Exploration of pathogenic microorganism within the small intestine of necrotizing enterocolitis
Yan Wang·Kun Jiang·Qiao Xia·Xia Kang·Shan Wang·Ji-Hong Yu·Wen-Feng Ni·Xiao-Qin Qi·Ying-Na Zhang·Jin-Bao Han·Gang Liu·Lei Hou·Zhi-Chun Feng·Liu-Ming Huang
Abstract Background Necrotizing enterocolitis (NEC) is the most common severe gastrointestinal emergency in neonates.We designed this study to identify the pathogenic microorganisms of NEC in the microbiota of the small intestine of neonates.Methods Using the 16S ribosomal DNA (rDNA) sequencing method,we compared and analyzed the structure and diversity of microbiotas in the intestinal feces of different groups of neonates: patients undergoing jejunostomy to treat NEC (NP group),neonates undergoing jejunostomy to treat other conditions (NN group),and neonates with NEC undergoing conservative treatment (NC group).We took intestinal feces and saliva samples from patients at different time points.Results The beta diversities of the NP,NN,and NC groups were all similar.When comparing the beta diversities between different time points in the NP group,we found similar beta diversities at time points E1 to E3 but signif icant differences between the E2—E3 and E4 time points: the abundances of Klebsiella and Enterococcus (Proteobacteria) were higher at the E1—E3 time points;the abundance of Escherichia-Shigella (Proteobacteria) increased at the E2 time point,and the abundance of Klebsiella decreased signif icantly,whereas that of Streptococcus increased signif icantly at the E4 time point.Conclusions Our results suggest that the pathological changes of intestinal necrosis in the small intestine of infants with NEC are not directly caused by excessive proliferation of pathogenic bacteria in the small intestine.The sources of microbiota in the small intestine of neonates,especially in premature infants,may be affected by multiple factors.
Keywords Microbiota·Necrotizing enterocolitis·Small intestine·16S rDNA
Introduction
Necrotizing enterocolitis (NEC) is the most common severe gastrointestinal emergency in neonates.NEC occurs mostly in premature infants and has the highest incidence rate and mortality of digestive system diseases in the neonatal intensive care center (NICU) [1,2].The pathogenesis of NEC remains unclear.Many scientif ic studies have found an association between the intestinal microbiome and the development of NEC [3— 6].Research on the colonic microbiota under normal and pathological conditions is vast,but few studies have focused on the small intestine due to the difficulties in obtaining samples.The intestinal tract is a continuous space,but local microenvironment differences determine the diversity of the microbial populations [7].For instance,the main microbiota in the large intestine are sugar-solubilizing Bacteroides and Clostridium [8— 10],which help absorb and ferment complex polysaccharides from undigested plant f ibers or food residues due to slow colonic peristaltic movement.In contrast,the small intestine has a relatively small amount of microbiota due to its rapid peristalsis and the secretion of bactericidal substances [11].These microbiota are readily inf luenced by changes in the environment,food type and passage time,and they are characterized by high volatility,instability,low species diversity,and unique dominant bacteria [12,13].
Commonly,fecal samples are used for NEC intestinal microbiome mainly fecal samples.In one study,prior to NEC onset,the relative abundance of proteobacteria in fecal bacteria in infants and young children increased,while that of f irmicutes and bacteroides decreased.Microbiota diversity and network structure changes with low lactobacillus abundance have been positively correlated with NEC risk in premature infants [10,14].
NEC often requires jejunostomy to save the affected neonates’ lives,and obtaining intestinal fecal samples is easy during the procedure.Some research models have been established on the premise that the occurrence of NEC may be deeply affected by the microbiota of the small intestine.NEC usually occurs in premature infants with immature small intestine microbiota colonization processes.Thus,gut microbiota variations may have a role in the early stage of NEC.Additionally,surgery,use of antibiotics,use of probiotics,enteral nutrition,jaundice,and other factors affect the development of the small intestine microbiota after jejunostomy procedures.In this study,we explored the small intestine microbiota changes before and after jejunostomy in infants with NEC.We collected fecal samples from patients with NEC who underwent jejunostomy at different time points to systematically study the composition changes and differences in the microbiota of the small intestine and colon.This study will help to reduce the mortality of premature infants with NEC and improve their prognosis.
