Mesenchymal stem cell-based TRAIL delivery inhibits the metastatic state of clinical therapy-resistant progressive neuroblastoma
Dinesh Babu Somasundaram·Andrew Maher·Sheeja Aravindan·Zhongxin Yu·Brian M. Besch ·Natarajan Aravindan,,,4
Progressive neuroblastomas (pNBs) that defy current intensive multimodal clinical therapy (IMCT) are a signif icant contributor to overall childhood cancer deaths [1 ].Neuroblastomas (NBs) that survive f irst-line IMCT actively evolve to acquire genetic and molecular rearrangements and display unparalleled plasticity and evolution,which causes frequent relapses in a rapidly accelerating timeline [2-4].While most of the recent eff orts focus on targeting acquired rearrangements pertaining to the proliferation of IMCT surviving tumor cells,it is equally crucial to target their migratory and invasive capabilities to prevent tumor evolution (metastasis and/or relapse).In this regard,TRAIL,a member of the TNF superfamily that interacts with fully functional death receptors (DRs,DR4 and DR5) leading to apoptotic cell death,has been investigated for the treatment of pNB [5,6].TRAIL could selectively kill cancer cells by activating DRs while sparing normal cells that express decoy receptors and low levels of DRs.Since TRAIL-induced killing is independent of any molecular rearrangements,all molecular subtypes (high-risk NB,HR-NB;pNB) are potentially amenable to TRAIL-induced killing.Although TRAIL-based NB cell killing has been realized [6-9],its function in the regulation of migration and invasion,particularly in IMCTresistant pNB cells,remains unknown.
For the cure of HR-NB,the benef it of stem cell (SC)purging or SC transplantation has been well realized [10].Crucially,recent eff orts indicate that SC-based molecular targeted cellular therapy (with engineered SCs) is a promising method for the treatment of pNB.Immunologically inert bone marrow-derived MSCs (BM-MSCs) have been recognized as suitable for the delivery of anticancer agents in immunocompetent patients.Despite the culture-associated impact on the phenotype of MSCs,studies have shown that MSCs have a life span of 24 hours in vivo with an induced systemic response as early as 2 hours after infusion [11].Using MSCs to deliver TRAIL would allow the protein to be continuously produced at the tumor site,overcoming the problem of its short life span.Engineered MSCs can migrate to and incorporate into the tumor stroma,delivering therapeutic molecules.TRAIL-loaded MSCs (MSCsTRAIL) have been shown to reduce the tumor and metastatic burdens in diverse tumor models.In NB,it is evident that MSCsTRAILeffi ciently killed DR-expressing tumor cells in vitro and selectively migrated to the tumor sites in vivo [9].Here,we investigated the prognostic/predictive signif icance of DRs in NB,their association with clinical outcomes,and their status in a panel of HR-NB patient-derived pNB cells to assess the ability of MSCsTRAILto counter pNB.Furthermore,we studied the crucial regulatory capabilities of MSCsTRAILin rearranging metastasis-related transcriptome machinery and its functional benef it in the regulation of pNB cell invasion,migration,and colony formation.
Correlation of DR4 and DR5 transcription with overall survival (OS),relapse-free survival (RFS),event-free survival (EFS),and progression-free survival (PFS) was performed using a web-based platform (http:// r2.amc.nl)that houses a comprehensive collection of NB studies with RNA-seq/microarray data.We examined the association of DR4 (seven studies,combinedn=2117) and DR5 (nine studies,combinedn,2470) with disease progression and clinical outcomes.DR4 mRNA displayed an equivocal lowis-worse (OS,4 cohorts;EFS,3 cohorts;PFS,1 cohort) and high-is-worse (OS,3 cohorts;EFS,3 cohorts;PFS,1 cohort)correlation with survival outcomes.Conversely,all nine cohorts displayed a signif icant high-is-worse association of DR5 with OS,EFS,PFS,and RFS when assessing its correlations with clinical outcomes.Together,these data clearly indicate the negative association of DRs (high-is-worse)with a poor prognosis for NB (Supplementary Table 1).
