Duodenal-jejunal bypass improves hypothalamic oxidative stress and inflammation in diabetic rats via glucagon-like peptide 1-mediated Nrf2/HO-1 signaling
Huai-Jie Wang,Li-Bin Zhang,Si-Peng Sun,Qing-Tao Yan,Zhi-Qin Gao,Fang-Ming Fu,Mei-Hua Qu
Abstract BACKGROUND Type 2 diabetes mellitus (T2DM) is often accompanied by impaired glucose utilization in the brain,leading to oxidative stress,neuronal cell injury and inflammation.Previous studies have shown that duodenal jejunal bypass (DJB) surgery significantly improves brain glucose metabolism in T2DM rats,the role and the metabolism of DJB in improving brain oxidative stress and inflammation condition in T2DM rats remain unclear.AIM To investigate the role and metabolism of DJB in improving hypothalamic oxidative stress and inflammation condition in T2DM rats.METHODS A T2DM rat model was induced via a high-glucose and high-fat diet,combined with a low-dose streptozotocin injection.T2DM rats were divided into DJB operation and Sham operation groups.DJB surgical intervention was carried out on T2DM rats.The differential expression of hypothalamic proteins was analyzed using quantitative proteomics analysis.Proteins related to oxidative stress,inflammation,and neuronal injury in the hypothalamus of T2DM rats were analyzed by flow cytometry,quantitative real-time PCR,Western blotting,and immunofluorescence.RESULTS Quantitative proteomics analysis showed significant differences in proteins related to oxidative stress,inflammation,and neuronal injury in the hypothalamus of rats with T2DM-DJB after DJB surgery,compared to the T2DM-Sham groups of rats.Oxidative stress-related proteins (glucagon-like peptide 1 receptor,Nrf2,and HO-1) were significantly increased (P < 0.05) in the hypothalamus of rats with T2DM after DJB surgery.DJB surgery significantly reduced (P < 0.05) hypothalamic inflammation in T2DM rats by inhibiting the activation of NF-κB and decreasing the expression of interleukin (IL)-1β and IL-6.DJB surgery significantly reduced (P < 0.05) the expression of factors related to neuronal injury (glial fibrillary acidic protein and Caspase-3) in the hypothalamus of T2DM rats and upregulated (P < 0.05) the expression of neuroprotective factors (C-fos,Ki67,Bcl-2,and BDNF),thereby reducing hypothalamic injury in T2DM rats.CONCLUSION DJB surgery improve oxidative stress and inflammation in the hypothalamus of T2DM rats and reduce neuronal cell injury by activating the glucagon-like peptide 1 receptor-mediated Nrf2/HO-1 signaling pathway.
Key Words: Duodenal jejunal bypass surgery;Type 2 diabetes mellitus;Neuron apoptosis;Inflammatory;Oxidative stress;Hypothalamic injury
lNTRODUCTlON
Type 2 diabetes mellitus (T2DM) is a condition characterized by high blood glucose and insulin resistance.It accounts for approximately 90% of adult diabetes patients worldwide[1,2].Long-term uncontrolled diabetes conditions with high blood glucose levels and whole-body insulin resistance are associated with neurodegeneration[3,4].This often results in impaired glucose utilization and energy metabolism,leading to oxidative stress in the brain[5-9].Prolonged oxidative stress in the brain can eventually lead to complications,such as inflammation of the central nervous system (CNS) and neuronal cell apoptosis[10,11].
Metabolic surgery (also known as bariatric surgery) has been proven to have a beneficial effect on the management of type 2 diabetes and obesity[12,13].For individuals with diabetes,this surgical procedure leads to substantial weight loss,improved management of blood glucose levels,and reduced reliance on medication[14-16].Duodenal jejunal bypass (DJB) surgery is one of the procedures based on metabolic surgery and can be used to investigate the mechanisms of metabolic surgery in the treatment of diabetes mellitus[17,18].DJB surgery significantly improves peripheral glucose metabolism in T2DM rats,but its effects on diabetes-induced central brain injury remain unclear[19,20].
Glucagon-like peptide 1 (GLP-1) is a hormone produced by L cells in the gastrointestinal tract,that serves multiple biological functions[21].The combination of this hormone and its receptor,GLP-1 receptor (GLP-1R),can increase insulin secretion,enhance glucose metabolism,and play an important role in controlling blood glucose homeostasis[22].Ruzeet al[23] showed that central GLP-1 improved cerebral glucose uptake in obese and diabetic rats after DJB[23].In addition to its metabolic effects,GLP-1 has also been shown to have neuroprotective effects,and upregulation of GLP-1 can protect cells from oxidative stress caused by hyperglycemia and limit brain inflammation[24,25].Kimetal[26] demonstrated that the activation of GLP-1R can enhance the expression of proteins related to oxidative stress and the cell metabolism regulatory protein NRF2,and upregulate antioxidant enzymes such as HO-1 and SOD to alleviate oxidative stress[26-28].According to research by Shan Yetal[29],GLP-1R agonists decrease blood-brain barrier breakdown and brain inflammation in an astrocyte-dependent manner[29].
In our previous studies,we found that DJB can increase serum GLP-1 Levels and enhance brain glucose utilization,playing a positive role in the treatment of diabetes[30-32].Therefore,GLP-1 might play a role in DJB surgery for T2DMrelated brain injury.In the current study,we investigated the role and mechanism of GLP-1 signaling in neuronal cell injury and inflammation in the hypothalamus of T2DM rats treated with the DJB procedure.This study can provide new insights into the role and molecular mechanisms of metabolic surgery in the treatment of diabetes and its central complications.
MATERlALS AND METHODS
Animals and treatment
The male Wistar rats (n=40,8 wk old) used in this study were obtained from Jinan Pengyue Animal Ltd.(Pengyue,Jinan,China).Rats were housed in individually ventilated cages with 12-h light/dark cycles at 21-23 °C and 30%-40% humidity.The rats had free access to food and water.T2DM rat models were established using a high-fat and highglucose diet induction paired with streptozotocin intraperitoneal injection (35 mg/kg,Sigma,United States),with random blood glucose > 16.7 mmol/L.We randomly divided the T2DM rats into two groups: The DJB group (T2DM-DJB,n=10) and the Sham group (T2DM-Sham,n=10).Normal control rats (control group,n=10) were fed a normal diet.All the research related to the use of animals in this study has complied with all relevant national regulations and institutional policies for the care and use of animals.The research plan was followed by the National Natural Science Foundation of China,No.82070856,and conducted in Laboratory Animal Center of Weifang Medical University.The experimental protocols were approved by the Institutional Animal Care and Use Committee (No.2021SDL574) and Institutional Review Board (No.2020SDL074).
