A Co3O4/graphdiyne heterointerface for efficient ammonia production from nitrates
CHEN Zhao-yang,ZHAO Shu-ya,LUAN Xiao-yu,ZHENG Zhi-qiang,YAN Jia-yu,XUE Yu-rui
(Shandong Provincial Key Laboratory for Science of Material Creation and Energy Conversion,Science Center for Material Creation and Energy Conversion,School of Chemistry and Chemical Engineering,Shandong University,Jinan 250100, China)
Abstract:The nitrate reduction reaction (NtRR) has been demonstrated to be a promising way for obtaining ammonia (NH3) by converting NO3− to NH3.Here we report the controlled synthesis of cobalt tetroxide/graphdiyne heterostructured nanowires(Co3O4/GDY NWs) by a simple two-step process including the synthesis of Co3O4 NWs and the following growth of GDY using hexaethynylbenzene as the precursor at 110 °C for 10 h.Detailed scanning electron microscopy,high resolution transmission electron microscopy,X-ray photoelectron spectroscopy,and Raman characterization confirmed the synthesis of a Co3O4/GDY heterointerface with the formation of sp-C―Co bonds at the interface and incomplete charge transfer between GDY and Co,which provide a continuous supply of electrons for the catalytic reaction and ensure a rapid NtRR.Because of these advantages,Co3O4/GDY NWs had an excellent NtRR performance with a high NH3 yield rate (YNH3) of 0.78 mmol h−1 cm−2 and a Faraday efficiency (FE) of 92.45% at−1.05 V (vs.RHE).This work provides a general approach for synthesizing heterostructures that can drive high-performance ammonia production from wastewater under ambient conditions.
Key words: Graphdiyne;Heterostructures;Electrocatalysis;Nitrate reduction reaction;Ammonia production
1 Introduction
Ammonia (NH3) is an important feedstock for modern industry and an ideal energy carrier.Unfortunately,industrial-scale NH3production is mainly based on the traditional energy-and emissions-intensive Haber-Bosch process from nitrogen (N≡N dissociation energy 941 kJ mol−1) and hydrogen under harsh conditions such as high temperatures (673–773 K) and high pressures (150–300 atm)[1–3].In view of this,the electrochemical conversion of nitrate (dissociation energy: 204 kJ mol−1) into NH3at room temperatures and ambient pressures has been regarded as the most promising route for ammonia production[4–7].For the complex eight-electron process from NO3− to NH3in NtRR,it is particularly necessary to optimize the adsorption and desorption behavior of both reactants and products simultaneously.The complex reaction processes of various reaction intermediates (NO2,NO,NOH,N2,NH2OH,NH2NH2) at the interface should also be considered to improve the selectivity of the catalysts.Till date,a wide variety of catalysts have been reported for efficient NH3production through electrocatalytic nitrate reduction reaction (NtRR)[8–13],but the NH3yield rates (YNH3) and Faraday efficiencies (FE) are still below industry standards due to the complex eight-electron process from NO3−to NH3conversion.It is therefore of great significance to design and synthesize new catalysts with highYNH3,selectivity,and stability for obtaining NH3from NtRR.
Among reported catalysts,heterostructured catalysts have shown many intrinsic advantages to catalysis with improved intrinsic activity and stability[14–15].The construction of a heterointerface is an important route to synergistically combine the advantages offered by multiple materials and adjust the catalyst’s properties such as electrical conductivity,hydrophilicity,interfacial electron modulation,and adsorption energy of intermediates,etc[16–23].Carbonbased materials have become one of the most used heterojunction materials due to their abundant natural reserves,high affinity and versatility[24–29].
Graphdiyne (GDY),a new rising star in the carbon family,has attracted considerable attention due to its specific sp-/sp2-hybridized all-carbon two-dimensional networks with unique properties such as a natural pore structure,a large specific surface area,the rich alkyne bonds,high intrinsic activity and excellent stability[30–58].In addition,the controllable growth of GDY on various material surfaces under mild conditions offers the advantage for growing high-performance active structures.
In this work,we successfully synthesized Co3O4/GDY heterostructured nanowires by in-situ growth of GDY on the surface of Co3O4(Fig.1).The newly-formed heterointerface between Co3O4and GDY has improved electric conductivity,increased active sites,the specific incomplete charge-transfer property.These unique advantages of Co3O4/GDY significantly promote the efficient conversion of NO3−to NH3,giving a highYNH3of 0.78 mmol h−1cm−2and FE of 92.45% at −1.05 V (vs.RHE).