Methods
Clinical case data and grouping: we enrolled neonates with NEC (or neonates requiring jejunostomy due to other causes)hospitalized in the NICU of the Pediatrics Department of PLA General Hospital between September 2019 and May 2021 for this study.We formed three different groups: 57 patients with NEC undergoing jejunostomy (the NP group),39 patients with NEC undergoing conservative treatment without a surgical operation (the NC group),and 24 patients with conditions other than NEC (volvulus,intestinal atresia)undergoing jejunostomy (the NN group).We collected fecal samples at the following four time points considering the clinical situations relevant for our comparative analysis: (a)infection period (E1,during jejunostomy/conservative treatments);(b) infection control period (E2,at the time of complete septic shock correction,improved infection index,and recovered intestinal function);(c) enteral nutrition period(E3,after two weeks of enteral nutrition stabilization and attainment of an intake of 30 mL/kg/day),and (d) f istula closing period (E4,within three days of complete enteral nutrition and f istula closing operation).
We extracted data from clinical records,including gestational ages,weights,and diseases at the time of the f irst stool specimen collections.We recorded the length of the proximal small intestine segment during the f irst jejunostomy (Table 1).In addition,we also extracted data on routine blood and biochemical examinations and antibiotic and probiotic use during the study.Between the enteral nutrition starting time and the end of the antibiotic treatment,we collected individual saliva and stool samples from 13 infants to test the microbiotas at those sites.We took stool specimens using a sterile cotton swab to extract >3 g of fecal sample from diapers or by opening f istula bags.During the jejunostomies,we extracted fecal samples from the proximal side of the cut end of the intestinal tube.We immediately placed each sample into a sterile cryopreservation tube,labeled it,and then stored it at -20 °C.We obtained 18 intestinal fecal samples at time points E1—E4 from the neonates in the NP and NN groups and used them to analyze continuous microbiome data.Other experiments for discontinuous studies involved the same selection of specimens.
We analyzed all samples using a NovaSeq6000 highthroughput sequencer to reveal the highly mutated regions of 16S ribosomal DNA (rDNA) (Online Supplementary Materials).Based on our sequencing results,we qualitatively and quantitatively analyzed the compositions and diversities of the microbes in the samples,obtaining the colony species and the relevant diversity indexes to evaluate intra-and intergroup species diversity differences.We used the Shannon index to analyze the alpha diversity of each group of samples and performed principal coordinated analysis (PCoA)clustering using a weighted UniFrac distance between different groups to detect beta diversity differences.We applied a Wilcoxon rank-sum test to identify signif icant differences;f inally,we found different species on the basis of rank-sum test (Kruskal-Wallis test) results among groups using a linear discriminant analysis (LDA) effect size analysis (LEfSe)among the different groups.
Results
Effects of necrotizing enterocolitis on gut microbiota
To explore the effects of NEC on the gut microbiota of neonates,we analyzed the microbiotas of 18 neonates in the NP,NN,and NC groups,assessing the species differences in patients with and without NEC.We collected fecal samples from the small intestine of infants in the NP and NN groups and from the colon of infants in the NC group.Figure 1 shows the microbiome analysis results.The main genera in each group includedKlebsiella(Proteobacteria),Streptococcus(Bacillota),andEnterobacteriaceaeandEnterococcus(Pseudomonadaceae) (Fig.1 a).A heatmap shows thatStaphylococcusandStenotrophomonaswere highly abundant in the NN group and low in abundance in the NP and NC groups,whereasGranulicatellawas highly abundant in the NC group but less abundant in the NP and NN groups;in addition,Enterobacteriaceaewere highly abundant in the NP group but low in abundance in the NN and NC groups (Fig.1 b).The main species varying in each group wereStaphylococcus,Streptomycetaceae,Bradyrhizobiaceae,Rhodospirillaceae,andActinomyces(Fig.1 c);the intra-group alpha diversities of the NP and NN groups were similar (P=0.72;Fig.1 d),whereas the intra-group alpha diversity of the NC group was signif icantly higher than those of the other two groups (P=0.016 andP=0.023).The beta diversities in all groups (Fig.1 e) exhibited similar species.