The detailed surgical and nursing procedures of the animals were performed as previously described[31].For the DJB operation,distal pyloric transection was performed,and the duodenal stumps were closed with nylon sutures (6-0,Zhejiang,China).The distal pylorus was anastomosed to the distal stump of the jejunal limb after the jejunum was transected 10 cm from the ligament of Treitz.The proximal stump of the small intestine was connected to the digestive limb 15 cm from the distal end of the gastrointestinal anastomosis.Sham operations were performed to transect the gastrointestinal tract and create an in-situ anastomosis,mimicking DJB surgery.The determination of glucose homeostasis pre-and postoperatively was performed as previously described[31].
Tissue processing and preparation
Six weeks after the operation,rats were sacrificed following the completion of testing for blood glucose homeostasis indicators,including blood glucose,serum GLP-1,and insulin levels.A portion of the hypothalamic tissues was collected from the rat hemisphere and frozen in liquid nitrogen for analysis of mRNA and protein expression.The other cerebral hemispheres of the rats were fixed in 4% formalin for immunohistochemistry (IHC) and immunofluorescence (IF) assays.
The expression of differential hypothalamic proteins analyzed by protein chip assay
Hypothalamic tissues were dissected and immediately placed into the SDT lysate buffer (100 mM Tris-HCL,2% SDS,100 mM DTT,pH 7.6.) and then homogenized.The quality of the supernatant proteins was analyzed using SDS-PAGE.The supernatant of the hypothalamus homogenate was prepared using filter-aided sample preparation filtration.The preparations were lyophilized and then redissolved in a 40 μL solution of 0.1% formic acid using a C18 cartridge.Peptide segments were separated using the Easy-nLCNano lift flow system and analyzed with the Q ExactiveTMPlus Mass Spectrometer.Quantitative analysis was performed using the label-free quantitation algorithm in this project.The significance level of protein enrichment for a specific gene ontology (GO) term or Kyoto encyclopedia of genes and genomes (KEGG) pathway was assessed using Fisher's exact test.Using Matplotlib software,we categorized the samples and proteins,and generated hierarchical clustering heatmaps simultaneously.
Western blotting analysis
Hypothalamus supernatants containing 30 μg of proteins were separated by 12% SDS-PAGE at 110 V for 2 h.The proteins were transferred onto a nitrocellulose membrane at 300 mA for 2 h at 4oC.TBS pH 8.0 with 0.5% Tween 20 was used to block the membranes for 1 h.After each membrane was incubated with the primary antibody overnight at 4 °C (Supplementary Table 1),it was washed three times with TBS for 30 min.The appropriate secondary antibody was then applied to the membrane and incubated for one hour at 23 °C.Protein analysis was conducted using western blotting detection equipment from Bio-Rad (United States) and ImageJ software (United States).
Flow cytometry
An ultrasonic sonicator (Virsonic 60) was used to lyse 50 mg of rat hypothalamus tissue.Cytokines interleukin (IL)-1β,IL-2,IL-4,IL-5,IL-6,IL-8,IL-10,IL-12p70,IL-17A,tumor necrosis factor α,interferon (IFN)-γ,and IFN-αwere measured in hypothalamic homogenate supernatants using cytokines combined detection kit (Jiangxi Cell-Gene Biotech CO.,Ltd) and were analyzed by FCAP Array 3.0 software (BD Biosciences) after collection of events in a flow cytometer (Beckman Coulter).
Immunohistochemistry and immunofluorescence assays
Sections were dewaxed,rehydrated,and then blocked with 10% fetal bovine serum for 30 min before undergoing an overnight incubation at 4 °C with the primary antibodies.Sections were incubated with a horse radish peroxidaseconjugated secondary antibody after being washed with phosphate buffered solution.The slides were exposed to diaminobenzidine for five minutes at room temperature prior to IHC examination.Hematoxylin was used for nuclear counterstaining.For the IF experiment,the slides were first incubated with the primary antibody.Then,the sections were incubated with goat anti-rabbit IgG H&L (Alexa Fluor®488) (ab150077,1:1000) secondary antibody for one hour.4',6-Diamidino-2-Phenylindole dye (blue) was used for nuclear staining.Image acquisition was performed using a microscope (IX71,Olympus,Japan).The primary antibodies are listed in Supplementary Table 1.
Quantitative real-time PCR
Total RNA was extracted from the hypothalamus using TRIzol reagent (Thermo Fisher,United States) and then reverse transcribed to cDNA using a reverse transcription kit (TOYOBO,Japan).SYBR Green (GeneCopoeia,United States) was used in the quantitative real-time (qRT)-PCR reaction,which was performed on a Roche Diagnostics (Germany) machine and analyzed using the Bio-Rad system.Supplementary Table 2 contains a list of the primer sequences that were used.
Statistical analysis
The data are presented as the mean ± SD by Graph Pad Prism 8.0.Differences between pre and post operation were evaluated with at-test.One-way ANOVA followed by Tukey's test was used to analyze differences between multiple groups.P< 0.05 indicated statistical significance.
RESULTS
The expression of different proteins in the hypothalamus after DJB intervention
The results of blood glucose and insulin detection pre and post operation showed that DJB surgery can significantly improve glucose homeostasis and alleviate insulin resistance in T2DM rats (Supplementary Figure 1).To investigate the expression of different proteins in the hypothalamus of rats between the T2DM-DJB and T2DM-sham groups,we conducted a quantitative proteomics (label-free) analysis.The results showed that at least 120 proteins were significantly altered (Figure 1A).KEGG enrichment and GO analysis revealed significant differences in signaling pathways and proteins related to oxidative stress,inflammation,and neuronal cell injury between the two groups of rats (Figure 1B and C).

