A chorismate mutase from Radopholus similis plays an essential role in pathogenicity
Sihua Yang ,Junyi Li ,Shuai Yang,Shiqiao Tang,Huizhong Wang,Chunling Xu ,Hui Xie
Laboratory of Plant Nematology and Research Center of Nematodes of Plant Quarantine, Department of Plant Pathology, College of Plant Protection, South China Agricultural University, Guangzhou 510642, China
Abstract In the process of infecting plants,plant parasitic nematodes release a series of proteins that play an essential role in the successful infection and pathogenesis of plant cells and tissues through stylets or body walls. In this study,based on transcriptome data,a chorismate mutase gene of Radopholus similis (RsCM) was identified and cloned,which is a single copy gene specifically expressed in the oesophageal gland and highly expressed in juveniles and females. Transient expression of RsCM in tobacco leaves showed that it was localised in the cytoplasm and nucleus of tobacco leaf cells,which inhibited the pattern-triggered immunity (PTI) induced by flg22,including callose deposition and defence gene expression,and cell death induced by immune elicitors BAX,but could not inhibit cell death induced by immune elicitors Gpa2/RBP-1. The RNA interference (RNAi) transgenic tomato of RsCM obviously inhibited the infection,pathogenicity,and reproduction of R.similis. However,the resistance of the overexpression transgenic tomato of RsCM to R.similis infection was significantly reduced,and the expression levels of two salicylic acid (SA) pathway genes (PR1 and PR5) in roots infected by the nematode were significantly down-regulated,which indicated that RsCM might be involved in the inhibition of SA pathway. The results of this study demonstrate that RsCM suppresses the host immune system and might be a new target for the control of R.similis,which also provides new data for the function and mechanism of CM genes of migratory parasitic plant nematodes.
Keywords: Radopholus similis,chorismate mutase,plant defense,transgenic tomato
1.Introduction
During the parasitic process of plant nematodes,a series of oesophageal gland secretions can be released into plant tissues through stylets,which play an essential role in the infection and pathogenesis of nematodes (Smantet al.1998;Daviset al.2004;Haegemanet al.2012). Chorismate mutase (CM),an essential enzyme in plant and microbial metabolism,catalyses the end product of the shikimate pathway to convert chorismic acid into prephenate and provides a precursor for the synthesis of chorismic acid derivatives phenylalanine and tyrosine. Many critical compounds in plants,such as salicylic acid (SA),are closely related to defence response;auxin indole-3-acetic acid is related to plant growth and development;and aromatic amino acids are chorismic acid-derived compounds (Parkinsonet al.2003).
The first CM gene found in animals wasMjCM1ofMeloidogynejavanica,localised in the oesophageal gland,and contained a signal peptide;thus,the product of this gene is likely to be secreted into plants and play a role (Lambertet al.1999). CM genes have been found in 11 plant parasitic nematodes: the sessile parasitic nematodes,Globoderarostochiensis(Luet al.2008),G.pallida(Joneset al.2003),G.ellingtonae(Chroniset al.2014),G.tabacumsolanacearum(Hanget al.2011),Heteroderaglycines(Bekalet al.2003),H.schachtii(Vanholmeet al.2009),M.incognita(Huanget al.2005;Wanget al.2018),M.javanica(Lambertet al.1999),andM.arenaria(Longet al.2006),and the migratory parasitic nematodes,Hirschmanniellaoryzae(Bauterset al.2014,2020) andPratylenchuscoffeae(Haegemanet al.2011).
Studies on the function and mechanism of CM genes of plant nematodes mainly focus on sessile parasitic nematodes,which has been proved to have the function of establishing and maintaining feeding sites and can interfere with the normal growth of plants and destroy the immune response of plants. For example,MjCM1overexpression in soybean restricted vascular bundle differentiation and lateral root formation (Doyle and Lambert 2003);the transient expression ofMiCM3ofM.incognitain tobacco leaves can enhance its susceptibility to pathogens and inhibit the expression of defence genePR1related to the SA pathway (Wanget al.2018). The competition hypothesis of chorismic acid in plant nematodes (Doyle and Lambert 2003),derived from CM studies of sessile parasitic plant nematodes,suggests that plant nematodes can secrete CM to their host through stylets. After these CM were secreted into the cytoplasm of plant cells,they competed with CM in plant plastids for chorismic acid,destroying the shikimate pathway of plants,and the synthesis of many chorismic acid derivatives is affected,affecting the immune response,normal growth,and development of plants. The CM genes of two species of migratory parasitic plant nematodes have been cloned (Haegemanet al.2011;Bauterset al.2014,2020),among which only the CM gene ofH.oryzae(HoCM) inhibited the immune response of the host.HoCMoverexpression in rice enhances the sensitivity of rice toH.oryzae(Bauterset al.2020). Therefore,further research on the CM genes of migratory parasitic plant nematodes is necessary,and the competition hypothesis of chorismic acid in plant nematodes needs to be verified by research on the CM genes of migratory parasitic plant nematodes.
