Bismuth nanoparticles anchored on N-doped graphite felts to give stable and efficient iron-chromium redox flow batteries
CHE Hang-xin ,GAO Yu-fei ,YANG Jia-hui ,HONG Song, ,HAO Lei-duan ,XU Liang,Sana Taimoor,Alex W.Robertson,SUN Zhen-yu,
(1.State Key Laboratory of Organic-Inorganic Composites,College of Chemical Engineering,Beijing University of Chemical Technology,Beijing 100029, China;2.School of Mechanical and Electrical Engineering,Guilin University of Electronic Technology,Guilin 541004, China;3.Department of Physics,University of Warwick,Coventry CV4 7AL,UK)
Abstract:Iron-chromium redox flow batteries (ICRFBs) use abundant and inexpensive chromium and iron as the active substances in the electrolyte and have great potential as a cost-effective and large-scale energy storage system.However,they are still plagued by several issues,such as the low electrochemical activity of Cr3+/Cr2+ and the occurrence of the undesired hydrogen evolution reaction (HER).We report the synthesis of amorphous bismuth (Bi) nanoparticles (NPs) immobilized on N-doped graphite felts(GFs) by a combined self-polymerization and wet-chemistry reduction strategy followed by annealing,which are used as the negative electrodes for ICRFBs.The resulting Bi NPs react with H+ to form intermediates and greatly inhibit the parasitic HER.In addition,the combined effect of Bi and N dopants on the surface of GF dramatically increases the electrochemical activity of Fe2+/Fe3+and Cr3+/Cr2+,reduces the charge transfer resistance,and increases the mass transfer rate compared to plain GF.At the optimum Bi/N ratio of 2,a high coulombic efficiency of up to 97.7% is maintained even for 25 cycles at different current densities,the energy efficiency reaches 85.8% at 60.0 mA cm−2,exceeding many other reported materials,and the capacity reaches 862.7 mAh L−1 after 100 cycles,which is about 5.3 times that of bare GF.
Key words: Iron-chromium flow battery;Bi;Negative electrode;Nitrogen doping;Graphite felt
1 Introduction
The development of clean energy sources is a current international priority.Renewable powers based on wind and solar generation are now becoming primary energy sources for powering our societies.However,the inherent intermittency of renewable energy sources brings about serious challenges for ensuring a consistent supply of power to the grid.Energy storage technology is an effective tool for achieving efficient renewable energy utilization and stable power system operation[1–2].Compared with physical energy storage technology such as pumped storage,chemical energy storage technology offers greater flexibility in terms of scale and location.Among the various chemical energy storage methods,redox flow batteries (RFBs) have become a large-scale battery energy storage technology with great potential owing to their long life,good safety,and high energy efficiency[3].
RFBs are considered as one of the best choices for megawatt-level power storage,and megawatt demonstration systems have been installed,for example,in China,the United States and Australia.The charge and discharge of the RFBs are realized mainly by the change in the redox state of the active substance in the solution on both sides of the positive and negative electrodes.An RFB consists of two electrodes,two electrolyte tanks,and an ion exchange membrane.The electrolyte is usually stored in an external tank and pumped into the reactor.The redox reaction is carried out on the electrode surface,and the active substance after the reaction flows back to the external storage tank with the electrolyte[4].Since the 1960s,a variety of RFBs have been developed,including all-vanadium flow batteries (VRFBs),vanadium-iron RFBs,zinc-bromine RFBs,and iron-chromium flow batteries (ICRFBs)[5].Among them,ICRFBs are the first real sense of flow battery in history.It uses cheap and abundant chromium and iron as the active substances in the electrolyte and is therefore a very cost-effective energy storage system compared with other kinds of flow batteries[6].