Figure 1 The expression of differential proteins in the hypothalamus after duodenal jejunal bypass intervention. A: Heat map of the expression of the 120 DEPs between the T [type 2 diabetes mellitus (T2DM)-Sham] rats and D [T2DM-duodenal jejunal bypass (DJB)] rats.The colored column represents the sample number,the row name indicates the DEGs,each rectangle in the graph corresponds to the expression value of a sample,red indicates high expression and blue indicates low expression;B: Statistics of significantly enriched Kyoto encyclopedia of genes and genomes pathways (T2DM-Sham vs T2DM-DJB);C: GO term statistics for significantly enriched genes (T2DM-Sham vs T2DM-DJB).
DJB surgery inhibits hypothalamic oxidative stress in rats with T2DM by activating the Nrf2/HO-1 signaling pathway
The effects of oxidative stress on various stages of diabetic encephalopathy have been demonstrated in recent studies,and supporting antioxidant stress therapy is beneficial for improving complications of diabetes in the central nervous system[6].We assessed the redox status of hypothalamic tissue using multiple assays (Figure 2A and B).The levels of MDA decreased,while the content of SOD increased in the hypothalamus of the T2DM-DJB rats after the operation,compared to the T2DM-Sham rats.Nrf2 is involved in antioxidation by upregulating the expression of HO-1 and is inhibited under high glucose (HG) conditions,resulting in inflammatory responses and cell injury[33].We evaluated the effects of DJB surgery on the expression of Nrf2 and HO-1 in the hypothalamus of rats with T2DM.As shown in Figure 2C-E,the number of Nrf2-and HO-1-positive cells in the hypothalamus of T2DM-DJB rats decreased significantly after the DJB operation.The qRT-PCR and western blot data indicated a significant increase in Nrf2 and HO-1 expression following DJB surgery in the hypothalamus of T2DM rats (Figure 2F and G).These results indicate that DJB surgery improves diabetic hyperglycemia-induced hypothalamic oxidative stress in T2DM rats,possibly achieved by activating the Nrf2/HO-1 signaling pathway.

Figure 2 Duodenal jejunal bypass surgery inhibits hypothalamic oxidative stress in rats with type 2 diabetes mellitus by activating the Nrf2/HO-1 signaling pathway. A: Analysis of SOD content in the hypothalamus of rats;B: Analysis of MDA content in the hypothalamus of rats;C: Nrf2 and HO-1 expression in the hypothalamus detected by immunofluorescence (scale bar,130 µm);D: The percentage of cells expressing Nrf2;E: The percentage of cells expressing HO-1;F: Expression levels of Nrf2 and HO-1 in hypothalamus detected by Western blotting;G: The quantitative densitometric analysis of Nrf2 and HO-1.aP < 0.05,bP < 0.01,cP < 0.001.T2DM: Type 2 diabetes mellitus;DJB: Duodenal jejunal bypass.
DJB surgery exerts antioxidant effects by activating hypothalamic GLP-1 signaling in T2DM rats
GLP-1R has been shown to be expressed in neurons in key regions of the brain and has neuroprotective and anti-inflammatory properties,which enhance the expression of the oxidative stress regulatory protein Nrf2/HO-1 to alleviate oxidative stress[26].We measured serum GLP-1 Levels and the expression of hypothalamic GLP-1R in each group of rats.The results showed that the fasting serum GLP-1 Level of T2DM-DJB rats after DJB surgery was significantly higher than that of T2DM-Sham rats (Figure 3A).In addition,we found that GLP-1R was highly expressed in the hypothalamic tissue of T2DM-DJB rats after DJB surgery (Figure 3B-D).These results indicate that DJB surgery enhances the expression of Nrf2/HO-1 by upregulating GLP-1 signaling in T2DM rats and plays a role as an antioxidant.
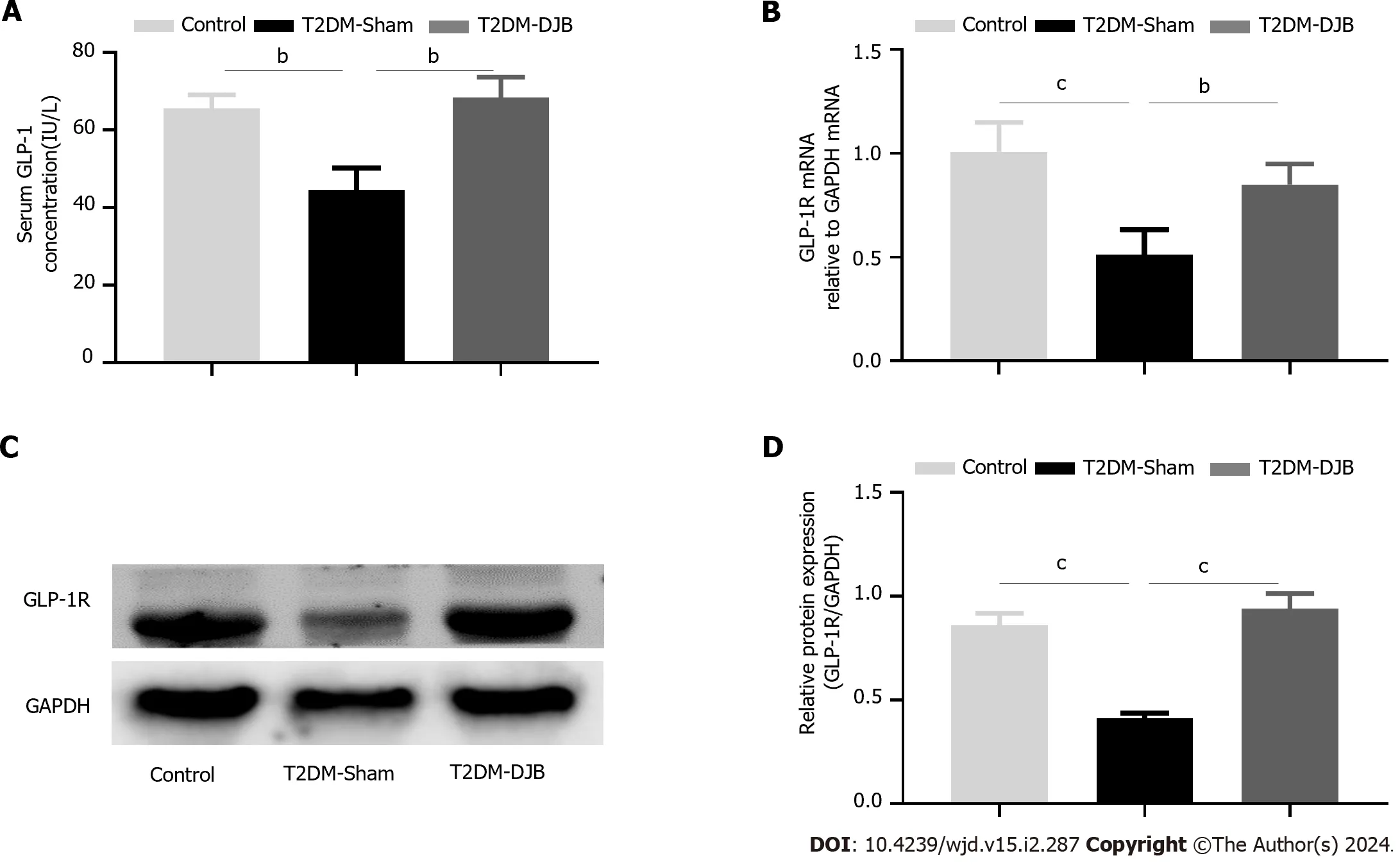