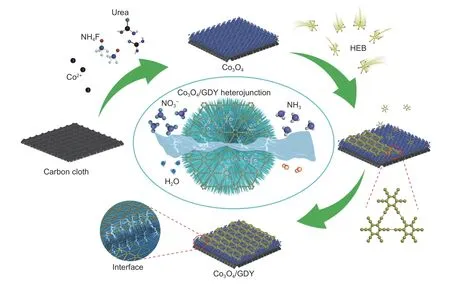
Fig.1 The synthetic routes of the Co3O4/GDY
2 Experimental
2.1 Materials
Cobalt (II) nitrate hexahydrate (Co(NO3)2·6H2O),ammonium fluoride (NH4F),urea and tetrabutylammonium fluoride (TBAF) were purchased from Energy Chemical.Unless otherwise specified,the reagents utilized in this study were used directly.The carbon cloth was thoroughly cleaned before use.All water used was purified with a Millipore system (typically 18.2 MΩ cm resistivity).
2.2 Synthesis of Co3O4
Co3O4was synthesized by a solvothermal method.Typically,a piece of carbon cloth (CC) (3 cm×3 cm) was added to a Teflon-lined stainless-steel autoclave containing 30 mL aqueous solution of Co(NO3)2·6H2O (0.36 g),NH4F (0.09 g) and urea(0.375 g) and kept at 120 °C for 6 h.The precursor sample was obtained and washed thoroughly by deionized water and dried at 80 °C in a vacuum oven.The products were annealed at 400 °C for 2 h to obtain Co3O4samples.
2.3 Synthesis of Co3O4/GDY
The freshly-prepared Co3O4was added to the Teflon-lined stainless-steel autoclave containing 30 mL hexaethynylbenzene pyridine solution (0.11 mg mL−1)and kept at 110 °C for 10 h.After the completion of the reaction,the obtained Co3O4/GDY was cleaned and used for electrochemical measurements.
2.4 Characterizations
Scanning electron microscopy (SEM) images were recorded using FEI Apreo SEM.High-resolution (HRTEM) images were taken on Talos F200X TEM.X-ray photoelectron spectroscopy (XPS,Nexsa)with Al Kα radiation was employed to determine the chemical composition and element states.Raman spectra were collected by a HORIBA Raman spectrometer at 473 nm laser excitation wavelength.In situ attenuated total reflection Fourier transformed infrared spectroscopy (ATR-FTIR) was measured using INVENIO S (BRUKER).The1H NMR signal was acquired using a Bruker 400 MHz system.(NMR,AVANCE III HD 400 MHz).X-ray diffraction (XRD)was acquired using X’Pert3 Powder (Malvern Panalytical).
2.5 Electrochemical tests
Electrochemical tests were performed on CHI 760D (there-electrode system;Shanghai CH.Instruments,China) in 0.5 mol L−1K2SO4+0.1 mol L−1KNO3using H-type cell separated by Nafion 117 membrane.Linear sweep voltammetry (LSV) measurements were carried out at a scan rate of 2.0 mV s−1.Chronoamperometry tests were conducted in Ar-saturated 0.5 mol L−1K2SO4+0.1 mol L−1KNO3aqueous solution (30 mL) under the Ar atmosphere.The chronoamperometry tests were used to measure the performance of the catalyst electrode at −1.5,−1.6,−1.7,−1.8 and −1.9 V (vs.SCE).Electrochemical impedance spectra (EIS) were recorded in a frequency range spanning from 100 kHz to 0.01 Hz.
3 Results and discussion
Fig.1 shows the synthesis route of Co3O4/GDY.In brief,the as-synthesized Co3O4was directly used as supporting material for growing GDY to obtain Co3O4/GDY samples.In this process,Co3O4acts as both the growing template and the catalyst for the coupling reaction of hexaethynylbenzene (HEB).HEB molecules gradually polymerize under the catalytic action of Co species on the surface of Co3O4,ultimately obtaining a GDY layer at the interface.The size,morphology,and structure of the samples were characterized by SEM and HRTEM measurements.After hydrophilic treatment and cleaning of raw CC,the smooth CC without any impurities was obtained(Fig.S1a-b).After the hydrothermal reaction,Co precursors were successfully loaded onto the surface of the CC (Fig.S2a-b).Next,it was annealed at 400 °C to obtain the Co3O4with a nanowires-like structure.