Radopholussimilisis an important migratory parasitic plant nematode with a wide host range that can parasitise more than 360 plant species (Xie 2006). It is also one of the top 10 plant pathogenic nematodes worldwide and seriously harms many horticultural crops such as bananas,citrus,and ornamental plants (Joneset al.2013). The reported pathogenic genes ofR.similisare β-1,4-endoglucanase (Rs-eng) (Haegemanet al.2010),cathepsin B (Rs-cb-1) (Liet al.2015b),cathepsin s (Rscps) (Wanget al.2016),xylanase (Rs-xyl) (Haegemanet al.2009),fatty acid-and retinoid-binding proteins (Rsfar) (Zhanget al.2015),calreticulin (Rscrt) (Liet al.2015a),and serine carboxypeptidase (Rs-scp-1) (Huanget al.2017). All these genes are closely related to the parasitism and pathogenicity ofR.similis,but no research has shown that the pathogenic genes ofR.similiseffectively inhibit the defence response of plants.
In this study,we screened and amplified the CM gene ofR.similis(RsCM) based on its transcriptome data (Huanget al.2019). To study the function ofRsCMby bioinformatics analysis,insituhybridisation,expression pattern analysis,subcellular localisation,pathogenicity analysis of nematodes to RNAi transgenic tomato and overexpression transgenic tomatoes,and inhibition analysis of tobacco defence response,etc.,provide evidence and data for elucidating the pathogenic mechanism ofRsCMand finding new control targets.
2.Materials and methods
2.1.Materials
Radopholussimiliswas identified and preserved by Plant Nematode Laboratory of South China Agricultural University and was cultured and propagated on carrot callus at 25°C,according to the method of Fallas and Sarah (1994).
The seeds ofLycopersiconesculentumMill.(Zhongshu 4) used in the experiment were purchased from Guangzhou Changhe Seed Co.,Ltd.,China. The tomato seeds were planted in a flowerpot (d=15 cm) filled with sterilised soil,cultured in a greenhouse with a relative humidity of 60–80% and (26±1)°C (photoperiod: 16 h light/8 h dark),and nematodes were inoculated when the height of the tomato plant was approximately 20 cm.The seeds ofNicotianabenthamianawere preserved in our laboratory. The culture conditions for tobacco were the same as those for tomato,and the experiment was conducted after the tobacco grew for 4–6 weeks.
2.2.Total RNA and DNA extraction
The mixed-stage nematodes were collected for total RNA and total DNA extraction. Two extraction methods for the total RNA and DNA of nematodes refers to one from the Trizol Reagent manual and the phenol/chloroform extraction method (Niuet al.2016),respectively. The synthesis of the first strand of cDNA refers to the instructions of One-step gDNA Removal and cDNA synthesis SuperMix kit (TransGen Biotech,Beijing).
2.3.Gene cloning and sequence analysis
The transcript of the suspected CM gene was screened from the transcriptome data ofR.similisby using the local BlastX method. The 5´ and 3´ end sequences of theRsCMgene were amplified according to the instructions of the SMART RACE cDNA amplification kit (Clontech,TaKaRa Biotechnology (Dalian) Co.,Ltd.,Dalian,China),and the amplified sequences were sequenced and spliced. The open reading frame (ORF) ofRsCMwas predicted by the ORF finder (http://www.ncbi.nlm.nih.gov/gorf/orfig.cgi),and a pair of full-length primers Full-CMF/Full-CMR (Appendix A) were designed to amplify theRsCMgene.
The similarity ofRsCMwas aligned in the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) with the BlastX tool. The signal peptide (SP) and transmembrane domain ofRsCMwere predicted using SignalP-4.0(http://www.cbs.dtu.dk/services/SignalP-4.0/) and TMHMMServer v.2.0 (http://www.cbs.dtu.dk/services/TMHMM/). The phylogeny tree was reconstructed using the Neighbour-Joining method by MEGA 7 software with a bootstrap test (1,000 replicates) (Kumaret al.2016).
2.4.Developmental expression analysis
The mixed state ofR.similiswas washed into a petri dish from carrot callus,and then 3,000 females,males,juveniles and eggs were collected from the petri dish into centrifuge tubes with a needle pick or a pipette;extracted the total RNA of the nematode;and reversed transcribed to synthesise cDNA. Next,we designed a pair ofRsCMspecific primers (Appendix A) for RT-qPCR amplification,witheif5aas the internal reference gene.
2.5.In situ hybridisation
A 301-bp sequence was selected from theRsCMsequence,and templates of sense probes and antisense probes were obtained by PCR amplification using primers CM-T7F/CM-R and CM-F/CM-T7R (Appendix A).
According to the operating manual of DIG RNA Labelling Mix (Roche,Chiyoda),the sense RNA probe and antisense RNA probe of theRsCMgene were synthesised.Insituhybridisation was conducted according to the method of de Boeret al.(1998). The results were observed and photographed by a Nikon Eclipse 90i microscope (Nikon Company,Tokyo,Japan).
2.6.Southern blot
A 268-bp sequence was selected from theRsCMsequence and amplified using the primer pairs CM-SF/CM-S-R (Appendix A). The Southern blot probe was synthesised according to the instructions of the PCR DIG probe synthesis kit (Roche,Switzerland). The nematode genome was digested by restriction enzymes,PteI andMfeI,and the pEASY:RsCMplasmid was used as the template for digestion as the control. Southern blot was performed with reference to DIG high prime DNA labelling and detection kit I (Roche,Basel,Switzerland).