Figure 3 Duodenal jejunal bypass surgery increases type 2 diabetes mellitus glucagon-like peptide 1 signals and enhances the expression of Nrf2/HO-1. A: Serum glucagon-like peptide 1 (GLP-1) secretion of rats in the three groups;B: The quantitative real-time PCR results of GLP-1 receptor (GLP-1R) expression;C: Expression levels of GLP-1R in the hypothalamus detected by Western blotting;D: The quantitative densitometric analysis of GLP-1R.aP < 0.05,bP < 0.01,cP < 0.001.T2DM: Type 2 diabetes mellitus;DJB: Duodenal jejunal bypass;GLP-1: Glucagon-like peptide 1;GLP-1R: Glucagon-like peptide 1 receptor.
DJB blocks the activation of NF-κB signaling and inhibits the production of proinflammatory cytokines in the hypothalamus of T2DM rats
Oxidative stress triggers an inflammatory response by activating the NF-κB signaling pathway,which regulates the release of numerous inflammatory cytokines[34].To determine whether DJB blocked oxidative stress-induced NF-κB activation,we used western blotting and qRT-PCR to compare the total NF-κB and its phosphorylation levels in the hypothalamus of the three groups of rats.Figure 4A-C shows that the expression of NF-κB and p-NF-κB in the hypothalamus of T2DM-DJB rats was significantly reduced compared to that in T2DM-Sham rats after DJB surgery.To confirm whether DJB surgery could ameliorate the inflammatory state of the hypothalamus by inhibiting NF-κB signaling,we used flow cytometry to measure the levels of the proinflammatory cytokines IL-1 and IL-6.As shown in Figure 4D-F,DJB surgery resulted in a significant reduction in the abnormally high expression of IL-1β and IL-6 observed in the hypothalamic tissue of T2DM-DJB rats.The qRT-PCR and Western blot results showed a similar trend in the gene and protein expression of IL-1β,and IL-6 as observed in the flow cytometry assay (Figure 4G-I).These findings suggest that DJB surgery significantly inhibits the NF-κB signaling pathway,effectively improving the inflammatory state of the hypothalamus.
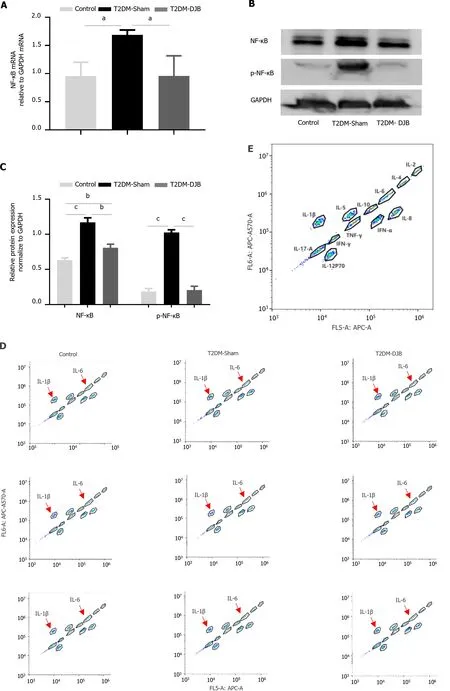
Figure 4 Duodenal jejunal bypass inhibits the production of proinflammatory cytokines by blocking the activation of NF-κΒ signaling in the hypothalamus of type 2 diabetes mellitus rats. A: The quantitative real-time (qRT)-PCR results of NF-κB and p-NF-κB expression;B: The expression levels of NF-κB and p-NF-κB detected by Western blotting;C: The quantitative densitometric analysis of NF-κB and p-NF-κB;D: Flow cytometry results for standards;E: Flow cytometry results for samples;F: Flow cytometry results for the levels of proinflammatory cytokines interleukin (IL)-1β and IL-6;G: The qRT-PCR results of IL-1β and IL-6 expression;H: Expression levels of IL-1β and IL-6 detected by Western blotting;I: The quantitative densitometric analysis of IL-1β and IL-6.aP < 0.05,bP < 0.01,cP < 0.001.T2DM: Type 2 diabetes mellitus;DJB: Duodenal jejunal bypass;IL: Interleukin.
DJB surgery improves hypothalamic nerve injury induced by oxidative stress
Glial fibrillary acidic protein (GFAP) is a specific marker of mature astrocytes,and its elevated expression indicates the onset of CNS injury[35].To determine whether DJB surgery ameliorates oxidative stress-induced neuronal injury in the hypothalamus of T2DM rats,the number of GFAP-positive glial cells was determined,and the expression levels of GFAP were measured using IF,qRT-PCR and western blot analysis.The number of GFAP-positive astrocytes was significantly reduced in T2DM-DJB rats after DJB surgery compared to T2DM-Sham rats (Figure 5A and B).The expression of GFAP mRNA and protein in the hypothalamus was significantly reduced in T2DM-DJB rats after DJB surgery,as indicated by qRT-PCR and western blot analysis (Figure 5C-E).These results suggest that DJB surgery ameliorates oxidative stressinduced hypothalamic neuronal cell injury.