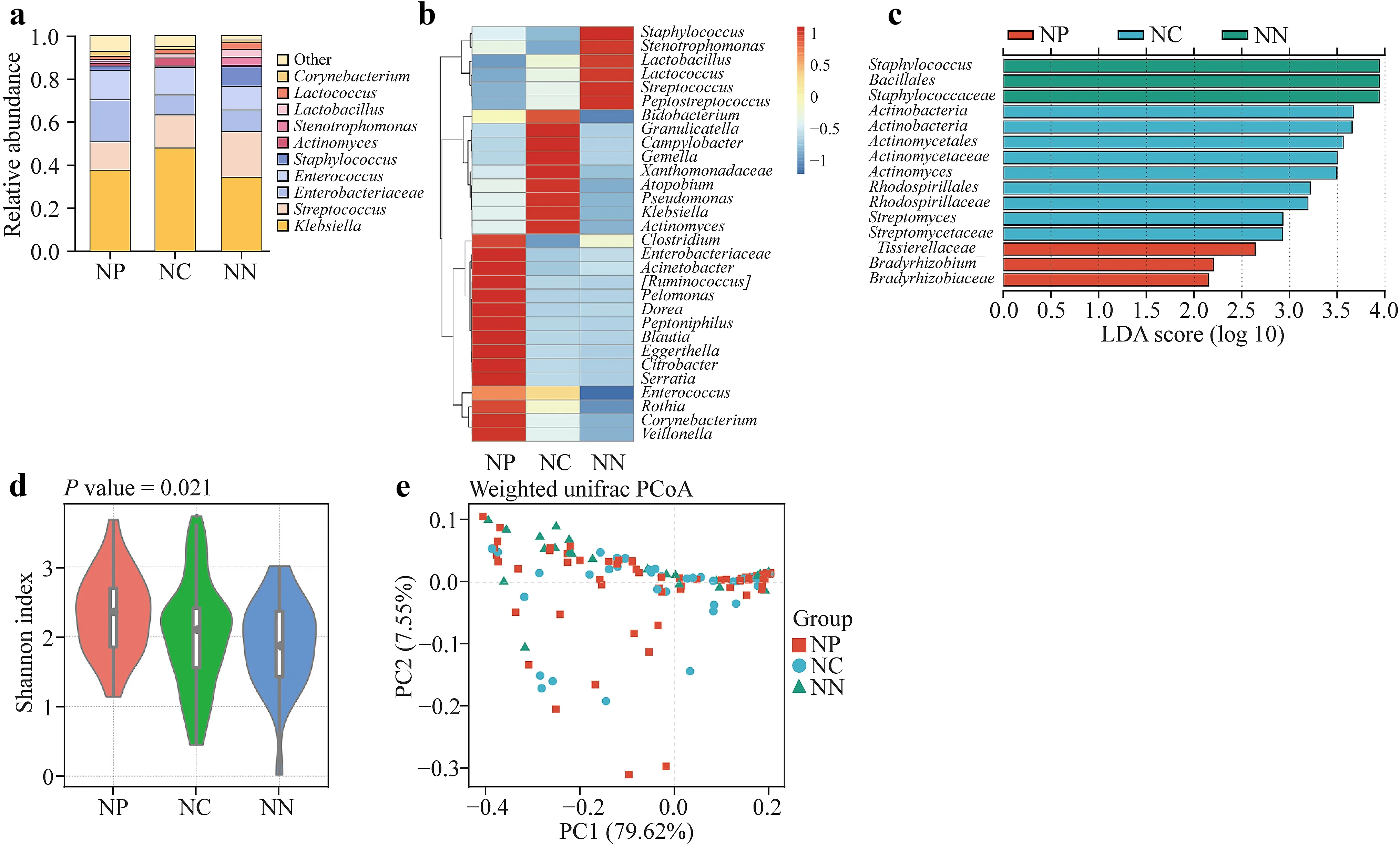
Fig.1 Effects of necrotizing enterocolitis (NEC) on the intestinal microbiota of neonates.a The main genera of the NP,NN,and NC groups were Klebsiella,Streptococcus,Enterobacteriaceae,Enterococcus, and others.b Staphylococcus,Stenotrophomonas and others were highly abundant in the NN group and less so in the NP and NC groups;Granulicatella and others were highly abundant in the NC group but less so in the NP and NN groups;and Enterobacteriaceae and others were highly abundant in the NP group but less so in the NN and NC groups.c The abundance of Staphylococcus,Streptomycetaceae,Bradyrhizobiaceae,Rhodospirillaceae,and Actinomyces dis-playing the most remarkable differences in each group. d Intragroup alpha diversities of the NP and NN groups were similar,while intragroup alpha diversities in the NN group were signif icantly higher than those in the other two groups.e Beta diversities showed similar species diversities in all the groups.NP patients undergoing jejunostomy to treat NEC,NN neonates undergoing jejunostomy to treat other conditions,NC and neonates with NEC undergoing conservative treatment,LDA linear discriminant analysis,PCoA principal coordinated analysis,PC principal coordinate
Gut microbiota trends at different time points
We studied the microbiota changing trends in the small intestinal digesta of 18 patients with NEC undergoing jejunostomy at E1—E4 time points.Figure 2 shows the microbiota sequence comparisons of the small intestine samples of these patients at different time points.
