Elucidating the cardioprotective mechanisms of sodium-glucose cotransporter-2 inhibitors beyond glycemic control
Ke-Xin Zhang,Cheng-Xia Kan,Fang Han,Jing-Wen Zhang,Xiao-Dong Sun
Abstract Sodium-glucose cotransporter-2 (SGLT2) inhibitors have emerged as a pivotal intervention in diabetes management,offering significant cardiovascular benefits.Empagliflozin,in particular,has demonstrated cardioprotective effects beyond its glucose-lowering action,reducing heart failure hospitalizations and improving cardiac function.Of note,the cardioprotective mechanisms appear to be independent of glucose lowering,possibly mediated through several mechanisms involving shifts in cardiac metabolism and anti-fibrotic,anti-inflammatory,and anti-oxidative pathways.This editorial summarizes the multifaceted cardiovascular advantages of SGLT2 inhibitors,highlighting the need for further research to elucidate their full therapeutic potential in cardiac care.
Key Words: Diabetes;Sodium-glucose cotransporter-2;Cardiovascular diseases;Empagliflozin
lNTRODUCTlON
The global increase in diabetes represents a significant public health challenge and is closely associated with an increased risk for cardiovascular diseases (CVD)[1].The lack of specific treatments to prevent its progression has left a significant gap in therapeutic strategies.Consequently,there is an urgent need for novel approaches to prevent and manage diabetes-related cardiac complications.Sodium-glucose cotransporter-2 (SGLT2) inhibitors (e.g.,empagliflozin),primarily known for their glucose-lowering capability,have emerged as unexpected protective agents against CVD in patients with diabetes.SGLT2 inhibitors may have beneficial effects on heart failure,including cases with dilated cardiomyopathy,by improving cardiac function and reducing hospitalization rates for heart failure[2].However,the unresolved cardioprotective mechanisms of these inhibitors have stimulated considerable scientific interest.The study by Lietal[3] provides an interesting insight into the molecular dynamics through which empagliflozin may exert its therapeutic effects on the diabetic heart.
Clinical trials have demonstrated that SGLT2 inhibitors significantly reduce the risk of hospitalization for heart failure and cardiovascular mortality.Notably,the DAPA-HF and EMPEROR-Reduced trials highlighted the positive effects of SGLT2 inhibition in patients with heart failure with a reduced ejection fraction,including those with and without diabetes[4-10].A comprehensive meta-analysis further reinforced these findings,indicating that SGLT2 inhibitors decrease the risk of cardiovascular mortality or first hospitalization for heart failure across a broad spectrum of left ventricular ejection fractions[2,4].Additionally,a meta-analysis involving over 21000 participants revealed consistent reductions in the risk of composite cardiovascular mortality or hospitalization for heart failure,as well as all-cause mortality[11].Evidence from clinical studies also indicated that SGLT2 inhibitors can improve diastolic function,particularly in heart failure with a preserved ejection fraction,a condition commonly observed in diabetic heart disease[12].
Animal studies have similarly provided evidence to support the cardioprotective role of SGLT2 inhibitors.A metaanalysis of preclinical animal models found that SGLT2 inhibitors reduced myocardial infarct size independent of diabetes,indicating a potential for broad cardioprotective applications beyond glucose-lowering effects[13].Our studies demonstrated that empagliflozin could also alleviate obesity-related cardiac dysfunction and attenuate ischemia/reperfusion injury[14,15].These studies provided evidence that SGLT2 inhibitors could benefit a wide population of heart failure patients,not just those with a reduced ejection fraction.
On the basis of the experimental data provided by Lietal[3],empagliflozin treatment displays therapeutic potential in mitigating diabetic cardiomyopathy in db/db mice.The treatment improved cardiac function,reduces myocardial apoptosis,and beneficially modulates signaling pathways associated with cardiac health,such as increased adenosine monophosphate-activated protein kinase (AMPK)/peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1) protein phosphorylation and decreased myosin phosphatase target subunit 1 phosphorylation.Furthermore,in vitrostudies supported these findings,demonstrating that empagliflozin protects cardiomyocytes from high-glucoseinduced mitochondrial damage,oxidative stress,and apoptosis,effects that were partly reversed by the addition of compound C,an AMPK inhibitor.The results were corroborated by the use of Rho kinase inhibitors and PGC-1α overexpression,which further validates the role of these pathways in cardiac protection.Interestingly,no SGLT2 protein expression was detected in cardiomyocytes,suggesting that the cardioprotective effects of empagliflozin may be independent of its glucose-lowering action and possibly mediated by AMPK/PGC-1α pathways.This indicates a potential non-glycemic beneficial effect of SGLT2 inhibitors on cardiac function in the context of diabetes,meriting further investigation.This study highlights novel mechanisms regarding the effectiveness of SGLT2 inhibitors in treating diabetic cardiomyopathy.