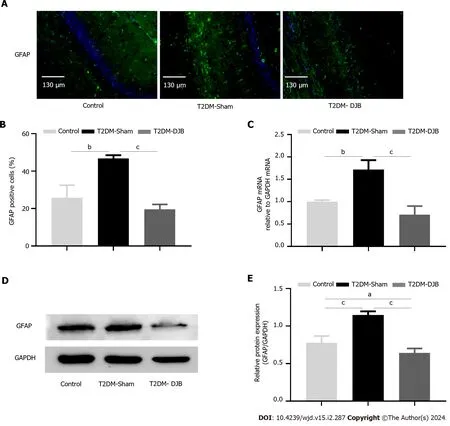
Figure 5 Duodenal jejunal bypass surgery improves hypothalamic cell injury induced by oxidative stress. A: The expression of glial fibrillary acidic protein (GFAP) in the hypothalamus determined by immunohistochemistry (scale bar,130 µm);B: The percentage of cells expressing GFAP;C: The quantitative real-time PCR results of GFAP expression in the hypothalamus;D: Expression levels of GFAP detected by Western blotting;E: The quantitative densitometric analysis of GFAP.aP < 0.05,bP < 0.01,cP < 0.001.T2DM: Type 2 diabetes mellitus;DJB: Duodenal jejunal bypass;GFAP: Glial fibrillary acidic protein.
DJB surgery improves diabetes-induced hypothalamic cell apoptosis in T2DM rats
Oxidative stress induced by diabetes mellitus is a key factor in promoting apoptosis[24].To evaluate the impact of DJB surgery on diabetes-induced hypothalamic cell apoptosis,we analyzed the expression of Cleaved-caspase-3,Caspase-3,and Bcl-2 in the hypothalamus of three groups of rats using qRT-PCR and western blot techniques.As depicted in Figure 6,the expression of Cleaved-caspase-3 and Caspase-3 was significantly reduced,whereas the expression of Bcl-2 was increased in the hypothalamus of T2DM-DJB rats following DJB surgery,in comparison to T2DM-sham rats.These results confirm that DJB surgery can inhibit the apoptosis of hypothalamic cells and reduce oxidative stress damage.
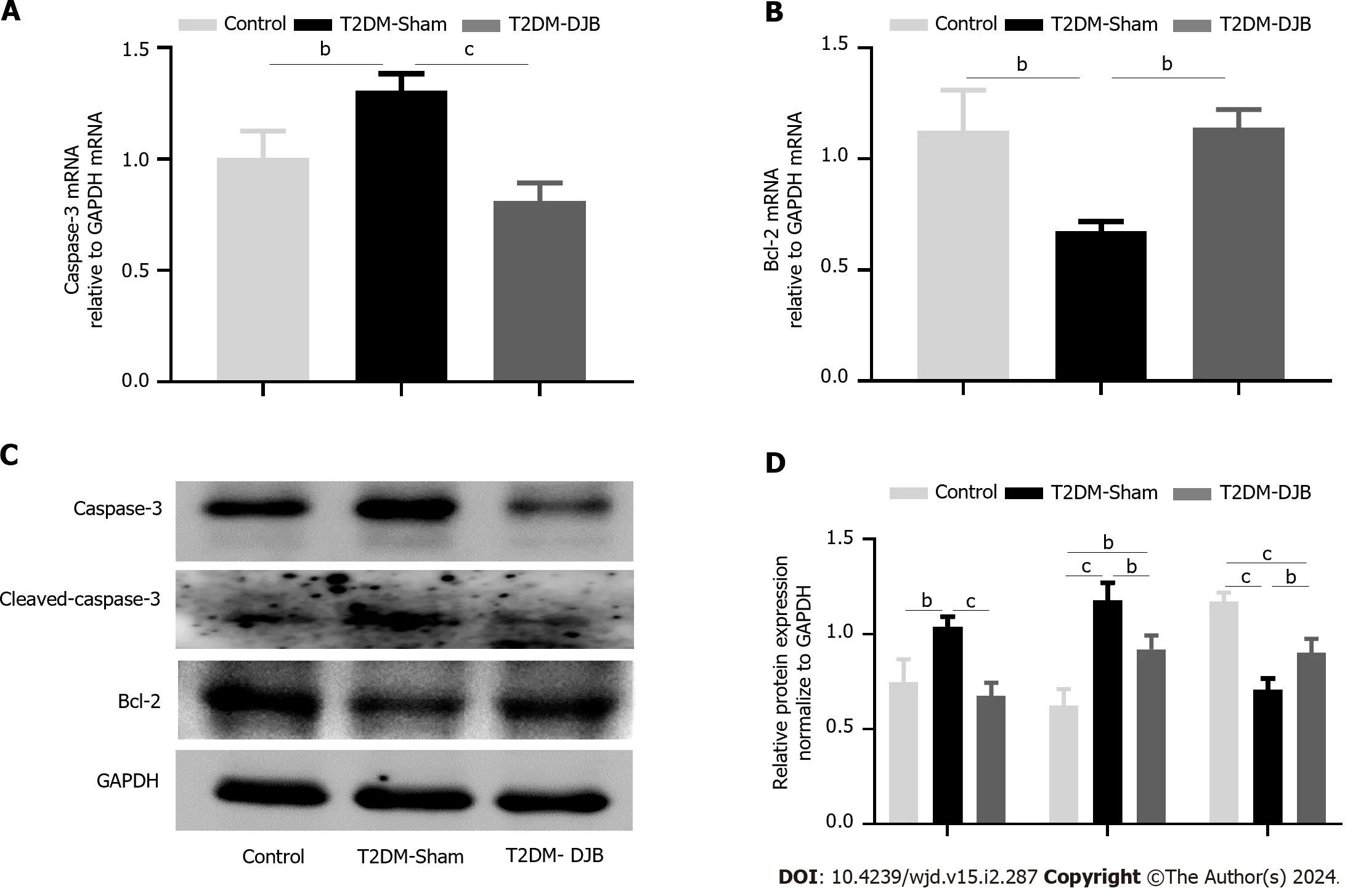
Figure 6 Duodenal jejunal bypass surgery improves diabetes-induced hypothalamic cell apoptosis in type 2 diabetes mellitus rats. A: The quantitative real-time (qRT)-PCR results of Caspase-3 expression;B: The qRT-PCR results of Bcl-2 expression;C: Expression levels of apoptosis-related proteins in the hypothalamus by Western blotting;D: The quantitative densitometric analysis of apoptosis-related proteins.aP < 0.05,bP < 0.01,cP < 0.001.T2DM: Type 2 diabetes mellitus;DJB: Duodenal jejunal bypass.