Immunohistochemical (IHC) assessment of the levels of DRs in our custom-archived tissue microarrays [12],with tumors from primary/metastatic sites from patients during both diagnosis and progressive disease,revealed def inite and measurable surface expression of DRs in human NB (Supplementary Fig.1).For the 104 specimens,from a total of 79 NB patients,DR4 expression ranged from 14.65% to 92.13%(mean,57.11%),and DR5 expression ranged from 8.40% to 78.16% (mean,38.45%).All protocols were approved by the OUHSC Institutional Review Board (IRB) for the research use of de-identif ied specimens,and all studies were performed in accordance with OUHSC IRB guidelines and regulations for the protection of human subjects.The expression of DRs was signif icantly higher in metastatic disease than in primary disease (Supplementary Fig.1).Survival analysis computed for levels of DR4 (high,> 60%;low,< 60%)displayed a signif icant high-is-worse association with poor OS [hazard ratio (HR)=3.142],RFS (HR=5.449) and PFS (HR=6.297) (Fig.1).Interestingly,we found a strong high-is-worse correlation of DR4 with the survival of children with high-risk (HR=3.557),metastatic (HR=3.639)and progressive (HR=2.147) disease (Supplementary Fig.2).Similarly,high DR5 expression was strongly and signif icantly associated with poor OS (HR=1.707),RFS(HR=5.449) and PFS (HR=2.543) (Fig.1).In addition,a high-is-worse correlation of DR5 was observed with the survival of children with high-risk (HR=1.701),metastatic(HR=2.672) and progressive (HR=1.467) disease (Supplementary Fig.2).Together,this new information strongly suggests a signif icant negative relationship between the expression of DRs and poor outcomes in NB patients.
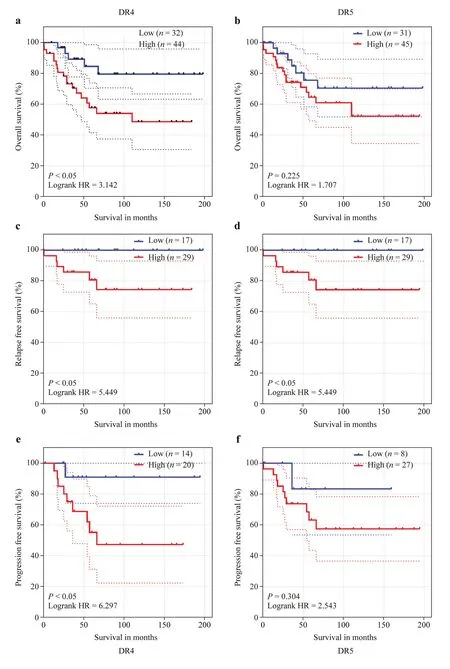
Fig.1 Death receptors expression with NB clinical outcomes.Kaplan-Meier curves showing the association of the expression or DR4 and DR5: a,b overall survival,c,d relapse-free survival and e,f progression-free survival in a cohort of 76 NB patients.All survival analyses[log rank Mantel-Cox test and hazard ratio (HR)] and Kaplan-Meier curves were performed in Graphpad Prism.Blue: low expression;red: high expression.NB neuroblastoma
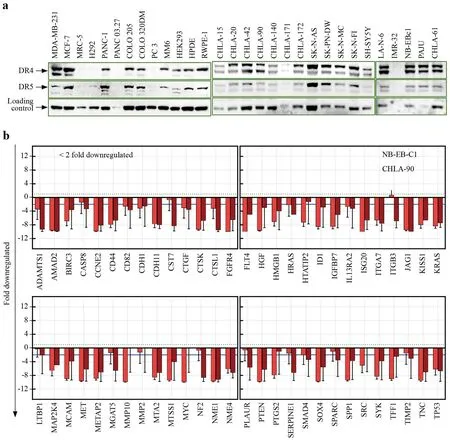
Fig.2 Death receptors expression in normal and cancer cells,and function of MSCs TRAIL in regulating metastasis transcriptome.a Increased expression of death receptors in progressive neuroblastoma cells.Immunoblots showing DR4 and DR5 levels in normal(MM6,HEK293,HPDE,RWPE-1,MRC-5),other cancer (breast,MDA-MB-231,MCF-7;colon,COLO-205,COLO-320DM;pancreatic,PANC-1,PANC 03.27;lung,H292;prostate,PC-3;Ewing sarcoma,SK-N-MC;neuroectodermal,SK-PN-DW) cells and in cell lines derived from stage-4 NB patients during diagnosis (CHLA-15,CHLA-42) and progressive disease (CHLA-20,NB-EBc1,IMR-32,CHLA-90,CHLA-140,SK-N-FI,LA-N-6,CHLA-172,SHSY-5Y,SK-N-AS,CHLA-171,CHLA-61,and PAJU).b TRAIL-modif ied mesenchymal stem cells (MSCs) regulate the tumor invasion and metastasis (TIM) transcriptome machinery.Gene comparison analysis on the qPCR prof iling of 93 TIM genes in progressive NB (NBEBc1;CHLA-90) cells exposed to TRAIL-modif ied MSCs were compared to untreated controls.TRAIL-modif ied MSCs exerted a cell-line independent transcriptional repression of 56 TIM genes in clinical therapy-resistant pNB cell lines.Of these,cell-line independent conserved downregulation of 40 genes were signif icant (> twofold).DR death receptor,NB neuroblastoma,pNB progressive neuroblastoma,TRAIL TNF-related apoptosis-inducing ligand