However,practical implementation of ICRFBs must address the problems of slow Cr3+/Cr2+kinetics and suppress the competing HER.Graphite felt (GF)is typically the first choice of ICRFB electrode materials owing to its favorable conductivity,stability,high specific surface area,porosity,and corrosion resistance[7–9].However,GF also has drawbacks such as poor hydrophilicity,insufficient electrochemical activity,and low reversibility.The surfaces of GF electrodes have been modified in an attempt to circumvent these issues and thus improve the ICRFB performance.For example,indium ions[10]have been demonstrated to inhibit the HER and accelerate Cr3+/Cr2+kinetics.By loading a small amount of Pb(100-200 μg cm−2) and Au (12-25 μg cm−2) onto GF[11],the kinetics of the Cr3+/Cr2+redox reaction has been promoted[12–13].Alternatively,Bi[5,14],Tl[15],SiO2[16],CoO[17–18],and other modified electrodes have also been utilized to accelerate the Cr3+/Cr2+redox reactions.
Here,we propose a synthesis method for the preparation of Bi nanoparticles (NPs) immobilized on Ndoped graphite felt (Bi/N-GF) by a combined interfacial copolymerization and wet-chemistry reduction approach followed by annealing,which is promising as an efficient negative electrode for ICRFBs.As-obtained catalyst significantly promotes the reactivity of Cr3+/Cr2+electrodes,improves mass transfer kinetics,and alleviates the HER of the anode.During galvanostatic charge and discharge,the Bi/N-GF exhibits excellent rate performance and long stability during cycling.The Coulombic efficiency (CE) is maintained at 97.7% after 25 cycles at various current densities.At 60.0 mA cm−2,the energy efficiency (EE) approaches 85.8%.The capacity reaches 862.7 mAh L−1after 100 cycles,which is about 5.3 times that of plain GF.
2 Experimental
2.1 Materials and preparation
FeCl2·4H2O,CrCl3·6H2O and Bi(NO3)3·5H2O were purchased from Macklin.HCl was bought from Beijing Tong Guang Fine Chemicals Company.Tris and dopamine (DA) hydrochloride were provided by Shanghai Aladdin Biochemical Technology.Deionized water (18.2 MΩ·cm) was obtained from a Millipore system.N2gas (99.999% purity) was bought from Beijing Haipu Gas Co.,Ltd.Graphite felts (GFs)were bought from Liaoning Jin Gu carbon material Co.LTD,China.Single cells were provided by Wuhan Zhi sheng New Energy Co.,LTD.Lines and peristaltic pumps were purchased from Baoding River Fluid Technology Co.,LTD.
The electrolyte of 1 mol L−1Fe/Cr (containing 1 mol L−1FeCl2·4H2O and 1 mol L−1CrCl3·6H2O) +3 mol L−1HCl was prepared by dissolving FeCl2·4H2O and CrCl3·6H2O in a concentrated HCl solution,and then diluted quantitatively with deoxidized water at room temperature.Deoxygenated water was prepared by gradually heating up deionized water to 80 °C,then introduced into the solution with a vacuum pump.The internal pressure increased,with steady oxygen bubble formation observed until it ceased,yielding deoxygenated water.The prepared electrolyte was left for 3 h before electrochemical tests.
Graphite felts (3 cm × 3 cm) were cleaned and calcined in air at 500 ºC for 5 h[19].100 mg dopamine hydrochloride solution was dissolved in a Tris−HCl buffer solution (10 mmol L−1Tris,pH=8.5),and the pre-treated GF was added and stirred for 1 h at room temperature.Then 200 mg Bi(NO3)3·5H2O was dissolved in 50 mL glycol solution,and then added to the above solution and stirred at room temperature for 7 h.Finally,the prepared samples were dried in an oven at 60 ºC for 24 h.The resulting samples were subsequently calcined at 450 ºC for 1 h and further at 700 ºC for 2 h[19–20]in a quartz furnace under N2atmosphere.As a consequence,Bi/N-GF was obtained,as illustrated in Fig.1.The loaded Bi in Bi/N-GF was estimated to be about 10.1% based on energy-dispersive X-ray spectroscopy (EDS) measurements during transmission electron microscopy (TEM) observation.By replacing glycol with distilled water while remaining the other conditions unchanged,bigger Bi nanoparticles supported on N-doped GF (named as Bi_bigger/N-GF) were obtained.The number Bi/NGF-0.5,Bi/N-GF-1 and Bi/N-GF-2 refers to a theoretical content of Bi/N.
