,CD4+naïve T cells,CD4+Th,and CD8 +naïve T cells in incomplete and complete KD groups before or after treatment.DEG differentially expressed genes,GO gene ontology,CD cluster of differentiation,Th helper T,Teffeffector T
Excessive immune response is the most notable clinical hallmark of KD.Before therapy,many DEGs enriched for regulating immunity were upregulated in both incomplete and complete KD patients compared to the control,especially in monocytes and CD8+Teffcells.GO and pathway analyses suggested that these DEGs enriched in several functional categories including activation and chemotaxis of immune cells,positive regulation of immune response and processes,regulation of interferon signaling pathway,positive regulation of cytokine production,related functionalities covering monocyte and granulocyte chemotaxis,response to interferon,tumor necrosis factor (TNF) production,regulation of extracellular regulated protein kinases 1(ERK1) and ERK2 cascade,regulation of Fc receptor-mediated stimulatory signaling pathway,regulation of toll-like receptor signaling pathway,and regulation of dendritic cell differentiation and chemotaxis.After IVIG therapy,most of these upregulated DEGs was downregulated.It seems that IVIG therapy reduced the difference in DEG expression,and further analysis of these genes may identify potential diagnostic markers and therapeutic targets for KD.The different expression patterns of the same gene in different cell types were variable,which is rare in other studies.
As shown in Fig.4 and Supplementary Fig.2,before treatment,monocytes were the cell type with the largest number of DEGs,exhibiting upregulated toll-like receptor signaling,IκB/NFκB signaling,NFκB-inducing kinase(NIK)/NFκB signaling and p38 MAPK cascade to inf lammatory factor release,and positive regulation of IL-1β,IL-8,and TNF-α production among incomplete KD patients.The toll-like receptor signaling pathway can activate macrophages and increase the production of type I interferon,leading to severe inf lammation [32,33].IκB/NFκB signaling,NIK/NFκB signaling,and the p38 MAPK cascade play important roles in mediating immune cell activation and inf lammatory factor production [34].Analysis of the DEGs enriched for immune cell activation and responsiveness revealed that patients with incomplete KD displayed more active immune responses and stronger chemotaxis than patients with complete KD.The expression levels of most of these DEGs in incomplete KD were not only higher than those in complete KD but also signif icantly altered after treatment.Following therapy,the DEGs enriched for positive regulation of IκB/NFκB signaling,NIK/NFκB signaling and p38 MAPK cascade in monocytes were downregulated in incomplete KD patients.It appears that monocytes are important target cells for IVIG therapy,which is more evident in incomplete KD.
The DEGs responsible for the Fc receptor-mediated stimulatory signal pathway [35],dendritic cell differentiation,and positive regulation of dendritic cell chemotaxis were upregulated in incomplete KD patients,consistent with enhanced dendritic cell antigen processing and presentation.The DEGs related to the regulation of platelet aggregation,platelet activation,and blood coagulation were upregulated in KD patients vs.controls and in incomplete KD vs.complete KD.This can explain the increase in platelets in KD patients [13] and suggests an increased risk of thrombosis with CALs in incomplete KD vs.complete KD patients.
Arteriopathy associated with KD includes three pathological processes [2]: (1) necrotizing arteritis consisting of a synchronized neutrophilic process that progressively destroys the arterial wall,causing aneurysms;(2) subacute/chronic vasculitis characterized by asynchronous inf iltration of lymphocytes,plasma cells and macrophages;and (3) luminal myof ibroblastic proliferation (LMP) characteristic of a unique medial smooth muscle cell-derived myof ibroblastic process with the potential to cause progressive arterial stenosis.As revealed by GO and pathway analyses,the DEGs related to inf lammatory responses,f ibroblast growth factor promoting the myof ibroblastic process [36],ERK1 and ERK2 signaling pathways related to f ibroblast proliferation [37],positive regulation of myoblast differentiation,and cellular response to vascular endothelial growth factor stimulus were all upregulated in incomplete KD than complete KD,implying that the risk of vascular necrosis,subacute/chronic vasculitis,LMP,and coronary artery damage [38] is higher in patients with incomplete KD.
In addition,the expression of chemokine receptor type 4 (CXCR4) in CD8+Teffcells,S100A12 and S100A8 in monocytes,S100A11 in neutrophils and MYC in CD4 +Th cells was upregulated before therapy,and the expression of S100A12,S100A8,S100A11,and MYC was downregulated after therapy in both incomplete and complete KD patients (Fig.5).MYC is essential in metabolic reprogramming and activated T cells require a substantial increase in energy production [39,40].Since S100A12,S100A8,S100A11,and CXCR4 exert pro-inf lammatory actions [41— 44] and the blood levels of IL-1β,IL-2,IL-6,IL-8,IL-12,TNF-α,complement 3 (C3),and C4 were all increased in both incomplete and complete KD patients before treatment (Fig.6).Our results suggest an overreacted immune system coupled by excessive production of inf lammatory factors.Meanwhile,the levels of IL-1β,IL-8,and TNF-α in incomplete KD were signif icantly increased compared with those in complete KD,as evidenced by the upregulation of DEGs related to IL-1β,IL-8,and TNF-α production;both of which are crucial inf lammatory mediators in the pathogenesis of KD [45— 47]and have been targeted in the treatment of IVIG-resistant patients,such as inf liximab (a monoclonal antibody against TNF-α) and anakinra (recombinant IL-1β receptor antagonist) [2,48].Compared with incomplete KD,the level of C4 was elevated in complete KD patients,indicating more pronounced innate immunity.