Among the species with the highest order of abundance,the main genera in each group includedKlebsiella(Proteobacteria),Streptococcus(Bacillota),Enterobacteriaceae,Enterococcus(Pseudomonadaceae),KlebsiellaandEnterococcus(Firmicutes) at time points E1,E2,and E3,and
Streptococcusincreased significantly at time point E4(Fig.2 a).The heatmap of abundance showed thatStaphylococcus,Haemophilus,Pelomonas,andRothiawere highly abundant at the E4 time point and low in abundance at the E1—E3 time points,whereasKlebsiellaand Enterobacteriaceae were highly abundant at the E1—E3 time points but low in abundance at the E4 time point (Fig.2 b).The main species varying in each group wereEnterobacteriales,Proteobacteria,Clostridiales,Acidobacteria,Lactobacillales,andStreptococcaceae(Fig.2 c).

Fig.2 Composition and diversity of the microbiota in the small intestine of patients with necrotizing enterocolitis at different stages.a The most abundant genera in each group were Klebsiella,Streptococcus,Enterobacteriaceae, and Enterococcus.b Staphylococcus,Haemophilus,Pelomonas,and Rothia were highly abundant at the E4 time point and less abundant at the E1—E3 time points,and Klebsiella and Enterobacteriaceae were highly abundant at the E1—E3 time points but less abundant at the E4 time point.c The abundance of Enterobacteriales,Proteobacteria,Clostridiales,Acidobacteria,Lactobacilla- les,and Sterptococcaceae displaying the most remarkable differences at each time points.d Intragroup alpha diversities were all similar.e Beta diversities were similar among groups at the E1—E3 time points,and they varied signif icantly between the E2-E3 time points and the E4 time point.NP patients undergoing jejunostomy to treat NEC,NN neonates undergoing jejunostomy to treat other conditions,NC and neonates with NEC undergoing conservative treatment,LDA linear discriminant analysis,PCoA principal coordinated analysis,PC principal coordinate
We evaluated the alpha diversities between the four groups by calculating the Shannon index;and our results showed that the intra-group microbiota diversities in the small intestine were all similar at the E1—E4 time points(Fig.2 d).According to the PCoA clustering results based on weighted UniFrac distance,the beta diversities were also similar at the E1—E3 time points (E1vs.E2,P=0.344;E1vs.E3,P=0.682;and,E2vs.E3,P=0.380),and the results at the E4 time point were relatively far from those at the E1—E3 time points (E1vs.E4,P=0.126;E2vs.E4,P=0.003;E3vs.E4,P=0.007;Fig.2 e).
Composition and diversity of microbiota in the oral cavity-small intestine-large intestine
We analyzed saliva,small intestinal digesta at E1—E3,and small intestinal digesta and feces samples at E4 from 13 patients with NEC to assess their microbiota diversities and compositions and identify the source of the microorganisms in the small intestine.Actinomyces(Actinobacteria) andRothiaandStreptococcus(Firmicutes) showed a higher abundance in saliva and small intestinal digesta at the E4 time point;Enterobacter,Klebsiella,andCitrobacter(Proteobacteria) had a higher abundance in feces and small intestinal digesta at the E1—E3 time points (Fig.3 a,b);andActinobacteria,β-proteobacteria,Bif idobacterium,andProteobacteriawere the main genera varying in abundance in the different groups (Fig.3 c).We found no signif icant differences between alpha and beta diversities (Fig.3 d &e).