2.7.Subcellular localisation
TheRsCMsequence without the native SP was amplified and cloned into pCAMBIA1300 vector to generate pCAMBIA1300:RsCMΔSP-GFP,and pCAMBIA1300:GFP was used as the control. All constructs were transformed into anAgrobacterium tumefaciensstrain GV3101 and transiently expressed inN.benthamianaleaves by agroinfiltration (Wydroet al.2006). After infiltration in the dark at 28°C for 48 h,the tobacco leaves were observed and photographed under a fluorescence microscope.
2.8.Western blot
The total proteins of tobacco leaves were extracted using the plant protein extraction kit. Sample proteins were denatured and separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane (PALL,Washington,NY,USA). After blocking with 5% (w/v) skim milk at room temperature for 1 h,the membrane was incubated with a primary mouse anti-GFP antibody or anti-Flag antibody (1:5,000 dilution) (Rotche,Rotkreuz,Switzerland) for 2 h. Next,the membrane was incubated with an anti-mouse horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution) (China Beijing Biosynthesis Biotechnology Co.,Ltd.) for 1 h. The proteins were visualised with the 3,3´-diaminobenzidine(DAB) colour developing kit (Beijing Solebao Biotechnology Co.,Ltd.,China) and photographed with a gel imager.
2.9.Plant immune response assay
The construct pCAMBIA1300:Flag-RsCMΔSPwas transformed into anA.tumefaciensand then infiltrated intoN.benthamianaleaves by agroinfiltration (Wydroet al.2006). After infiltration in the dark at 28°C for 48–72 h,the tobacco injection area analysis for the calluses of tobacco was conducted according to the methods of Fabroet al.(2011) and Marcoet al.(2016).
2.10.Analysis of defence gene expression
For determining the defence-related gene expression levels,the constructs pCAMBIA1300:Flag-RsCMΔSPwas transformed into anA.tumefaciensand then infiltrated intoN.benthamianaleaves by agroinfiltration for 48 h. The tobacco injection area was soaked in 10 μmol L–1flg22 for 3 h,the total RNA of tobacco leaves was extracted using the HiPure plant RNA mini kit,and the cDNA was obtained by reverse transcription by using one-step gDNA removal and cDNA synthesis supermix. The transcript abundance of three tobacco PTI marker genes,NbPti5,NbGras2,andNbacre31(Nguyenet al.2010),was determined by RT-qPCR (Bio-Rad CFX-96),andNbEF1a(Rajputet al.2014) was used as the reference gene. The tobacco leaves soaked with the same concentration of flg22 for 3 h after 48 h injection of pCAMBIA1300:GFP construct and tobacco leaves soaked with clean water as control,and the experiment conducted three biological repetitions.
2.11.Detection of inhibitory effect of RsCM on immune elicitor-induced cell necrosis
The BAX and Gpa2/RBP-1 immunoinducers were used to test the inhibitory effect ofRsCMon cell necrosis. The constructs pCAMBIA1300:Flag-RsCM,pCAMBIA1300:flag-RsCMΔSP,and pCAMBIA1300:GFP were transformed into anA.tumefaciensand then infiltrated into tobacco leaves by agroinfiltration at 28°C for 48 h,respectively. The BAX or Gpa2/RBP-1 immunoinducer was infiltrated into the same infiltration sites at 28°C for 3 to 6 d. After infiltration,the necrosis of tobacco leaves was examined and photographed with a Canon camera DS126181 (Canon,Tokyo,Japan). The necrosis index was counted by the method of Gilroyet al.(2011). Each treatment was infiltrated five tobacco leaves and the experiment was repeated twice.
2.12.In planta RsCM silencing and overexpression
A 450-bp fragment ofRsCMwas selected as the template for tomato-mediated silencing,and a silencing vector pFGC5941:RsCM225–675containing a silencing hairpin structure was constructed;moreover,a designatory peptideRsCMwas used as the template for tomatomediated overexpression,and an overexpression vector pCAMBIA1300:RsCMΔSPwas constructed.Agrobacteriumtumefacienscontaining RNAi vector pFGC5941:RsCM225–675,pFGC5941 empty vector,overexpression vector pCAMBIA1300:RsCMΔSP,and pCAMBIA1300 empty vector were transferred into tomato explants byA.tumefacienstransformation,respectively(Velchevaet al.2005). The wild-type tomatoes,transgenic pFGC5941 empty vector tomatoes and the transgenic pCAMBIA1300 empty vector tomatoes were used as controls. The positive T0generation transgenic tomatoes were obtained through PCR amplification and Southern blot hybridisation detection. We detected the relative expression amount of the target gene by qPCR and selected the T0generation transgenic tomato with top three highest expression amount as a female parent to cultivate the T1generation transgenic tomato trains. PCR validation was performed on T1generation RNAi and overexpression transgenic tomatoes using CMIF/CMIR and Full-CMF/Full-CMR primers,respectively (Appendix A) to obtainRsCMRNAi and overexpression transgenic tomatoes.
2.13.Pathogenicity determination of transgenic plants
Three T1generation transgenic tomato trains seedlings with growth conditions,which were basically consistent,were inoculated withR.similisat the inoculation amount of 1,000 nematodes/pot. We watered the tomato seedlings appropriately to moisten the soil before inoculation with nematodes: the nematode suspension was inoculated into a well drilled in a radius of 1.5 cm from the tomato root using a pipette (Wanget al.1997). After inoculation with nematodes,the tomatoes were returned to the incubator for culture. For nematode loss prevention,no water was provided within 3 d after inoculation. Five replicates were set for each treatment,and the experiment was repeated twice. After 60 d of nematode inoculation,the tomato seedlings were removed from the soil,and the nematode was isolated from the soil and roots using the Berman funnel method (Hooperet al.2005) and Kaplan’s method(1994). Additionally,the fresh root weight was measured,and the root damage was recorded and photographed.