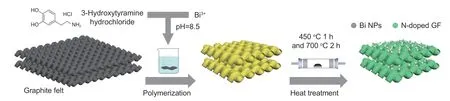
Fig.1 A schematic illustration of the synthesis of Bi/N-GF
For the fabrication of Bi-GF,200 mg Bi(NO3)3·5H2O was dissolved in 50 mL glycol solution and the pre-treated GF was added and stirred for 8 h.The subsequent drying and thermal treatment steps were the same as those used for the preparation of Bi/N-GF.
For the preparation of N-GF,100 mg dopamine hydrochloride solution was dissolved in a Tris-HCl buffer solution (10 mmol L−1Tris,pH=8.5),and the pre-treated GF was added and stirred for 8 h at room temperature.The subsequent drying and thermal treatment steps were the same as those used for the preparation of Bi/N-GF.
2.2 Characterization
Scanning electron microscopy (SEM) was performed on an S-4800 microscope with a 3 kV accelerating voltage.TEM and aberration corrected highangle annular dark-field scanning TEM (HAADFSTEM) were done with a JEOL ARM200 microscope with a 200 kV accelerating voltage.STEM samples were prepared by depositing a droplet of suspension onto a Cu grid coated with a lacey carbon film.X-ray diffraction (XRD) patterns were recorded on a D/MAX-RC diffractometer operated at 30 kV and 100 mA with CuKαradiation (λ=0.154 18 nm) at a scanning rate of 5° min–1.X-ray photoelectron spectroscopy (XPS) measurements were carried out using a Thermo Scientific ESCALAB 250Xi instrument.The instrument was equipped with an electron flood and a scanning ion gun.No special treatment was applied to the samples before XPS analysis.The C―C binding energy of 284.8 eV was used for internal calibration.Peak deconvolution was performed with the Avantage software.
2.3 Electrochemical measurement
Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were tested at 65.0 ºC using a three-electrode system consisting of a graphite working electrode (6.0 mm in diameter),a saturated calomel reference electrode (SCE),and a platinum network (1 cm2) counter electrode on a CHI660 electrochemical workstation (Shanghai Chen Hua Instrument Co.,LTD).The volume of electrolyte that was used during the tests was 20 mL.CV measurements with different sweep rates were performed in the potential ranges from −0.8 to 0.8 V.EIS was conducted under a direct current voltage range of −0.5 V and 0.35 V,a frequency range of 100 kHz and 10 MHz,and an amplitude of 5 mV.The test results were fitted with the Zview software.
2.4 Flow battery test
As-obtained Bi/N-GF (diameter: 3 cm × 3 cm)and Nafion 117 were used as the anode and cation exchange membranes,respectively.1 mol L−1FeCl2·4H2O and 1 mol L−1CrCl3·6H2O dissolved in a 3 mol L−1HCl solutions were utilized as the analyte and catholyte,respectively.To eliminate any residual air,the storage tank linked to the cell was purged with N2for 30 min and was subsequently heated in a water bath to 65 ± 3 ºC.The electrolyte was introduced into a singular cell at a flow rate of 120.0 mL min−1.All voltage measurements were versus SCE unless otherwise stated.Electrochemical tests were conducted using a potentiostat/current meter,with a cut-off voltage of 0.8 V for the charge test and 1.2 V for the discharge test.The CE was calculated by the ratio of the number of electrons transferred during discharge to the number of electrons transferred during charge.In the process of charge and discharge,the cross contamination of positive and negative electrolytes normally leads to low coulombic efficiency.Voltage efficiency(VE) was determined by the ratio of average discharge voltage to average charge voltage.EE was estimated by the ratio of the output energy of the discharge process to the stored energy of the charge process.