Substantiating the prognostic relevance of DRs in pNB would allow us to assess the feasibility of an MSCTRAIL-based therapeutic strategy.For this,we studied the expression prof ile of DRs in a panel of stage-4 patientderived therapy-resistant pNB cells and compared them to normal healthy cell lines,cell lines from other tumor types,and NB cells derived during diagnosis (Supplementary Table 2;Fig.2 a).Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) normalized immunoblot analysis in normal healthy cells (human monocytes,MM6 28%;embryonic kidney,HEK 26%;pancreatic ductal,HPDE 24%;prostate epithelial,RWPE-1 47%;lung f ibroblast,MRC-5 0% cells)displayed a baseline expression of DR4 (Supplementary Fig.3a).On a comparative note,the expression of DR4 in human cancer cells was highly variable,with relatively high levels (55%-68%) in breast cancer (MDA-MB-231,MCF-7),basal levels (29%-32%) in colon cancer (COLO-205,COLO-320DM),marginal levels (2%-38%) in pancreatic cancer(PANC-1,PANC 03.27),and near-zero expression in lung(H292) and prostate (PC-3) cancer cells.Conversely,NB cell lines derived during diagnosis (CHLA-15 from primary tumor,pT-NBCs;CHLA-42 from BM,BM-NBCs) and from pNB (CHLA-20,NB-EBc1,IMR-32 from pT;CHLA-90,CHLA-140,SK-N-FI,LA-N-6,CHLA-172,SHSY-5Y,SKN-AS,from BM;CHLA-171 and CHLA-61 from peripheral blood,PB-NBCs;and Paju from pleural eff usion off brain metastasis) displayed varied levels of DR4 ranging from 39% to 588% (maximum in the extremely therapy-resistant Paju) (Fig.2 a).In NB,pT-NBCs (CHLA-15,CHLA-20,NB-EBc1,IMR-32) displayed a 103% to 391% range of DR4 expression,and BM-NBCs (CHLA-90,CHLA-140,SK-NFI,LA-N-6,CHLA-172,SHSY-5Y,SK-N-AS) ranged from 80% to 228%.Interestingly,PB-NBCs showed low (CHLA-171,39%) and high (CHLA-61,198%) DR4 expression(Supplementary Fig.3b).Crucially,in response to therapy pressure,a twofold increase in DR4 expression was evident in pNB cells (CHLA-20,NB-EBc1,IMR-32) when compared to the cells derived during diagnosis (CHLA-15,CHLA-42).It is pertinent to mention that CHLA-15 and CHLA-20 derived from the same patient conf irmed this therapy pressure-induced twofold DR4 increase.

Fig.3 MSCs TRAIL -dependent TRAIL delivery and its function on NB cell survival and metastatic state.Histograms from ELISA showing a robust TRAIL secretion in the media and b increased levels of TRAIL in pNB cells (SK-N-FI,CHLA-90) cocultured with TRAILloaded MSCs.Mock cocultures without MSC TRAIL were used as control.c Immunoblots analysis showing increased levels of TRAIL in pNB cells (SK-N-FI,CHLA-90) cocultured with TRAIL-loaded MSCs.GAPDH was used as loading controls.d MTT analysis showing measurable reduction in survival of pNB cells (SK-N-FI,CHLA-90) cocultured with TRAIL-loaded MSCs.Plain MSCs are used as mock coculture controls.e Line plot from scratch wound gap measurements (mean,SEM) showing the cell migration patterns of NBEBc1 cells cocultured with MSC TRAIL .TRAIL delivery signif icantly inhibited pNB cell migration.f Line plot from Matrigel invasion assay (mean,SEM) showing robust invasion of CHLA-90,SK-NFI cells.MSC TRAIL completely abrogated invasion in both cell lines investigated.ELISA enzyme-linked immunosorbent assay,TRAIL TNF-related apoptosis-inducing ligand,pNB progressive neuroblastoma,MSC mesenchymal stem cell,GAPDH glyceraldehyde-3-phosphate dehydrogenase,MTT methylthiazolyldiphenyl-tetrazolium bromide,SEM standard error of mean
Examining DR5 expression in normal cells showed a baseline level in HEK293 (9%),MM6,HPDE,RWPE-1 (12%)and MRC-5 (23%) cells (Fig.2 a).The expression of DR5 was marginal in breast (MDA-MB-231,13%;MCF-7,31%);colon (COLO-205,17%;COLO-320DM,16%);pancreatic(PANC-1,35%;PANC 03.27,18%);lung (H292,17%) and prostate (PC-3,7%) cancer cells (Supplementary Fig.s3c).In contrast,NB cells displayed robust expression of DR5 (25%to 154%),with a maximal level (154.8%) in the Paju cells.pT-NBCs (CHLA-15,89%;CHLA-20,143%;NB-EBc1,84%,IMR-32,96%) and BM-NBCs (CHLA-90,CHLA-140,SK-N-FI,LA-N-6,CHLA-172,SHSY-5Y,SK-N-AS;ranging from 59 to 113%) exhibited high DR5 expression when compared to the healthy controls and cells from other tumor types (Fig.2 a).In contrast,PB-NBCs showed relatively low(CHLA-171,25%) to moderate (CHLA-61,58%) DR5 expression.As was seen in DR4,we observed a twofold increase in DR5 with therapy pressure (Supplementary Fig.3d).Critically,the outcomes demonstrate that HR-NB cells express high levels of DRs,that NB cells that survive IMCT acquire a robust increase in their DR levels and that DRs can be exploited for developing novel therapeutic strategies for pNB.