2.14.Detection of RsCM relative expression level of nematode in RNAi transgenic tomato
For detecting whether theRsCM,which was inoculated with T1generation RNAi transgenic tomato,was effectively silenced,approximately 1,000R.similiswere isolated and collected from the rhizosphere of the T1generation RNAi transgenic tomato,the transgenic pFGC5941 empty vector tomatoes,and the wild-type tomatoes,respectively.By using the aforementioned method,the RNA of the nematodes was extracted and reversely transcribed into cDNA. Next,the RT-qPCR experiment was conducted to detect the relative expression level ofRsCM,and the experiment was repeated three times.
2.15.Detection of SA pathway gene expression of overexpression transgenic tomatoes
To test whetherRsCMcould effectively inhibit the SA pathway in plants,we collected the roots from the T1generation overexpression transgenic tomatoes,the transgenic pCAMBIA1300 vector tomatoes,and the wildtype tomatoes,which inoculated with nematodes for 30 d.The RNA of tomato roots was extracted using the HiPure plant RNA mini kit and reversely transcribed into cDNA by the aforementioned method. The RT-qPCR method was used to detect the relative expression levels of two SA pathway-related genes,PR-1andPR-5,in tomato(Leonettiet al.2017),withβ-actinin tomato as the internal reference gene (Li 2019). The experiment was repeated three biological times.
2.16.Data processing
Experimental data were processed using GraphPad Prism software for one-way ANOVA and multiple comparisons at the significance levelP=0.05 (Duncan). Preliminary analyses showed that there was no significant difference(P>0.05) between the date from two runs of each experiment with three tomato strains using in dependentsamplet-tests,and that allow date from two runs to be combined for analyses.
3.Results
3.1.Sequence analysis of RsCM gene from R.similis
A 1,277-bp genomic DNA sequence and a 1,125-bp coding region ofRsCM(GenBank accession number: MN176124.1) were amplified fromR.similis(Fig.1-A). The complete open read frame (ORF)encodes a 374-amino-acid (aa) protein with a predicted molecular mass of 37.1 kDa. Signal analysis ofRsCMidentified a 23-aa N-terminal SP (Fig.1-D),no putative transmembrane domain was predicted (Fig.1-B),and a conserved domain of the chorismate mutase family was observed (Fig.1-C).
A multiple sequence alignment of the deduced amino acid sequence ofRsCMwith chorismate mutase sequences from other plant-parasitic nematodes is present in Fig.1-D,the comparison showed thatRsCMcontains highly conserved regions of type II chorismate mutase,and its similarity with HgCM1ofH.glycinesandGpCM1ofG.pallidawas 43.90 and 42.86%,respectively.
The phylogenetic tree composed ofRsCMand 41 CM sequences from other species showed (Fig.2) thatRsCMis clustered into one branch with the CM genes of cyst nematode,indicating that it is closely related to cyst nematode. Moreover,CM genes of plant-parasitic nematodes have a closely related to some bacteria;thus,RsCMmight be transferred horizontally from bacteria.
3.2.Developmental expression analysis and in situ hybridisation
To analyse the developmental expression pattern ofRsCM,we performed RT-qPCR using RNA isolated from different developmental stages ofR.similis. The relative expression levels ofRsCMin different developmental stages were detected (Fig.3).RsCMexpression fold values in the juveniles,relative to the egg,was significantly highest (5.11) (P<0.05),and that in the females was significantly higher than that in the males and eggs (2.46vs.1.18 and 1.15) (P<0.05). There was no significant difference in expression levels between males and eggs.
Tissue localisation ofRsCMinR.similiswas determined byinsituhybridisation. A hybridisation signal was observed in the subventral oesophageal gland cells with the DIG-labelled antisense RNA probe ofRsCM. No hybridisation signal was found in the tissue ofR.similiswith the sense RNA probe ofRsCM(Fig.4).
3.3.Southern blot of RsCM
The completely digested nematode genome DNA was hybridised with the Southern blot probe labelled with digoxigenin. The genomic DNA and plasmid DNA digested by two types of endonucleases have obvious hybridisation signals,and both have only one hybridisation band,which indicates thatRsCMexists as a single copy in theR.similisgenome (Fig.5).
3.4.RsCM subcellular localisation analysis
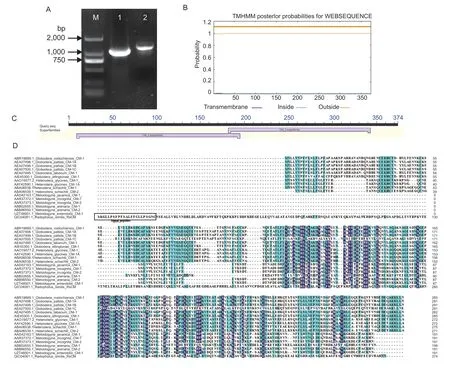
Fig. 1 Sequence information of chorismate mutase gene of Radopholus similis (RsCM). A,DNA and cDNA full-length amplification of RsCM. M,DS2000 marker;1,cDNA full-length amplification of RsCM;2,DNA full-length amplification of RsCM. B,prediction of transmembrane domain. C,prediction of conserved domains. D,multiple sequence alignment of the predicted RsCM protein with other chorismate mutase sequences from plant-parasitic nematodes.