3 Results and discussion
To fabricate Bi/N-GF,GF was first coated with polydopamine (PDA) from the self-polymerization of oxidized DA.The nitrogen-enriched layer facilitated the deposition of Bi NPs resulting from glycol reduction at elevated temperature.After further heat treatment,Bi NPs adhered to the surface of N-doped GF were attained.The morphologies of GF,N-GF,Bi-GF,and Bi/N-GF (the Bi/N mass ratio is 2.0 unless stated otherwise) were characterized by SEM (Fig.S1a−d).At low magnifications,a large number of interconnected one-dimensional fibers were observed in all cases.In contrast to the relatively smooth surface of bare GF,N-GF and Bi-GF samples,the outer surface of Bi/N-GF was seen to be densely deposited with aggregates.This indicates that the introduction of additional N species in GF favors the anchoring of Bi particles on the surface of GF.The microstructure of the resulting Bi/N-GF was further investigated by (S)TEM.It is apparent that almost all graphite felts (existing as graphite flakes) were decorated with Bi NPs with an irregular shape (Fig.2a,b).The average size of Bi NPs was estimated to be about 25 nm.In addition to some big aggregates,a high proportion of NPs of less than 15 nm were identified (Fig.2c,d).These formed NPs lack crystallinity,as confirmed by fast Fourier transform (FFT) (Fig.2e).The formation of Bi and N species on GF was validated by the STEM images and corresponding EDS elemental maps (Fig.2f−i).
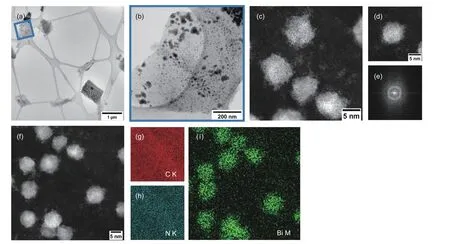
Fig.2 STEM characterisation of Bi/N-GF.(a) Low magnification bright field (BF)-STEM image.(b) Magnified view of the indicated area in (a),showing nanoparticle decoration of the graphite flake.(c) High magnification HAADF-STEM image of the particles,showing amorphous aggregation of atoms and some loose single atoms.(d) HAADF-STEM image and (e) fast Fourier transform of a nanoparticle,confirming lack of crystallinity.(f) HAADF-STEM image and(g−i) accompanying EDS mapping,confirming Bi aggregate nanoparticles dispersed on the N-doped graphite felt
As illustrated in the XRD patterns of GF and Bi/N-GF (Fig.3a),no characteristic diffraction peaks of Bi were observed.The three pronounced peaks appearing at 26.6°,44.7° and 55.1° can be ascribed to the (004),(102) and (105) crystal reflections of C in GF,respectively (PDF#26−1080).The surface composition and oxidation state of the samples were analyzed by XPS (Fig.3b).The C 1 s XPS spectra for both GF and Bi/N-GF can be deconvoluted into 3 peaks corresponding to C=C (284.8 eV),C―C(285.3 eV) and C―O (286.2 eV) (Fig.S2).Note that the content of C=C in Bi/N-GF is similar to that in GF but the content of C―O is higher.More oxygencontaining functional groups can boost the Cr3+/Cr2+redox reaction,benefiting the ICRFB.As shown in Fig.3c,in contrast to GF that contains solely graphitic N (originating from the polyacrylonitrile precursor used for the preparation of GF),Bi/N-GF exhibits three N 1s peaks attributable to graphitic N (401.0 eV),pyrrolic N (399.6 eV),and pyridinic N (398.3 eV),resulting from the PDA dopant.The surface atomic content of N in Bi/N-GF was estimated to be about 2.6%.The percentage of pyridinic N is higher than that of pyrrolic N.This is beneficial since pyridinic N doping offers lone electron pairs,which favor the adsorption of the positively charged cadmium ions to promote the redox reaction of Cr3+/Cr2+[20].Two prominent peaks centered at 162.5 and 157.0 eV were observed for Bi 4f XPS spectrum,which correspond to the 4f2/5and 4f2/7peaks of Bi0(Fig.3d),suggesting the formation of metallic Bi.