Fig.5 Analysis of gene expression in incomplete and complete KD patients.Gene expression analysis of MYC,CXCR4,HLA-DRB5,S100A8,S100A12,and S100A11 in healthy,incomplete,and complete KD patients,before or after treatment.Comparisons between groups were made by ANOVA (t test with Bonferroni correction) and P <0.05 was considered statistically signif icant.KD Kawasaki disease,CXCR4,chemokine receptor type 4,HLA human leukocyte antigen

Fig.6 Analysis of the expression of inf lammatory factors.a tSNE analysis of IL1-β,IL-2,IL-6,IL-8,IL-12,TNF-α,C3,and C4 in scRNA-seq samples from incomplete and complete KD patients before treatment.b ELISA analysis of IL1-β,IL-2,IL-6,IL-8,IL-12,TNF-α,C3,and C4 in incomplete and complete KD patients before treatment.tSNE t-distributed stochastic neighbor embedding,IL interleukin,TNF tumor necrosis factor,C3 complement 3,C 4 complement 4,scRNA-seq single-cell RNA sequencing,KD Kawasaki disease,ELISA enzyme-linked immunosorbent assay
In summary,the possible biological processes potentially underlying the changes in the peripheral cellular landscape in incomplete and complete KD patients involving both innate and adaptive immunity and the global and dynamic immune responses unique to each cell type were described.Our f indings not only provide valuable insights into the immunological basis of KD that may facilitate better diagnosis and treatment but are also expected to contribute to deciphering the difference in pathogenesis between incomplete and complete KD.In incomplete KD patients,CD4+naïve T,CD4+Th,memory B cells,and plasma cells were increased;whereas,monocytes,neutrophils,NK cells,and CD8+Teffcells were decreased.The clonal expansions of CD4+Th and especially memory B cells were increased,while CD8 +Teffcells were decreased in incomplete KD.DEG assessment revealed that functionalities of toll-like receptor signaling,IκB/NFκB signaling,NFκB-inducing kinase (NIK)/NFκB signaling,p38 MAPK cascade,Fc receptor-mediated pathway,ERK1 and ERK2 signaling,dendritic cell antigen processing and presentation were upregulated;while,MHC class I was downregulated in incomplete KD.The expression of HLA-DRB5 encoding MHC class II was upregulated.The levels of IL-1β,IL-8,and TNF-α were signif icantly high,consistent with the upregulated relating DEGs in incomplete KD patients.The immunological and molecular biological characteristics may be helpful for the diagnosis of incomplete KD.
This study had a limitation with limited sample size.More patients at earlier stages will be included to expand the sample size to observe if more signif icant differences exist between incomplete and complete KD,along with the above immunological and molecular changes.This may allow us to clarify whether the detected changes are the manifestation of the course of KD or KD patients have inherent features in their immune responses,thereby facilitating disease development.
Supplementary lnformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s12519-023-00752-4.
AcknowledgementsWe would like to thank the work done by the National Center for Drug Screening,Shanghai Institute of Materia Medica,Chinese Academy of Sciences and Accuramed Technology(Shanghai),and Department of Pharmacology,School of Basic Medical Sciences,Fudan University.
Author contributionsSSN: writing—original draft.ZY,ZYZ,XC,YDH: data curation.FX,ZD,LCL,QGY: data analysis.CC,WF:collecting samples.ZQT,LF: writing—review.GYH,WMW: study designing,writing—review and editing.SSN and ZY contributed equally.All authors read and approved the f inal manuscript.
FundingThis work was partially supported by the National Natural Science Foundation of China 81872915 (WMW),82073904 (WMW),82121005 (YDH),81973373 (YDH),21704064 (ZQT) and 82270313(SSN);National Science &Technology Major Project of China—Key New Drug Creation and Manufacturing Program 2018ZX09735-001(WMW) and 2018ZX09711002-002-005 (YDH);National Major Project of China Science and Technology Innovation 2030 for Brain Science and Brain-Inspired Technology 2021ZD0203400 (ZQT);the National Key Basic Research Program of China 2018YFA0507000(WMW);Shanghai Municipal Education Commission—Shanghai Top-Level University Capacity Building Program DGF817029-04(WMW);Hainan Provincial Major Science and Technology Project ZDKJ2021028 (YDH and ZQT) and Shanghai Municipality Science and Technology Development Fund 21JC1401600 (YDH).
Data availabilityThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interestNo f inancial or non-f inancial benef its have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approvalThe study was approved by the institutional Ethics Committee of Children’s Hospital of Fudan University (IRB protocol number: 2022-106).
 World Journal of Pediatrics2024年2期
World Journal of Pediatrics2024年2期
- World Journal of Pediatrics的其它文章
- Editors
- Information for Readers
- Instructions for Authors
- Changes in children’s cardiorespiratory f itness and body mass index over the course of the COVID-19 pandemic: a 34-month longitudinal study of 331 primary school children
- Preoperative serum cortisone levels are associated with cognition in preschool-aged children with tetralogy of Fallot after corrective surgery: new evidence from human populations and mice
- Exploration of pathogenic microorganism within the small intestine of necrotizing enterocolitis