For the investigation of the localisation of RsCM in plant cell walls,two constructs,pCAMBIA1300:RsCMΔSP-GFP and pCAMBIA1300:GFP,were transiently expressed inN.benthamianaleaves. At 2 d post-infiltration,the pCAMBIA1300:RsCMΔSP-GFP and pCAMBIA1300:GFP fusion proteins were observed in the cytoplasm and nucleus (Fig.6-A). Protein blotting on two infiltrated leaves further confirmed the correct expression of these fusion proteins in tobacco cells (Fig.6-B).
3.5.lnhibition of RsCM on tobacco immune response
First,in the investigation of the inhibitory effect of RsCM on cell necrosis induced by immune elicitors(BAX and Gpa2/RBP-1),no necrosis was observed in the leaves area infiltrated with the constructs pCAMBIA1300:GFP and pCAMBIA1300:Flag-RsCMΔSP,respectively;82.33 and 3.26% leaf necrosis were observed in the leaves area infiltrated with the constructs pCAMBIA1300:GFP+BAX and pCAMBIA 1300:Flag-RsCMΔSP+BAX,the difference between the two treatments was significant (P<0.05) (Fig.7-A and C). The leaf necrosis areas accounted for 86.85 and 85.89% of the total injected area in the treatments infiltrated with the constructs pCAMBIA1300:GFP+Gpa2/RBP-1 and pCAMBIA1300:Flag-RsCMΔSP+Gpa2/RBP-1,respectively,there was no significant difference in the two treatments(P>0.05) (Fig.7-B and D). These results indicated that RsCM was effective in inhibiting BAX-induced cell necrosis,but no cell necrosis was caused by the immunoblaster combination Gpa2/RBP-1. Western blot results showed that all target proteins were expressed(Fig.7-E and F).

Fig. 2 Neighbour-joining phylogenetic trees of chorismate mutase of Radopholus similis (RsCM) and other organisms. Phylogenetic tree for proteins with conserved domains of chorismate mutase from cyst nematodes,root-knot nematodes,Hirschmanniella oryzae,bacteria,plants,and fungi generated by MEGA6.0. The RsCM amino acid sequence is marked in gray background,and each sequence is followed by its accession number on GenBank.
Second,the results of the investigation of the effect of RsCM on callose deposition in tobacco leaves showed that the callose deposition of the treatment of RsCMΔSP+flg22 was significantly less than that of GFP+flg22 treatment,and the expression of RsCM inhibited the callose deposition caused by flg22 (Fig.8).
Finally,the transcription levels of tobacco defencerelated genes,includingNbPti5,NbGras2,andNbacre31,were detected. RT-qPCR showed that compared with the water soaking treatment,the expression levels ofNbPti5,NbGras2,andNbacre31in tobacco leaves treated with pCAMBIA1300:GFP were significantly up-regulated(P<0.05),by 13.48 times,13.97 times,and 48.65 times,respectively;while those in tobacco leaves treated with pCAMBIA1300:flag:RsCMΔSPwere 1.69 times,1.09 times,and 4.17 times higher than those in the water soaking treatment,respectively,with no significant difference(P>0.05) (Fig.9). These results indicated that RsCM obviously inhibited the up-regulation of tobacco defence genes induced by flg22,and the expression of RsCM inhibited the immune response of tobacco.
3.6.Silencing and overexpressing of RsCM affect pathogenicity of R.similis on tomatoes

Fig. 3 Expression of chorismate mutase gene of Radopholus similis (RsCM) in different life stages of R.similis. X-axis represents the life stages of R.similis;Y-axis represents the fold change values of RsCM which were presented as the change in expression relative to the egg. Bars indicate standard errors of mean data (n=3);different letters indicate significant differences(P<0.05) among groups.

Fig. 4 Tissue localisation of chorismate mutase gene of Radopholus similis (RsCM) in nematode via in situ hybridisation. A,no hybridisation signal was detected in negative control nematodes with digoxigenin-labelled sense RsCM RNA probe. B and C,specific hybridisation signals were detected in oesophageal glands of R.similis with digoxigeninlabelled antisense RsCM RNA probe. mb,medium bulb;eg,oesophageal glands. Scale bar=10 μm.

Fig. 5 Southern blot analysis of chorismate mutase gene of Radopholus similis (RsCM). 1 and 2,Southern blot results of R.similis genome DNA digested by PteI and MunI;3 and 4,Southern blot results of pEASY:RsCM plastid digested by PteI and MunI,respectively.

Fig. 6 Subcelluar localisation of chorismate mutase of Radopholus similis (RsCM) in Nicotiana benthamiana cells.A,Agrobacterium stain GV3101 carrying fusion constructs RsCMΔSP-GFP and GFP control were transiently expressed in N.benthamiana. B,Western blot confirmed the expression of RsCMΔSP-GFP and GFP in N.benthamiana leaves. Scale bar=50 μm.