Fig.3 Composition and diversity of microbiota in saliva-small intestinal digested-feces of patients with necrotizing enterocolitis(NEC).a Main bacterial compositions of each group.b Relative genus abundance heatmap showing that in saliva and small intestinal digesta at E4 time points had higher Actinomyces,Rothia, and Streptococcus abundances than others.In feces and small intestinal digesta at the E1—E3 time points,the more abundant bacteria included Enterobacter,Klebsiella,and Citrobacter.c The abundance of Actinobacteria(from top to bottom,the f irst is phylum name,and the second is class name),Betaproteobacteria,Bif idobacterium,and Proteobacteria displaying the most remarkable differences between feces and saliva samples.d We found similar alpha diversity and e beta diversity.NP patients undergoing jejunostomy to treat NEC,N N neonates undergoing jejunostomy to treat other conditions,NC and neonates with NEC undergoing conservative treatment,LDA linear discriminant analysis,PCoA principal coordinated analysis,PC principal coordinate
Discussion
Many studies have conf irmed that the gut microbiota is associated with the development of NEC.However,taking samples from the small intestine is difficult under normal conditions,and an association between the microbiota in the small intestine and the development of NEC has not been clarif ied.Most studies on the association between NEC and gut microbiota are based on colonic microbiotas obtained by taking fecal samples.The functional differences between the small intestine and the colon inevitably lead to their microbiotas being different.NEC involves mostly the small intestine,and an association between the microbiota in the small intestine and NEC onset has been hypothesized.Additionally,after jejunostomy,intestinal failure (or the inability to establish normal enteral nutrition after 42 consecutive days) secondary to short bowel syndrome is common.In these cases,in which the colonic microbiota disappears or cannot provide its normal biological function,the small intestine microbiota becomes important and can impact intestinal function.
This study showed that the microbiota diversities in the small intestine of neonates in the NP and NN groups were similar,and their main predominant species were basically the same.We found small differences in the abundances ofStaphylococcusandStreptococcus(Firmicutes),Prevotella(Bacteroides),andActinomycesandRothia(Actinomycetes).Our results suggest that NEC and other conditions,such as volvulus,intestinal atresia,and other noninfectious intestinal diseases,produce no signif icant impacts on the gut microbiota diversity in the small intestine of neonates.Intestinal diseases of neonates,especially in premature infants,are likely to be caused by endogenous factors such as intestinal hypoxia and ischemia rather than by pathogenic microorganisms in the intestinal tract.During the progression of NEC,the small intestine microbiota is not characterized by a predominant pathogen,and its bacterial composition remains varied.We believe that the pathological intestinal necrosis changes in the small intestine during NEC are not directly caused by the excessive proliferation of pathogenic bacteria.We found a signif icant difference in the microbial diversities of small intestinal digesta samples of neonates in the NP group and the colonic feces samples of those in the NC group.The alpha diversity of the colonic fecal samples was higher than that of the small intestinal digesta samples,suggesting that microbiota in the small intestine and colon of patients with NEC differ in their diversity and major dominant species composition abundances.Thus,the colonic microbiota cannot be used to replace the small intestine microbiota during NEC,and detection of the colonic microbiota can only be used as a reference.
We studied the composition and diversity of the microbiota in the small intestinal digesta of patients with NEC at different time points.The microbiota diversity changes in the small intestine of patients with NEC were relatively small at the E1—E4 time points,and the alpha and beta diversities of the microbiota in the small intestine exhibited no signif icant differences at those same time points.However,we found different dominant species at different time points.For instance,KlebsiellaandEnterococcus(Proteobacteria) were highly abundant during the infection,infection control,and enteral nutrition periods,and the abundance ofEscherichia-Shigella(Proteobacteria) and Enterobacteriaceae increased during the enteral nutrition period,whereasStreptococcus(Firmicutes) showed a higher abundance in the f istula closing/recovery period.During the jejunostomy process,the abundance ofKlebsiella(Proteobacteria) was higher at the E1—E3 time points,and it decreased signif icantly at the E4 time point,whereasStreptococcus(Firmicutes) increased signif icantly at the E4 time point,which is consistent with those of other studies [10,14].Considering the clinical jejunostomy stages,the E2 time point corresponds to the period when the infectious shock is completely corrected,infection indicators have improved,and intestinal function is recovered.We observed no signif icant difference between small intestine microbiota diversity or dominant species at the E2 and E1 or E3 and E4 time points,and we did not detect a dominant pathogen at any time point.This further demonstrates that NEC originates from endogenous factors in neonates.Our PCoA clustering results based on weighted UniFrac distance showed that the E4 time point was relatively distant from the other three time points,and we found some dominant species abundance differences between the time points,suggesting that the microbiota in the small intestine gradually colonized it.During the colonization process,the small intestine’s function also develops gradually.Therefore,we performed our microbiota analyses considering the differences in time points (E1—E3vs.E4 in subsequent analyses).