As shown in Fig.2a-c,Co3O4nanowires with homogeneous size and distribution were uniformly grown on carbon fibers.The exposed Co sites become ideal sites for GDY growth and subsequent catalytic reactions.After the in-situ growth of GDY,the Co3O4/GDY exhibits a rougher surface and maintains original nanowires morphology as compared to those of pristine Co3O4(Fig.2d-f).The 3D structure and rough surface can enlarge the specific area for catalysis.Fig.2g shows the even dispersion of C,Co and O in Co3O4/GDY.Compared to CC,the hydrophilicity of the Co3O4/GDY is significantly enhanced (Fig.2h),which facilitates the transportation of reaction substrates,intermediates,and products at the interface.The optical images are shown in Fig.2i.The as-prepared Co3O4/GDY is quite flexible,which makes it a potential candidate for flexible devices that can be applied in micro ammonia production.These characteristics make GDY an ideal catalytic material.
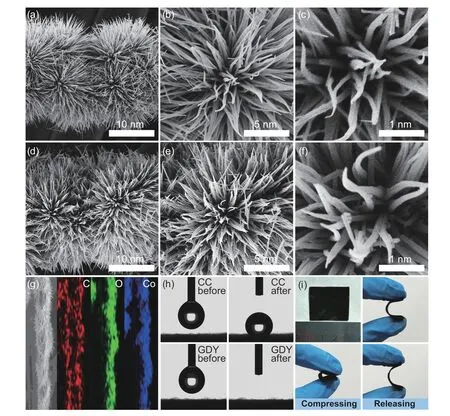
Fig.2 Morphological characterizations of samples.(a) Low-and (b,c) high-magnification SEM images of the Co3O4.(d) Low-and (e,f) high-magnification SEM images of the as-prepared Co3O4/GDY.(g) EDS mapping of C,O and Co in Co3O4/GDY nanowires.(h) Contact angle measurements of Co3O4/GDY.(i) Optical photos of Co3O4/GDY
HRTEM images (Fig.3a-c) reveal the crystalline nature of Co3O4,featuring (200),(211),(112) and(103) planes.The lattice spacings of Co3O4/GDY changes slightly (Fig.3d) due to the interaction between Co3O4and GDY.The uniform distribution of Co3O4species can be observed in Fig.3e.A clear interface between Co3O4and GDY is marked in Fig.3f,indicating the heterointerface between different crystalline phases,consistent with the above results.To further explore the characteristics of GDY in Co3O4/GDY,the regions where only GDY exists are selected (Fig.3g).The lattice spacings of GDY were found to be 0.370 and 0.395 nm (Fig.3h.) The stacked GDY layers are marked in Fig.3i.These observations prove that Co3O4/GDY heterointerfaces were formed successfully.This was further confirmed by XRD measurements (Fig.S4).
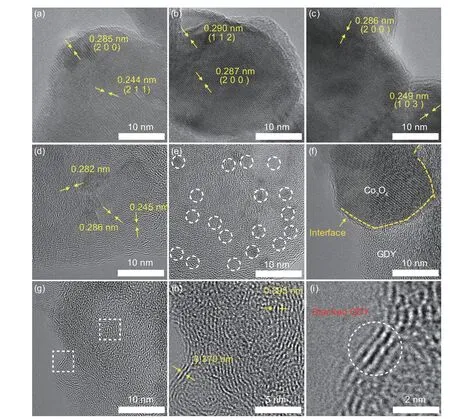
Fig.3 (a-c) HRTEM images of the Co3O4.(d-f) HRTEM images of the Co3O4/GDY.(e) The high distribution of Co3O4 in Co3O4/GDY.(f) Boundary between Co3O4 and GDY.(g) Low-and (h,i) high-magnification HRTEM images of GDY on the surface of Co3O4/GDY
XPS survey spectra (Fig.S3) show the coexistence of Co,C and O in both Co3O4and Co3O4/GDY.The Raman peaks corresponding the alkyne bonds(1 877 and 2 096 cm−1) were observed in Co3O4/GDY[59–60].Compared with GDY (Fig.S5),the red shift of Co3O4/GDY is ascribed to the tensile strains of the alkyne bond in Co3O4/GDY.These results prove the existence of the interaction between Co3O4and GDY,which is consistent with the HRTEM.Compared with the initial Co3O4,three new peaks of sp-C(285.02 eV),Co―C (283.16 eV),and π-π*interaction (290.68 eV) appear in Co3O4/GDY (Fig.4b).Fig.4c shows the existence of a mixed valence state of Co2+with Co3+in Co3O4/GDY.Compared with pure Co3O4,the Co2+binding energy of Co3O4/GDY slightly decreases.This might be due to the electron transferred from GDY species.Fig.4d shows that the surface-OH ratio in Co3O4/GDY is higher than Co3O4,which is beneficial to electrochemical mass transfer(Fig.S6).The conversion of valence between Co3+and Co2+occurs in the as-formed interface (Fig.4e).To investigate the intrinsic mechanism of high catalytic activity in Co3O4/GDY,CV and EIS were applied.The results show that Co3O4/GDY possesses a larger electrochemical active surface area and lower resistance (Fig.4f-g,Fig.S7).The C 1s,O 1s and Co 2p XPS analysis of Co3O4/GDY obtained after NtRR(Fig.S8,Fig.4h) reveal that sp-C,Co―C,and π-π*don’t disappear,affirming the structural stability of the Co3O4/GDY.The Co 2p XPS spectra of Co3O4/GDY (Fig.4c,4h) show a substantial transition from Co2+to Co3+during the NtRR process,indicating that the valence state of Co species changes during the catalytic process (Fig.4i).The abundant electrons of the alkyne bond in the GDY undergo incomplete charge transfer behavior with Co3O4,stabilizing electron transport at the interface.In addition,GDY can serve as a continuous electron storage space,which guarantees sufficient electron supply at the interface,thus supporting the complex eight-electron transfer process from NO3− to NH3.These characteristics enable Co3O4/GDY to become the ideal material of NtRR.