After screening and verification,three independent T1generation transgenic tomato strains of each treatment were obtained. For the investigation of study,the pathogenicity ofR.similison tomato after silencing and overexpressing ofRsCM,R.similiswas inoculated into the roots of the T1generation transgenic tomato for 30 d,plant root damage was detected,and the fresh weight of root and the number of rhizosphere nematodes were measured and counted. The severity of injury symptoms of the T1generation RNAi transgenic tomato was significantly higher than that of the control wild-type tomatoes and transgenic empty vector tomatoes,while the fresh root weight was significantly higher than those treatments (0.36 gvs.0.16 g and 0.36 gvs.0.18 g)(P<0.05). The root of the T1generation overexpression transgenic tomato was more affected than that of the wild-type tomatoes and transgenic empty vector tomatoes,while the fresh root weight was significantly lower than those treatments (0.09 gvs.0.16 g and 0.09 gvs.0.18 g) (P<0.05). There was no significant difference in root weight between the wild-type tomatoes and transgenic empty vector tomatoes (0.16 gvs.0.18 g)(Fig.10-A and B). The number of nematodes in the rhizosphere of the T1generation RNAi transgenic tomato was significantly lower than that of the wild-type tomatoes and transgenic empty vector tomatoes (1,393vs.2,847 and 1,393vs.2,800) (P<0.05),that in the rhizosphere of the T1generation overexpression transgenic tomato was not significantly different from that of the two treatments(3,007vs.2,847 and 3,007vs.2,734) (Fig.10-C),and there was no significant difference in the number of nematodes in the rhizosphere in the two treatments (2,800vs.2,734).

Fig. 7 Inhibitory analysis of BAX-and Gpa2/RBP-1-induced Nicotiana benthamiana cell death by chorismate mutase of Radopholus similis (RsCM). A and B,inhibitory effect of RsCM on BAX (A)-and Gpa2/RBP-1 (B)-induced N.benthamiana cell necrosis. C and D,mean percentage of cell death lesion of different treatments. Bars indicate standard errors of mean data (n=5). Different letters indicate significant differences (P<0.05) among groups. E and F,detection of protein expression by Western blot. 1,pCAMBIA1300:Flag→48h→BAX;2,pCAMBIA1300:Flag;3,pCAMBIA1300:Flag-RsCMΔSP;4,pCAMBIA1300:Flag-RsCMΔSP→48h→BAX;5,pCAMBIA1300:Flag→48h→Gpa2/RBP-1;6,pCAMBIA1300:Flag;7,pCAMBIA1300:Flag-RsCMΔSP;8,pCAMBIA1300:Flag-RsCMΔSP→48h→Gpa2/RBP-1.

Fig. 8 Inhibition of flg22-induced callose deposition by chorismate mutase of Radopholus similis (RsCM). RsCM+flg22,tobacco leaves were soaked in 10 μmol L–1 flg22 solution after 48 h of RsCMΔSP injection;GFP+flg22,tobacco leaves were soaked in 10 μmol L–1 flg22 solution after 48 h of GFP injection;WT,wild-type tobacco leaves. Scale bar=100 μm.

Fig. 9 Inhibition of flg22-induced Nicotiana benthamiana defence gene expression by chorismate mutase gene of Radopholus similis (RsCM). The fold change values of NbPti5,NbGras2,and Nbacre31 were presented as the change in expression relative to water treatment;RsCM+flg22,tobacco leaves were soaked in 10 μmol L–1 flg22 solution after 48 h of RsCMΔSP injection;GFP+flg22,tobacco leaves were soaked in 10 μmol L–1 flg22 solution after 48 h of GFP injection;water,tobacco leaves with water injection. Bars indicate standard errors of mean data (n=3). Different letters indicate significant differences (P<0.05)among groups.
The RT-qPCR assay was applied to detect the relative expression level ofRsCMinR.similiscollected from the rhizosphere of tomato and the relative expression levels of two SA pathway genes,PR-1andPR-5,in the roots of T1generation overexpression transgenic tomatoes inoculated withR.similisfor 30 d. TheRsCMexpression fold values ofR.similiscollected from roots of T1generation RNAi transgenic tomatoes were significantly lower than those collected from roots of the wild-type tomatoes and transgenic empty vector tomatoes (1vs.5.7 and 1vs.5.5)(P<0.05),and there was no significant difference inRsCMexpression fold values between the two control treatments(5.7vs.5.5) (Fig.11). The expression fold values ofPR-1andPR-5in roots of overexpression transgenic tomatoes inoculated withR.similiswere significantly lower than those in the wild-type tomatoes and transgenic empty vector tomatoes (1vs.6.7,1vs.7.1,and 1.1vs.6.8,1.1vs.6.2) (P<0.05),and there was no significant difference in expression levels between the two control treatments(6.7vs.7.1 and 6.8vs.6.2) (Fig.12).
These results indicated that the T1generation RNAi transgenic tomato effectively inhibited the expression ofRsCM,effectively inhibiting the pathogenicity ofR.similisand significantly reducing the damage degree of tomato.The T1generation overexpression transgenic tomato inhibited the SA pathway of the plants,leading to the obvious aggravation of tomato victimisation.