SGLT2 inhibitors,beyond their role in glucose excretion,confer cardiac protection through several mechanisms[16,17] (Figure 1).Primarily,they act as mild diuretics,which reduce cardiac preload and afterload by promoting natriuresis and osmotic diuresis,thereby lessening the cardiac load[18].They also beneficially shift cardiac metabolism away from fatty acid oxidation,which is less oxygen-efficient,towards glucose utilization and potentially towards ketone body utilization,thus improving the heart’s energy efficiency[19].These drugs may also protect against cardiac fibrosis by several means.They reduce hyperglycemia-related advanced glycation end-products,downregulate transforming growth factor-beta,and inhibit the cardiac sodium-hydrogen exchanger,which together help to prevent hypertrophy and fibrosis[20,21].
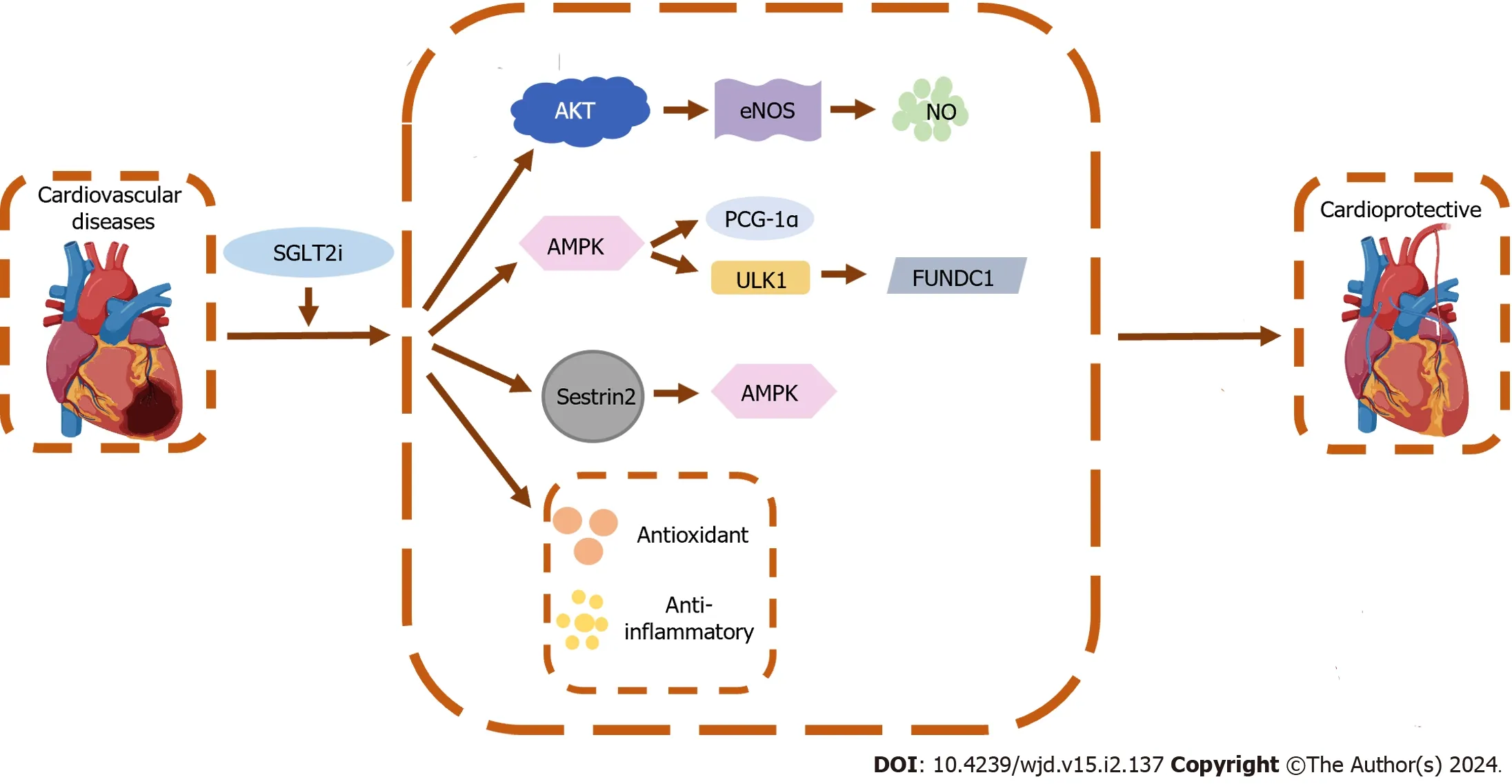
Figure 1 The cardioprotective mechanisms of sodium-glucose cotransporter-2 inhibitors beyond glycemic control. SGLT2i: Sodium-glucose cotransporter-2 inhibitor;AMPK: Adenosine monophosphate-activated protein kinase;Akt: Protein kinase B;eNOS: Endothelial nitric oxide synthase;NO: Nitric oxide;PGC-1α: Peroxisome proliferator-activated receptor gamma coactivator-1alpha;ULK1: Unc-51 like autophagy activating kinase 1;FUNDC1: FUN14 domain containing 1.