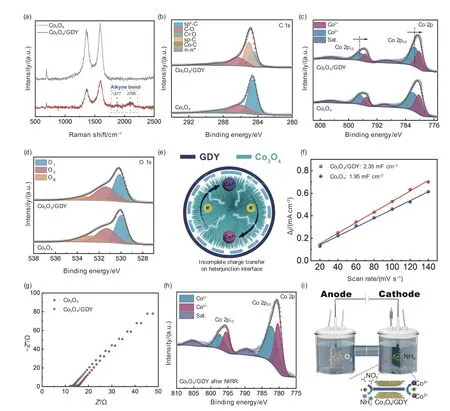
Fig.4 (a) Raman spectra of Co3O4 and Co3O4/GDY.(b) C 1s XPS spectra of Co3O4 and Co3O4/GDY.(c) Co 2p XPS spectra of Co3O4 and Co3O4/GDY.(d) O 1s XPS spectra of Co3O4 and Co3O4/GDY.OⅠ represents the Co-O bond;OⅡ is assigned as the ―OH on the surface;OⅢ is considered to originate from environmental contamination.(e) Schematic illustration of the electron transfer at the GDY-Co3O4 interfaces of Co3O4/GDY.(f) The current density differences(Δj=ja−jc) are plotted against scan rates.(g) Nyquist plots of Co3O4 and Co3O4/GDY.(h) Co 2p XPS spectra of Co3O4/GDY after NtRR.(i) Schematic diagram of charge changes in Co3O4/GDY during the NtRR process
Next,the NtRR performance of Co3O4/GDY was measured at 300 K and 101 kPa.The Ar-saturated 0.5 mol L−1K2SO4+0.1 mol L−1KNO3was used as the electrolyte.All potentials were converted to a reversible hydrogen electrode (RHE).The schematic representation of the NO3−-to-NH3conversion is shown in Fig.5a.Fig.5b shows the LSV curves of Co3O4and Co3O4/GDY in electrolytes with or without KNO3,respectively.As the applied potential increases,both Co3O4and Co3O4/GDY exhibit a strong current response to NO3− reduction,indicating the occurrence of NtRR in the presence of NO3−.Compared with Co3O4,Co3O4/GDY exhibits a stronger response trend,which indicates that the combination of the GDY and Co3O4can significantly improve catalytic activity due to the formation of highly active heterointerfaces between GDY and Co3O4with incomplete charge transfer and enhanced conductivity.Fig.5c shows the chronoamperometric curves of Co3O4/GDY at different potentials.As expected,the current density of Co3O4/GDY is significantly increased with a more negative potential,followed by an increasing competition for hydrogen evolution reaction (HER).Ultraviolet-visible (UV-Vis) spectrophotometry was employed to determine the concentration of the produced NH3for the electrolyte after electrolysis by the indophenol blue method.Co3O4/GDY exhibits superior catalytic performance with Faraday efficiency (FE) reaching over 80% at full potential(Fig.5e).The FE and ammonia production rate (YNH3)of Co3O4/GDY reach the highest values of 92.45% and 0.78 mmol h−1cm−2,respectively,which greatly exceeds bare Co3O4before the in-situ growth of GDY(Fig.5d).To investigate the stability of the catalyst,Co3O4/GDY was tested for 8 catalytic cycles (Fig.5f,Fig.S10).The results show that the Co3O4/GDY maintained highYNH3and FE during the testing period,without significant performance degradation.These results demonstrate the stabilizing effect of GDY on the Co3O4interface.Without the addition of NO3−,no ammonia was detected after electrolysis for the same time (Fig.5g,Fig.S14),proving that all the generated ammonia originated from the nitrate.The15N isotopic labeling tracer experiments confirmed the N in the synthesized ammonia originated from the reduction of NO3−(Fig.S15).Analysis results also show that there is no N2H4and negligible NO2−formed during the reaction (Fig.S16),indicating