4.Discussion
In this study,the phylogenetic analysis ofRsCMshowed thatRsCMis clustered into one branch with the CM genes of cyst nematode,indicating that it is closely related to cyst nematode,moreover,CM genes of plantparasitic nematodes has a closely related to some bacteria. Mathew and Opperman (2019) clustered various orthologous genes shared byR.similiswith other nematodes showed thatR.similishas a higher overlap with the cyst nematodes than with the root-knot or other migratory endoparasitic nematodes,which indicated thatR.similisis evolutionarily closer to the cyst nematodes.Noon and Baum (2016) reported that the CM of plantparasitic nematodes,including cyst nematodes and rootknot nematodes,were horizontally acquired from bacteria most similar toBurkholderiasoil bacteria. The results of this study supported these studies.

Fig. 10 In planta RNA interference (RNAi) and overexpression of chorismate mutase gene of Radopholus similis (RsCM)influence the pathogenicity of R.similis on tomato. A,pathogenicity of R. similis on T1 generation transgenic tomato.B,the fresh root weight of tomato. C,number of nematodes collected from tomato roots. CK,healthy tomato;WT,wild type tomato;RNAi,T1 generation RNAi transgenic tomato;PFGC5941,empty vector PFGC5941 transgenic tomato;pCAMBIA1300,empty vector pCAMBIA1300 transgenic tomato;OE,T1 generation overexpression transgenic tomato.Data are the average standard error of three tomato strains with five replicates. The same lowercase letters in the figure indicate that there is no significant difference among the treatments.

Fig. 11 Expression of chorismate mutase gene of Radopholus similis (RsCM) after in planta RNA interference (RNAi) RsCM.The fold change values of RsCM were presented as the change in expression relative to RNAi treatment. WT,relative expression of RsCM in R.similis after 30 d inoculation with wildtype tomatoes;EV,relative expression of RsCM in R.similis after 30 d inoculation with empty vector PFGC5941 transgenic tomatoes;RNAi,relative expression of RsCM in R.similis wildtype tomato after 30 d inoculation with T1 generation RsCM RNAi transgenic tomato. Data are the average standard error of three tomato strains with three replicates;the same lowercase letters in the figure indicate that there is no significant difference among the treatments.
RsCMwas specifically expressed in the oesophageal gland ofR.similis,and its expression in juveniles and females,which are infestation stages of the nematode,was significantly higher than that in males and eggs,indicating thatRsCMplayed an important role in the parasitism and infection ofR.similisto plants. In the process of infecting plants,plant parasitic nematodes can secrete a series of substances into plant cells through stylets or body walls. Most of these secretions are proteins produced by oesophageal glands of nematodes,and a few are produced by head sensilla or the tail (Perryet al.1996;Haegemanet al.2012). These secreted proteins play an important role in the successful infection of nematodes (Hogenhoutet al.2009). The CM genes of 11 other plant nematodes,such asRsCM,are specifically expressed in oesophageal glands (Bekalet al.2003;Joneset al.2003;Huanget al.2005;Longet al.2006;Luet al.2008;Vanholmeet al.2009;Haegemanet al.2011;Hanget al.2011;Bauterset al.2014,2020;Wanget al.2018),and these CMs may be secreted into plants to play a role (Doyle and Lambert 2003). Different developmental stages ofR.similisplay different roles in the process of infecting the host: juveniles and females have infectivity,and males have no infectivity because of the degeneration of stylets and oesophageal glands (Joneset al.2013).Other effector protein genes ofR.similishave been reported,such asRs-eng1b(Zhanget al.2012),Rs-cb1(Liet al.2015b),Rs-crt(Liet al.2015a),Rs-far1(Zhanget al.2015),Rs-cps(Wanget al.2016),andRs-scp1(Huanget al.2017),conform to the characteristics of high expression in juveniles and females but have low or no expression in males. Therefore,it is inferred thatRsCMwere secreted into plants through stylet and plays an essential role in the parasitic and pathogenic process ofR.similis.

Fig. 12 Expression of two salicylic acid pathway marker genes in diseased roots of overexpression transgenic tomato by in planta overexpressed chorismate mutase gene of Radopholus similis (RsCM). A,expression of LePR1 gene. B,expression of LePR5 gene. The fold change values of LePR-1 and LePR-5 were presented as the change in expression relative to OE treatment. WT,roots of wild-type tomato 30 d after inoculation of R. similis;EV,roots of empty vector pCAMBIA1300 transgenic tomato 30 d after inoculation of R.similis;OE,roots of T1 generation RsCM overexpression transgenic tomato 30 d after inoculation of R.similis. Data are the average standard error of three tomato strains with three replicates;the same lowercase letters in the figure indicate that there is no significant difference among the treatments.
During their long-term contact with pathogens,plants evolved PTI (PAMP-triggered immunity) and ETI(effector-triggered immunity) immune systems (Jones and Dangl 2006). PTI refers to a series of immune responses,such as cell necrosis,reactive oxygen species explosion,callose deposition,and up-regulation of defence gene expression,which are recognised by plant cells when pathogens invade plants;ETI refers to a new round of immune response initiated by plant cells recognising specific effector proteins after a pathogen escapes or overcomes PTI reaction of plants through the produced effector proteins. BAX protein is the elicitor of plant allergic necrosis,and its expression in tobacco can induce the basic immune response of tobacco,leading to cell necrosis (Lacomme and Cruz 1999). Effectors that inhibit cell necrosis induced by BAX are considered to have the effect of inhibiting plant basic immune response (Wanget al.2011). Gpa2/RBP-1 is also an elicitor of allergic necrosis in plants. RBP-1 is a nontoxic protein of potato cyst nematode,and Gpa2 is a resistance gene from plants. Their co-expression in tobacco can cause an ETI reaction and induce cell necrosis (Saccoet al.2009). In this study,RsCM was transiently expressed in tobacco leaves,which significantly inhibited the defence gene expression and callose deposition induced by flg22,and cell necrosis induced by BAX,but not the cell necrosis induced by Gpa2/RBP-1. Therefore,RsCM inhibits PTI but not ETI in plants.