DJB surgery promotes hypothalamic neurogenesis in T2DM rats
The occurrence of CNS degenerative diseases in diabetic patients is probably caused by a decrease in activated neurons and an increase in cell apoptosis.A common method of evaluating a cell's proliferation capacity is to stain it with Ki67.Immunohistochemical analysis showed that T2DM-DJB rats had significantly more Ki67-positive cells in the hypothalamus post operation than T2DM-Sham rats (Figure 7A and B).The C-fos proteins are often used as markers of neuronal activation.IHC staining indicated a significant increase in the number of hypothalamic C-fos positive cells in T2DM-DJB rats compared to T2DM-Sham rats (Figure 7C and D).DJB surgery significantly increased the expression of Cfos and BDNF in the hypothalamus of T2DM-DJB rats compared to T2DM-Sham rats,according to the qRT-PCR and western blot analysis (Figure 7E-H).These results suggest that DJB surgery promotes hypothalamic cell growth and neuronal activation in T2DM rats.
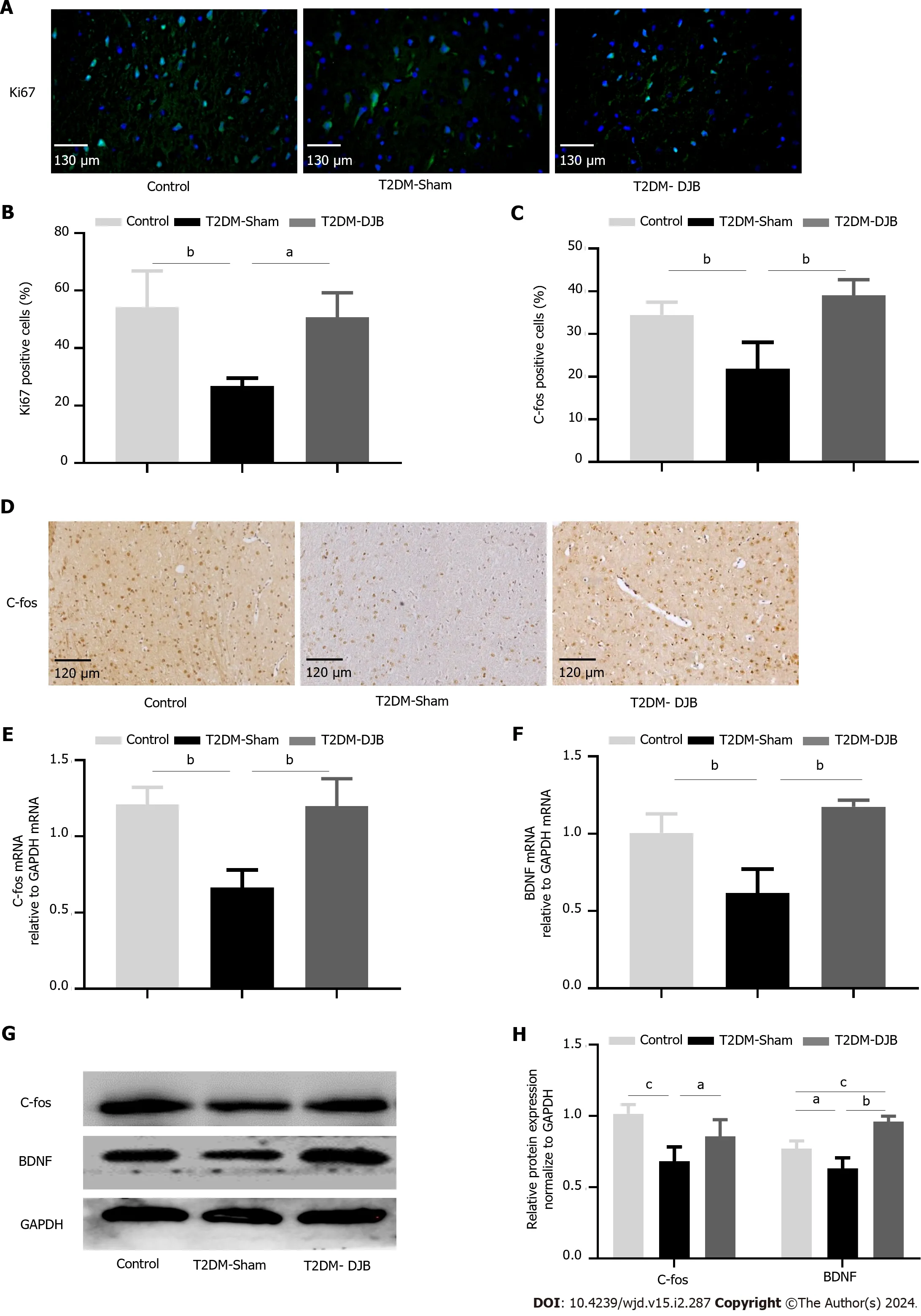
Figure 7 Duodenal jejunal bypass surgery promotes hypothalamic neurogenesis in type 2 diabetes mellitus rats. A: Immunofluorescence analysis the expression of Ki67 (scale bar,130 µm);B: The percentage of cells expressing Ki67;C: The percentage of cells expressing C-fos;D: Immunofluorescence analysis the expression of C-fos (scale bar,120 µm);E: The quantitative real-time (qRT)-PCR results of C-fos expression;F: The qRT-PCR results of BDNF expression;G: Expression levels of C-fos and BDNF detected by Western blotting.The quantitative densitometric analysis of C-fos and BDNF.aP < 0.05,bP < 0.01,cP < 0.001.T2DM: Type 2 diabetes mellitus;DJB: Duodenal jejunal bypass.