the high selectivity of the Co3O4/GDY for NtRR.Fig.5h shows the product ratio at different potentials for Co3O4/GDY.Fi g.5i shows that Co3O4/GDY exhibits higher NH3yield and FE than reported electrocatalysts.The intermediates of Co3O4/GDY in the NtRR process were examined by in-situ FTIR spectroscopy.From Fig.5j-l,the spectral peaks detected at 1 346 and 1 224 cm−1can be ascribed to the generation of NH4+.The presence of a peak at 838 cm−1suggests the formation of an intermediate NO2−.Additionally,the concentration of NO2−demonstrates a gradual rise during the time span of 0−10 min.The presence of a peak at 957 cm−1suggests a decrease in the concentration of NO3−.Thus,the efficient degradation of NO3−can be achieved by using Co3O4/GDY,resulting in the conversion of NO3−into NH3through the intermediate NO2−.
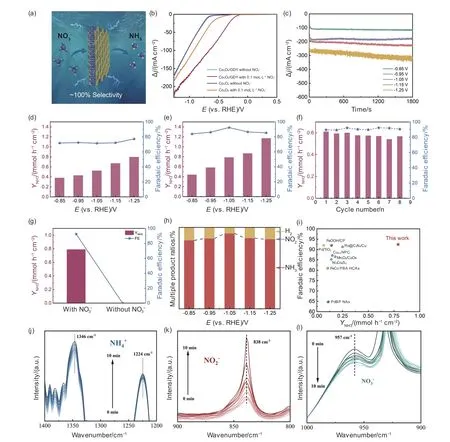
Fig.5 NtRR performance tests.(a) Schematic representation of the NO3−-to-NH3 conversion.(b) Linear sweep voltammetry curves of the samples in 0.5 mol L−1 K2SO4 +0.1 mol L−1 NO3−+ and pure 0.5 mol L−1 K2SO4 aqueous solutions.(c) Current density-time curves of Co3O4/GDY at different potentials in 0.5 mol L−1 K2SO4+0.1 mol L−1 NO3−.The YNH3 and FE of (d) Co3O4 and (e) Co3O4/GDY in 0.5 mol L−1 K2SO4+0.1 mol L−1 NO3−.(f) Stability tests of Co3O4/GDY at −1.05 V (vs.RHE).(g) YNH3 and FE of Co3O4/GDY at −1.05 V (vs.RHE) with and without NO3−.(h) Proportion of products.(i) NtRR performance comparison to other catalysts.(j-l) Insitu ATR-FTIR spectra of Co3O4/GDY during NtRR
4 Conclusion
In summary,we have successfully constructed Co3O4/GDY by growing GDY on Co3O4.The newlyformed heterointerface between Co3O4and GDY led to noticeably improved electric conductivity,increased active sites,and the specific incomplete charge-transfer property.These advantages endow the catalyst with high FE (92.45%) andYNH3(0.78 mmol h−1cm−2) for selective and efficient NtRR at room temperatures and ambient pressures.
Data availability statement
The data that support the findings of this study are openly available in Science Data Bank at https://doi.org/10.57760/sciencedb.j00125.00120 or https://cstr.cn/31253.11.sciencedb.j00125.00120.
Acknowledgements
This work was supported by the National Key Research and Development Project of China (2022YFA1204500,2022YFA1204501,2022YFA1204503,2018YFA0703501),the Taishan Scholars Youth Expert Program of Shandong Province (tsqn201909050),and the Natural Science Foundation of Shandong Province (ZR2020ZD38,ZR2021JQ07).
- 新型炭材料的其它文章
- Carbon-based electrocatalysts for water splitting at high-current-densities: A review
- 序
- Defect engineering of carbon-based electrocatalysts for the CO2 reduction reaction: A review
- Carbon-based metal-free nanomaterials for the electrosynthesis ofsmall-molecule chemicals: A review
- A review of carbon-based catalysts and catalyst supports for simultaneous organic electro-oxidation and hydrogen evolution reactions
- MOF-derived nanocarbon materials for electrochemical catalysis and their advanced characterization