Fig.3 (a) XRD patterns of GF and Bi/N-GF.(b) Wide-survey and (c) N 1s XPS spectra of GF and Bi/N-GF.(d) Bi 4f XPS spectrum of Bi/N-GF
We used CV to explore the reactivity of GF,NGF,Bi-GF,and Bi/N-GF for the redox reactions of Fe2+/Fe3+and Cr3+/Cr2+.The obtained electrochemical parameters were listed in Table S1.It can be seen from Fig.4a,that two peaks of Fe2+/Fe3+redox pairs were found in all samples.Compared with GF,the peak redox current of Bi/N-GF is significantly enhanced.The oxidation peak current densities (Ipa) of GF,N-GF,Bi-GF,and Bi/N-GF were 22.4,18.7,33.4 and 35.4 mA cm−2,respectively.The current densities(Ipc) of corresponding reduction peaks were −17.5,−13.8,−30.4 and −32.5 mA cm−2.The ratios of oxidation/reduction peak current density (−Ipa/Ipc) of GF,NGF,Bi-GF and Bi/N-GF were derived to be 1.3,1.4,1.1 and 1.1.It can be deduced that the introduction of Bi caused a great increase of the reduction peak current,and improved the electrochemical reversibility of Fe2+/Fe3+.As displayed in Fig.4b,only Bi/N-GF displayed an obvious redox peak.By comparison,only Bi/N-GF afforded remarkable Cr3+/Cr2+redox peaks (Table S2).Bi/N-GF exhibited the largestIpa(4.2 mA cm−2),compared with pristine GF(2.0 mA cm−2),N-GF (3.6 mA cm−2),and Bi-GF(2.3 mA cm−2).In contrast to the absence of reduction current peak,Bi/N-GF provided aIpcas high as−5.1 mA cm−2.The ratio of the oxidation/reduction peak current density (−Ipa/Ipc) was 0.8,indicating its good reversibility.This is likely due to the fact that Bi NPs are helpful to prevent the HER during charging by forming a BiHxintermediate to suppress the reduction of H+[5]and thus improved the electrochemical activity of Cr3+/Cr2+.Bi was also calculated to possess a stronger binding energy for H atoms and higher energy barrier for H2evolution from H ions relative to carbon[3].Compared to Bi-GF,more pyridine N species are present which increased the adsorption of Cd ions and promoted the redox reaction of Cr3+/Cr2+.In addition,Bi NPs with smaller sizes were observed to exhibit better electrochemical activity and reversibility(Fig.S4a,b).
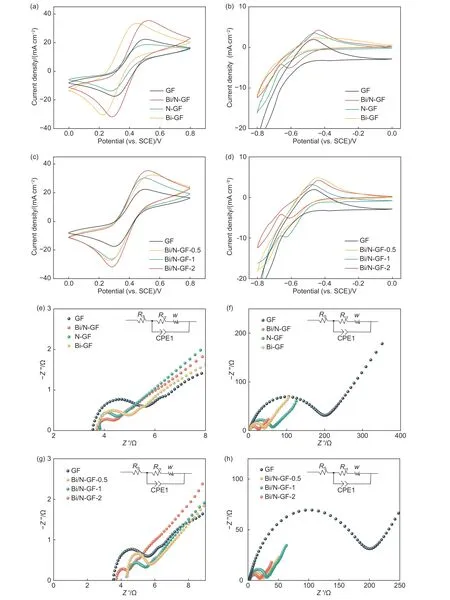
Fig.4 CV curves of GF,N-GF,Bi-GF and Bi/N-GF derived under (a) 40.0 mV s−1 and voltage range of 0−0.8 V (vs.SCE) at the positive electrode and(b) 6.0 mV s−1 and voltage range from −0.8 to 0 V at the negative electrode.CV curves of GF,Bi/N-GF-0.5,Bi/N-GF-1,and Bi/N-GF-2 obtained under (c) 40.0 mV s−1 and voltage range of 0−0.8 V at the positive electrode and (d) 6.0 mV s−1,−0.8−0 V at the negative electrode.EIS diagrams of GF,N-GF,Bi-GF,and Bi/N-GF measured under (e) 0.35 V at the positive electrode and (f) −0.5 V at the negative electrode.EIS diagrams of GF,Bi/N-GF-0.5,Bi/N-GF-1,and Bi/NGF-2 acquired under (c) 0.35 V at the positive electrode and (d) −0.5 V at the negative electrode