Moreover,SGLT2 inhibitors contribute to reducing arrhythmia risks and modulate ion homeostasis within the heart,suggesting a role in improving myocardial cell function and calcium handling[22].Their cardioprotective effects extend to anti-inflammatory and antioxidant actions,because they diminish nuclear factor-kappaB activity and enhance antioxidant system activity (e.g.,Sestrin2,nuclear factor erythroid 2-related factor 2,heme oxygenase-1)[14,23].This contributes to decreasing oxidative stress,another risk factor for heart failure.In addition,these drugs improve endothelial function and arterial compliance,partly through increased nitric oxide production,and affect the secretion of adipokines,which are involved in the pathophysiology of heart failure[24-26].This endothelial protection was confirmed by studies showing that empagliflozin suppresses endothelial apoptosis and maintains capillarization through the protein kinase B/endothelial nitric oxide synthase/nitric oxide pathway,thereby enhancing heart performance[27].Caietal[28] further demonstrated that empagliflozin mitigates endothelial oxidative stress and inhibits mitochondrial apoptosisviathe AMPK/unc-51 like autophagy activating kinase 1/FUN14 domain containing 1/mitophagy axis,thereby improving cardiac microvascular structure and endothelial function.SGLT2 inhibitors also induce protective autophagy and reduce apoptosis in cardiac cells,and they are being investigated for their potential effects on specific molecular pathways such as Sestrin2-AMPK,which are associated with heart failure management[14,23,29].Overall,the multifaceted approach to SGLT2 inhibitors highlight their potential as a therapeutic strategy for cardiovascular health,with ongoing research continuing to elucidate their complex mechanisms and benefits.
Nevertheless,the exact mechanisms by which SGLT2 inhibitors exert their cardioprotective effects remain under investigation,and it is likely that multiple mechanisms act in concert.Perhaps the most striking finding was empagliflozin's effectiveness in the absence of SGLT2 expression in cardiomyocytes.This clearly demonstrated the diabetesindependent action of this drug,highlighting its potential as a targeted therapy for CVD.The cardioprotective effects observed in patients with heart failure,including those with CVD,have led to an expansion of the indications for SGLT2 inhibitors beyond diabetes to include the treatment of heart failure with a reduced ejection fraction,with ongoing research potentially further broadening their therapeutic applications.Despite these promising findings,further research is necessary to fully elucidate the extent to which these mechanisms contribute to the cardiovascular benefits of SGLT2 inhibitors,the understanding of which will enhance the clinical application of these agents and potentially lead to more targeted treatments for patients with diabetic heart disease.
CONCLUSlON
SGLT2 inhibitors have become an essential therapeutic advancement in diabetes management due to their low risk of hypoglycemia and notable cardiovascular benefits.In addition to their glucose-lowering effects,SGLT2 inhibitors are recognized for their efficacy in treating heart failure through various non-glycemic mechanisms.These include hemodynamic changes and anti-inflammatory,anti-fibrotic,antioxidant,and metabolic effects,which together contribute to the cardiovascular advantages observed with SGLT2 inhibitor use.Further research is ongoing to fully understand the mechanisms through which these inhibitors exert their cardioprotective effects.
FOOTNOTES
Author contributions:All the authors contributed to this paper with conception and design of the study,literature review and analysis,drafting and critical revision and editing,and final approval of the final version.
Conflict-of-interest statement:The authors declare no conflict-of-interests in preparing this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Ke-Xin Zhang 0000-0002-9224-9465;Cheng-Xia Kan 0000-0002-4593-0303;Fang Han 0000-0002-8743-8763;Jing-Wen Zhang 0000-0002-6064-1374;Xiao-Dong Sun 0000-0001-7775-2823.
S-Editor:Gong ZM
L-Editor:A
P-Editor:Chen YX
 World Journal of Diabetes2024年2期
World Journal of Diabetes2024年2期
- World Journal of Diabetes的其它文章
- Diabetes is affecting everyone everywhere
- Glucagon-like peptide-1 receptor agonists as a possible intervention to delay the onset of type 1 diabetes: A new horizon
- Βalancing act: The dilemma of rapid hyperglycemia correction in diabetes management
- Duodenal-jejunal bypass improves hypothalamic oxidative stress and inflammation in diabetic rats via glucagon-like peptide 1-mediated Nrf2/HO-1 signaling
- Assessment of pathogenicity and functional characterization of APPL1 gene mutations in diabetic patients
- Long noncoding RNA protein-disulfide isomerase-associated 3 regulated high glucose-induced podocyte apoptosis in diabetic nephropathy through targeting miR-139-3p