DlSCUSSlON
Diabetic encephalopathy is one of the chronic complications of T2DM.The World Epidemiological Survey showed that patients with T2DM usually have by mild to moderate brain dysfunction[36,37].Growing evidence has demonstrated that an imbalance in glucose homeostasis in T2DM can exacerbate oxidative stress and tissue inflammation in the brain[38].Long-term brain inflammation is typically the main cause of neuronal cell apoptosis,resulting in mild cognitive impairment and even neurodegenerative diseases[39,40].
Previous studies have shown that metabolic surgery is effective for weight loss,remission of T2DM,and improvements in brain glucose metabolism[23,41].However,the role and mechanisms of DJB surgery in alleviating diabetes-induced central brain injury are unclear.In this study,we analyzed the differential protein expression in the hypothalamus between the T2DM-Sham group and the T2DM-DJB group of rats using quantitative proteomics (label-free).We found that at least 120 proteins in the hypothalamic tissues exhibited significant changes.KEGG enrichment and GO analysis revealed significant differences in signaling proteins associated with oxidative stress,inflammation,and neurological damage in the hypothalamic tissues of the two groups of rats.Therefore,DJB surgery may improve hypothalamic injury in diabetic rats by modulating oxidative stress,inflammation,and neuronal survival.
Recent studies have confirmed the impact of oxidative stress on various stages of diabetic encephalopathy,supporting the idea that treatments targeting antioxidant stress can help improve CNS complications associated with diabetes[42].Our results showed that DJB surgery significantly increased the level of SOD and inhibited MDA production in the hypothalamus of T2DM rats,thereby effectively reducing hypothalamic oxidative stress.The Nrf2/HO-1 signaling pathway is a significant regulator of oxidative stress[43,44].Yangetal[45] found that HG induced inflammation and apoptosis in cerebral microvascular endothelial cells,which may be a result of inhibiting the Nrf2/HO-1-mediated antioxidant pathway[45].Hence,we examined the impact of DJB surgery on the hypothalamic expression of Nrf2 and HO-1 in rats with T2DM.The expression of Nrf2 and HO-1 in the hypothalamus of T2DM rats was significantly upregulated after DJB surgery.Studies have shown that GLP-1 is involved in regulating oxidative stress and inflammation,in addition to its metabolic effects[46].Kimetal[26] demonstrated that EX4 exerts antioxidant effects and reduces damage to pancreatic β-cells by activating the Nrf2 signaling pathway[26,47].In this study,DJB surgery increased the serum levels of GLP-1 and upregulated the expression of GLP-1R in the hypothalamic tissue of T2DM rats.DJB surgery reduces hypothalamic oxidative stress in rats with T2DM by activating the GLP-1-mediated Nrf2/HO-1 signaling pathway.
Oxidative stress triggers the massive secretion and release of proinflammatory cytokines in the body,ultimately leading to an inflammatory response,which is probably mediated by NF-κB signaling activation[48].Nrf2 activation inhibits the accumulation of ROS and reduces NF-κB activation,thereby suppressing the inflammatory response.Tuetal[49] demonstrated that GEN,a novel agonist of GLP-1R,provides protection against the hyperglycemia-induced inflammatory response in Müller cells and the retinal blood barrier in DR mice.This protective effect is primarily attributed to the upregulation of the Nrf2 antioxidant signaling pathway through GLP-1R[49].The levels of inflammatory cytokines and NF-κB activation in the hypothalamus of the three groups of rats were assessed using western blot,flow cytometry,and qRT-PCR to investigate the role of DJB in hypothalamic inflammation induced by oxidative stress in T2DM rats.Therefore,DJB surgery improved the hypothalamic inflammatory state in T2DM rats by inhibiting NF-κB signaling activation and reducing the secretion of proinflammatory cytokines.
Increased expression of the astrocyte biomarker GFAP in the brains of diabetic patients is believed to be a significant indication of CNS damage caused by neuroinflammation and tissue changes[35,50].HG conditions significantly increased the expression of GFAP in Müller cells,as demonstrated by bothinvivoandinvitrostudies.Liraglutide treatment reduced oxidative stress and downregulated GFAP expression in Müller cells,protecting them from HG-induced injury[51].To investigate the effect of DJB surgery on hypothalamic cell injury in diabetic rats,we evaluated the expression of GFAP protein and compared the differences in the number of GFAP-positive cells in hypothalamic tissues among the three groups of rats.The experimental results showed that DJB significantly reduced GFAP expression in the hypothalamus of T2DM rats and alleviated hypothalamic cell injury.
Oxidative stress induced by chronic hyperglycemia in diabetes mellitus is an important factor that promotes neuronal cell apoptosis[52,53].Growing evidence suggests that Caspase-3 activation is essential for apoptosis because it regulates the translocation and activation of Bcl-2 family proteins,thereby promoting the final stages of apoptosis[50,54].In this study,DJB surgery significantly upregulated the expression of Bcl-2 in the hypothalamus of rats with T2DM,downregulated the expression of Caspase-3,and inhibited the apoptosis of hypothalamic cells.We evaluated the expression of Ki67,C-fos,and BDNF,which are associated with neuronal proliferation,differentiation,and maturation,in diabetic rats to determine the effects of DJB surgery on neuronal injury[55].We found that the number of C-fos positive and Ki67 positive nerve cells in the hypothalamus was significantly reduced in diabetic rats,accompanied by downregulation of hypothalamic BDNF expression.However,DJB significantly reversed these changes.These data suggest that DJB can inhibit hypothalamic cell apoptosis in T2DM rats,promote neurogenesis,and reduce diabetes-induced neuronal cell damage.
In the present study,we demonstrated that DJB enhanced hypothalamic antioxidant activity and alleviated hypothalamic neuronal apoptosis and inflammation in T2DM rats,partly through the upregulation of peripheral GLP-1 secretion (Figure 8).Since enteric GLP-1 can act on the CNS through two pathways (neural or humoral),our current study did not involve the specific pathways through which enteric GLP-1 affects central brain injury[56].Further investigation is needed to determine the influence of enteric neural or endocrine pathways after DJB surgery on the amelioration of diabetes-induced central brain injury by GLP-1.