Studies have shown that microbial colonization is important for immune maturation and organ development in neonates.Birth,mode of delivery,feeding patterns,and antibiotic usage have been shown to affect early microbiota acquisition in neonates [15— 21].The gut microbiota of neonates is most likely affected by food and other external factors.Therefore,we analyzed the composition and diversity of the microbiota in saliva,small intestinal digesta at the E1—E3 time points,and small intestinal digesta and feces samples at the E4 time point in 13 neonates with NEC.As observed,the alpha and beta diversities of saliva,small intestinal digesta,and feces samples were similar.The main differences included the high abundances ofActinomycetes,Rothia,andStreptococcusin saliva and small intestinal digesta at the E4 time point and the high abundances ofEnterobacter,Klebsiella,andCitrobacterin the feces and small intestinal digesta at the E1—E3 time points.The main genera among the samples includedActinobacteria,β-proteobacteria,Bif idobacterium,andProteobacteria.However,we found similar microbiotas in saliva and small intestine diversities and dominant species,suggesting that the establishment of the gut microbiota in neonates is associated with the saliva microbiota;that is,the small intestine microbiota probably originates from oral bacteria after food and saliva ingestion.
Intestinal infections have been proposed as the main causes of NEC in neonates [22— 24].In this study,the microbiotas without predominant pathogens were the norm in the small intestine,and the microbial diversities in the small intestine were not significantly different at different time points.Hence,we speculate that the pathogenesis of NEC in neonates may be caused by endogenous factors.Conceivably,the incomplete intestinal development of neonates,especially in premature infants,may lead to intestinal ischemia or injury causing NEC.Intestinal ischemia or injury,together with the incomplete establishment of gut microbiota in neonates and the instability of the microbiota composition,may lead to the loss of mucosal barrier integrity and the proliferation and diffusion of various bacteria,causing disease progression.The sources of microbes in the small intestine of neonates,especially in premature infants,may include saliva and food boluses,and our results suggest that the microbiota in the small intestine may originate from those sources.
We could not obtain samples of small intestinal digesta in healthy neonates for this study due to practical and ethical considerations.We established a control group (microbiota in the small intestine of healthy neonates) to directly compare the microbiotas with those of patients with NEC.Additionally,we failed to analyze all the different factors inf luencing the development and progression of NEC and its associated gut microbiota due to the disease complexity.NEC has a high incidence rate,and it has a sudden onset,rapid progression,and high mortality;therefore,obtaining small intestinal digesta samples of patients with NEC at different time points was impossible.As a result,the number of samples included in this study was small,and we were not able to compare the small intestine microbiota at early and late disease stages;thus,we failed to establish a correlation model for the severity of NEC and the small intestine microbiota or one for the association between the prognosis and beta diversities at different time points.In addition,ethical and safety considerations limited our ability to study enough cases to systematically assess the impact of different types of food,feeding methods,eating habits,and/or other factors affecting the gut microbiota of neonates.Future studies with large sample sizes are needed to conf irm our f indings.
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s12519-023-00756-0.
AcknowledgementsWe would like to thank the children who participated in the study and the support of the General Hospital of the People's Liberation Army for this work.
Author contributionsWY: conceptualization,investigation,formal analysis,writing—original draft,funding acquisition,writing—review&editing.JK,XQ: conceptualization,investigation,formal analysis,writing—original draft.KX,WS,YJH,GL: conceptualization,formal analysis.NWF,QXQ,ZYN,HJB: investigation,formal analysis.HL,FZC,HLM: funding acquisition,conceptualization,investigation,writing—review &editing.WY,JK and XQ contributed equally to this work.
FundingThis work was sponsored by the National Key R&D Program of China (No.2022YFC2703400,to Yan Wang).This work has been funded by the Health Bureau of the Logistics Support Department of the Military Commission (Grant no.21JSZ18,to Liu-Ming Huang).
Data availability statementThe datasets generated during and/or analyzed during the current study are not publicly available due to the rules of General Hospital of the People's Liberation Army but are available from the corresponding author on reasonable request.
Declarations
Conflict of interestNo f inancial or non-f inancial benef its have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approvalLl procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and approved by the Institutional Ethics Committee of PLA General Hospital.
 World Journal of Pediatrics2024年2期
World Journal of Pediatrics2024年2期
- World Journal of Pediatrics的其它文章
- Growth and development of children in China: achievements,problems and prospects
- Childhood sleep: assessments,risk factors,and potential mechanisms
- Childhood sleep: physical,cognitive,and behavioral consequences and implications
- Risk factors for long COVID in children and adolescents: a systematic review and meta-analysis
- SARS-CoV-2 variants are associated with different clinical courses in children with MlS-C
- NLRP3 activation in macrophages promotes acute intestinal injury in neonatal necrotizing enterocolitis