The RNAi transgenic tomato had obvious resistance and inhibition to the pathogenicity and reproduction of nematodes,and theRsCMofR.similisisolated from its roots was obviously silenced. The resistance of overexpressed transgenic tomatoes to nematode infection was significantly reduced,and the degree of damage was significantly increased,but the number of nematodes on tomato roots was not significantly increased,which might be due to the severe necrosis of the diseased roots of overexpressed transgenic tomatoes,which could not provide sufficient space and nutrition to breed additional nematodes. These results prove thatRsCMplays an important role in the infection and pathogenesis ofR.similis.
SA is one of the secondary metabolites of chorismates,which can regulate physiological and biochemical processes such as seed germination,stomatal closure,cell respiration,membrane permeability,and plant senescence (Raskin 1992),but its most important role is to induce plant disease resistance. PTI and ETI in plants are closely related to the accumulation of SA in cells(Matsuoka and Ohashi 1986). After plants are infected by pathogens,the content of SA in infected tissues increases rapidly. The accumulation of SA can spread the signal of pathogens from infected parts to the whole plant,induce plants to acquire systemic resistance to pathogens(Yuko and Makoto 1987),and induce the expression of disease-related genes (PR-1andPR-5) (Moreauet al.2012;Beatriceet al.2017). SA also plays an important role when plants against the infection of plant parasitic nematodes. Studies have shown that applying the proper amount of SA to tomato roots can enhance the resistance of tomato to root-knot nematodes (Kyndtet al.2012;Molinariet al.2014). CM is a key enzyme involved in modification of the host SA pathway (Wanget al.2018).The expression of thePR-1gene is regulated by SA,a plant hormone,and is often used as a molecular marker for systemic acquired resistance in many plant species(Mitsuharaet al.2008).PR-5is usually not expressed in healthy plants;it is only induced to express in response to injury or pathogen attack (Monteiroet al.2003). In this study,transgenic tomato with overexpression ofRsCMwas obtained,and its susceptibility toR.similisincreased significantly,and the expression levels of the two genesPR-1andPR-5(Molinariet al.2014) related to the SA pathway in the roots inoculated withR.similisdecreased significantly,which indicated thatRsCMmight be involved in the inhibition of the SA pathway. MiCM3 of parasiticM.incognitaalso inhibitsPR-1expression (Wanget al.2018).
The chorismate competition hypothesis of plant nematodes based on the research results of the CM function of sessile parasitic plant nematodes is that plant nematodes secrete CM into the cytoplasm of plants and compete with the original CM in the cytoplasm body of plants for chorismate,breaking the dynamic balance of plant cell chorismate between cytoplasm and plastid and affecting the normal growth of plants and the function of defence system (Doyle and Lambert 2003).RsCM,in the cytoplasm of tobacco cells,may have a similar mechanism of chorismate competition with the sessile parasitic nematode CM to affect the synthesis of SA by the host,that is,after being secreted into the plant cytoplasm,RsCMcompetes for the chorismate in the plant cytoplasm body and inhibits the synthesis of SA in plant cells,inhibiting the defence response of plants and finally benefiting the infection and parasitism of nematodes. In addition,RsCMwas also in the nucleus of tobacco cells and inhibited the expression of two genes,PR-1andPR-5,related to the SA pathway,indicating thatRsCMmight have other effects on SA;thus,the inhibition mechanism of migratory parasitic nematodes on host defence response may differ from that of sessile parasitic nematodes.
5.Conclusion
In this study,based on transcriptome data,RsCMwas identified and cloned. Further experiments proved thatRsCMplayed an important role in pathogenicity. It specifically expressed in the oesophageal gland and highly expressed in juveniles and females,which inhibited the PTI induced by flg22 and cell death induced by immune elicitors BAX. In addition,RsCMinhibited the SA pathway by competing for CM in plants,and then inhibited the defense response of plants,which was ultimately beneficial to the infection and parasitism of nematodes,but further research was needed to confirm.
Acknowledgements
This research was funded by the Guangdong Basic and Applied Basic Research Foundation,China(2021A1515011273) and the National Natural Science Foundation of China (31071665).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendixassociated with this paper is available on http://https://doi.org/10.1016/j.jia.2023.04.040
 Journal of Integrative Agriculture2024年3期
Journal of Integrative Agriculture2024年3期
- Journal of Integrative Agriculture的其它文章
- Molecular mechanisms of stress resistance in sorghum: lmplications for crop improvement strategies
- Artificial selection of the Green Revolution gene Semidwarf 1 is implicated in upland rice breeding
- Dynamics and genetic regulation of macronutrient concentrations during grain development in maize
- The NAC transcription factor LuNAC61 negatively regulates fiber development in flax (Linum usitatissimum L.)
- The underlying mechanism of variety–water–nitrogen–stubble damage interactions on yield formation in ratoon rice with low stubble height under mechanized harvesting
- Rice canopy temperature is affected by nitrogen fertilizer