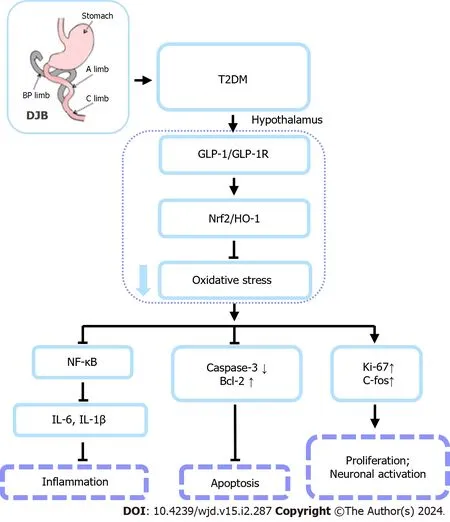
Figure 8 A schema summarizing the protective effects of duodenal jejunal bypass surgery on hypothalamic cell injury induced by diabetes. Duodenal jejunal bypass (DJB) surgery significantly reduces hypothalamic oxidative stress injury and inflammation in type 2 diabetes mellitus rats by a mechanism depending on glucagon-like peptide 1 (GLP-1)-mediated activation of Nrf2/HO-1 signaling pathway.DJB therapy effectively inhibits hypothalamic oxidative stress and inflammatory damage caused by diabetes by GlP-1-mediated activation of Nrf2/HO-1 to inhibit oxidative stress damage in the hypothalamus.In addition,DJB inhibits inflammation by upregulating Nrf2 expression,activating the Nrf2/HO-1 axis,and inhibiting the NF-κB pathway.T2DM: Type 2 diabetes mellitus;DJB: Duodenal jejunal bypass;GLP-1: Glucagon-like peptide 1;GLP-1R: Glucagon-like peptide 1 receptor;IL: Interleukin.
CONCLUSlON
DJB surgery can improve oxidative stress and inflammation in the hypothalamus of T2DM rats and reduce neuronal cell injury.This improvement is achieved by upregulating the GLP-1-mediated Nrf2/HO-1 signaling pathway.
ARTlCLE HlGHLlGHTS
Research background
Type 2 diabetes mellitus (T2DM) is often accompanied by impaired glucose utilization in the brain,leading to oxidative stress,neuronal injury and inflammation.Previous studies have shown that duodenal jejunal bypass (DJB) surgery significantly improves brain glucose metabolism in T2DM rats,but its role in brain injury and the underlying mechanisms are still unclear.
Research motivation
DJB can increase serum glucagon-like peptide 1 (GLP-1) Levels and enhance brain glucose utilization,playing a positive role in the treatment of diabetes.Therefore,GLP-1 signaling may play a significant role after DJB surgery in alleviating T2DM-related brain injury.
Research objectives
To investigate the role and metabolism of DJB in improving hypothalamic oxidative stress and inflammation condition in T2DM rats.
Research methods
A T2DM rat model was inducedviaa high-glucose,high-fat diet,and a low-dose streptozotocin injection.T2DM rats underwent DJB surgery or Sham surgery.Differential expression of hypothalamic proteins and genes was analyzed by protein microarray,flow cytometry,quantitative real-time PCR,western blot,and immunofluorescence.
Research results
Protein microarray results showed significant differences between the T2DM-Sham rats and the T2DM-DJB rats in signaling proteins related to oxidative stress,inflammation,and neuronal injury.DJB surgery increased the serum levels of GLP-1 and upregulated the expression of GLP-1 receptor and antioxidant signaling proteins (Nrf2 and HO-1) in the hypothalamic tissue of T2DM rats.DJB also reduced the expression of hypothalamic inflammatory and nerve cell injuryrelated factors,playing a neuroprotective role and reducing hypothalamic injury.
Research conclusions
DJB surgery improve oxidative stress and inflammation in the hypothalamus of T2DM rats and reduce neuronal cell injury by activating the GLP-1-mediated Nrf2/HO-1 signaling pathway.
Research perspectives
Further investigation is needed to determine the influence of enteric neural or endocrine pathways after DJB surgery on the amelioration of diabetes-induced central brain injury by GLP-1.
FOOTNOTES
Co-corresponding authors:Fang-Ming Fu and Mei-Hua Qu.
Author contributions:Qu MH and Fu FM had equal contribution to this paper;Qu MH and Fu FM designed the research scheme and directed the relevant experimental techniques and methods;Wang HJ,Zhang LB,Sun SP,Yan QT,Gao ZQ performed the research and data analysis;Wang HJ,Qu MH analyzed the data and wrote the manuscript;All authors have read and approve the final manuscript.Qu MH and Fu FM contributed to the experimental design.Qu primarily developed the research direction and experimental hypothesis based on literature and previous research.Fu FM participated in the design of the experimental verification scheme.Qu MH and Fu FM jointly supervised the modeling and duodenal jejunal bypass surgery-related experiments.They are co-corresponding authors of this study,and there is no conflict of interest between them.
Supported bythe Natural Science Foundation of China,No.82 070856;the Science and Technology Development Plan of Shandong Medical and Health Science,No.202102 040075;Scientific Research Plan of Weifang Health Commission,No.WFWSJK-2022-010 and No.WFWSJK-2022-008;and Weifang Science and Technology Development Plan,No.2021YX071 and No.2021YX070.
lnstitutional review board statement:This study was carried out following the recommendations of Weifang Medical University,China.The experimental protocol was approved by the Research Ethics Committee of Weifang Medical University,China,Approval No.2020SDL074.
lnstitutional animal care and use committee statement:The experimental protocols were approved by the Institutional Animal Care and Use Committee,No.2021SDL574.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
ARRlVE guidelines statement:The authors have read the ARRIVE guidelines,and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Mei-Hua Qu 0000-0003-2464-6773.
S-Editor:Li L
L-Editor:A
P-Editor:Chen YX
 World Journal of Diabetes2024年2期
World Journal of Diabetes2024年2期
- World Journal of Diabetes的其它文章
- Diabetes is affecting everyone everywhere
- Glucagon-like peptide-1 receptor agonists as a possible intervention to delay the onset of type 1 diabetes: A new horizon
- Βalancing act: The dilemma of rapid hyperglycemia correction in diabetes management
- Assessment of pathogenicity and functional characterization of APPL1 gene mutations in diabetic patients
- Long noncoding RNA protein-disulfide isomerase-associated 3 regulated high glucose-induced podocyte apoptosis in diabetic nephropathy through targeting miR-139-3p
- Application of non-mydriatic fundus photography-assisted telemedicine in diabetic retinopathy screening