To def nie the functional signif ciance of cell-based TRAIL delivery in regulating the metastatic state of pNB cells,we investigated the MSCTRAIL-dependent regulation (vs.MSC)of tumor cell invasion and metastasis (TIM) transcriptional machinery in cocultured BM-NBCs (CHLA-90) and pT-NBCs (NB-EBc1).In CHLA-90 cells,of the 93 genes analyzed,TRAIL delivery downregulated 67 TIM genes and increased another 10;16 genes did not show any modif ications.Likewise,TRAIL delivery in NB-EBc1 cells downregulated 64 TIM genes,increased nine genes,and did not alter 20 genes.Comparing the prof iles between cell lines,nineteen genes displayed cell-line-dependent changes,and another fourteen genes showed a cell-line-independent no change;hence,both were excluded from downstream analysis.Interestingly,56 TIM genes displayed cell-line-independent downregulation in pNB cells with MSC TRAIL -based TRAIL delivery (Fig.2 b;Supplementary Table 3).Among these genes,cell-line-independent conserved downregulation of 40 genes was signif icant (≥ twofold).IPA bioinformatic analyses considering only relationships where the “conf idence=experimentally observed” revealed that these molecules have def ined roles in > 350 canonical pathways with nearly 500 biological functions.In light of tumor progression and dissemination,we observed their signif icant [-log(Pvalue)] association in key pathways,including the tumor microenvironment,drug resistance,and molecular mechanisms of cancer (Supplementary Fig.4).Furthermore,these molecules are known to tightly interact and coordinate key cellular functions,including tumor cell invasion (42 genes),migration (46 genes),tumor growth (39 genes),angiogenesis (38 genes),metastasis (38 genes),and proliferation (49 genes),the hallmark events that orchestrate NB evolution (Supplementary Fig.5a).Evidently,this study revealed the direct relevance of the regulated TIM genes to NB evolution with a signif icant association in (1)extra-adrenal retroperitoneal tumors;(2) malignant neoplasms of the retroperitoneum;(3) advanced malignant solid tumors;(4) metastatic solid tumors;(5) invasive tumors;(6) advanced extracranial solid tumors;(7) growth of solid tumors;and (8)embryonal tumors (Supplementary Fig.5b).These outcomes clearly demonstrate that MSCTRAIL-dependent TRAIL delivery def initively reprograms the metastatic phenotype of pNB cells.
To determine the ability of MSCTRAIL-dependent TRAIL delivery to regulate the metastatic state,we compared the changes in the survival,migration,invasion,and tumorsphere formation capacities of pNB (SK-N-FI,CHLA-90)cells cocultured with MSCsTRAIL(vs.MSCs).Enzymelinked immunosorbent assay (ELISA) conf irmed robust secretion of TRAIL from MSCsTRAILin the media (Fig.3 a)and further conf irmed the corroborative increase in TRAIL in the target cells (Fig.3 b) (and validated with immunoblotting,Fig.3 c).Consistent with the documented evidence[9],MSCTRAIL-dependent TRAIL-mediated decreases in cell survival (MTT,Fig.3 d) and viability (Trypan blue inclusion) were evident.The scratch wound assay under proliferation-controlled conditions displayed a signif icant decrease in cellular migration in pNB (NB-EBc1) cells cocultured with MSCsTRAIL(Fig.3 e,Supplementary Fig.6).Substantiating further Matrigel invasion assays performed in CHLA-90 and SK-N-FI cells cocultured with MSCsTRAILshowed a marked inhibition of their invasive capabilities (Fig.3 f).Assessing the benef it of MSCsTRAILin inhibiting tumorsphere formation,NB-EBc1 cells cocultured with MSCsTRAILusing limiting dilution transplantation assay (LDTA) displayed organized tumorsphere formation (Supplemental-Video V1-left panels).Cells cocultured with MSCsTRAILexhibited a lack of tumorsphere formation and displayed monolayer cell spreading indicating diff erentiation (Supplemental-Video V1-right panels).Uniquely,these in vitro outcomes from coculture studies imply that MSCTRAILdelivery may serve as an eff ective strategy for ameliorating the metastatic state of pNB.
Here,we show the benef ti of MSC-based TRAIL delivery in regulating the metastatic state of pNB cells.Evidence from other cancers indicates a similar benef it of TRAIL [13,14] and recognizes that TRAIL-targeting strategies (e.g.,TRAIL-modif ied SCs,TRAIL-coated leukocytes,E-selectin-TRAIL liposomes,TRAIL gene therapy,soluble TRAIL,oAds-TRAIL,and extracellular vesicles from MSCsTRAIL)could be fruitful for treating metastatic cancers [15-20].In NB,studies have shown that cells expressing DRs succumb to MSCsTRAIL[9] and def ine the NB homing capabilities of systemically delivered MSCsTRAIL[9].Since MSCs can migrate to and incorporate into the tumor stroma,engineered MSCs can deliver therapeutic molecules.In conclusion,our f indings identif ied the prognostic signif icance of DR expression for NB and its association with clinical outcomes and acquired high levels of DRs in therapy-resistant pNB cells.Critically,we found that MSCsTRAILaff ected tumor invasion and metastasis transcriptional machinery regulation and the regulation of the metastatic state (migration,invasion,and tumorsphere formation) of pNB cells.This proof-of-concept study recognizes that TRAIL-modif ied MSCs could serve as an eff ective maintenance therapeutic strategy for pNB.
AcknowledgementsThe authors acknowledge the Stephenson Cancer Center (SCC) Cancer Tissue pathology core for all TMA and IHC services and the SCC Cancer Functional Genomics core for help with LDTA analysis.The authors acknowledge the Stephenson Cancer Center,Offi ce of Cancer Research Science Writer,Dr.Daniel J Morton for his help in critically reviewing this manuscript.
Author contributionsNA: conceptualization,formal analysis,writing-original draft.DBS and AM: investigation,data curation.SA:investigation,data curation,writing-review and editing.BMB: investigation.ZY: investigation,writing-review and editing.All the authors read and approved the f inal manuscript.
FundingThis work was funded by Oklahoma Center for the Advancement of Science and Technology,OCAST-HR19-045;Department of Defense,DoD Peer Reviewed Cancer Research Program,CA-210339 and the National Institutes of Health P20GM103639.The work was also supported by the National Cancer Institute Cancer Center Support Grant [P30CA225520,and a grant from the Oklahoma Tobacco Settlement Endowment Trust [R23-03] both awarded to the OU Health Stephenson Cancer Center.
Data availabilityAll data generated or analyzed during this study are included in this published article and as supplemental information.
Declarations
Conflict of interestNo f inancial or non-f inancial benef its have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approvalAll protocols were approved by the University of Oklahoma Health Sciences Center Institutional Review Board with permission for the research use of de-identif ied specimens (OUHSC IRB #6207).All experiments were performed in accordance with the University of Oklahoma Health Sciences Institutional Review Board guidelines and regulations for the protection of human subjects.
 World Journal of Pediatrics2024年3期
World Journal of Pediatrics2024年3期
- World Journal of Pediatrics的其它文章
- Insights intopostural orthostatic tachycardia syndrome afterCOVID-19 inpediatric patients
- Modeling tuberous sclerosis complex with human induced pluripotent stem cells
- Comparative effi cacy and optimal duration of f irst-line antibiotic regimens for acute otitis media in children and adolescents:a systematic review and network meta-analysis of 89 randomized clinical trials
- Improved survival at the cost of more chronic lung disease? Current management and outcomes in extremely preterm infants born in New South Wales and the Australian Capital Territory: 2010-2020
- Development and validation of a nomogram to predict allograft survival after pediatric liver transplantation
- Perioperative complication incidence and risk factorsfor retroperitoneal neuroblastoma in children: analysis of 571 patients