To investigate the optimal Bi-to-N loading ratio on the reactivity of Fe2+/Fe3+and Cr3+/Cr2+,CV tests were conducted,as illustrated in Fig.4c.The resulting electrochemical parameters were given in Table S3.Well-resolved Fe2+/Fe3+redox pair peaks were observed for all Bi/N-GF samples with different Bi/N ratios.TheIpavalues of Bi/N-GF with Bi/N ratios of 0.5,1.0 and 2.0 were 32.4,30.3 and 35.4 mA cm−2,respectively,and the correspondingIpcvalues were−27.5,−26.4 and −32.5 mA cm−2,which were all higher than GF (Ipa=22.4 mA cm−2,Ipc=−17.5 mA cm−2).Bi/N-GF with a Bi/N ratio of 2.0 was found to deliver the highest peak intensity with a−Ipa/Ipcvalue closest to 1.0,indicating its best chemical reversibility.Fig.4d shows the CV curves of the negative electrodes with different Bi/N ratios,and the resulting electrochemical parameters are provided in Table S4.Compared with bare GF,Bi/N-GF samples had substantially stronger Cr3+/Cr2+redox peaks.In stark contrast to theIpaof 2.0 mA cm−2for GF,theIpavalues for Bi/N-GF with a Bi/N ratio of 0.5,1.0 and 2.0 were 3.2,4.8 and 4.2 mA cm−2,respectively.The correspondingIpcwere −6.8,−9.7 and −5.1 mA cm−2.The Bi/N-GF with a Bi/N ratio of 2.0 exhibited a−Ipa/Ipcvalue closest to 1.0,reflecting its best chemical reversibility.
We further measured the CV curves of GF and Bi/N-GF samples during charge at the positive electrode by varying the scan rate from 20 to 100 mV s−1in the voltage range of 0 to 0.8 V.Their CV curves were acquired by altering the scan rate from 2 to 8 mV s−1in the voltage range of −0.8 to 0 V.The results are shown in Fig.S3a−f.In addition,the mass transfer rate was studied by plotting the change in the peak current densities of Fe2+/Fe3+and Cr3+/Cr2+with the square root of the sweep speed.As shown in Fig.S3g,the peak current densities of GF and Bi/N-GF samples are linearly related to the square root of the scanning rate,implying that all samples are associated with diffusion-controlled reactions.The slopes of GF,Bi/N-GF-0.5,Bi/N-GF-1 and Bi/N-GF-2 were 2.3,3.2,3.0 and 3.3,respectively.Bi/N-GF-2 possessed the largest slope,indicating that it had the highest mass transfer rate,consistent with its CV result.As revealed in Fig.S3h,GF did not show a reduction peak during the test,while Bi/N-GF had an obvious and regular peak current density.This verifies that Bi/N-GF significantly improves the electrochemical activity of Cr3+/Cr2+and also has a more rapid mass transfer rate.
As displayed in Fig.4e−h,EIS profiles consist of a semicircle in the high frequency region corresponding to the charge transfer reaction at the electrode/electrolyte interface,and a straight line in the low frequency region,ascribed to the material diffusion process in the electrolyte.It can be concluded that the Fe2+/Fe3+and Cr3+/Cr2+redox reactions at the electrodes are mainly controlled by diffusion and charge transfer.The charge transfer resistance (Rct),ohmic resistance (Rs) and diffusion capacitance of the electrolyte (Wo) in the equivalent circuit after fitting by Zview were provided in Tables S5 and S6.TheRctfollowed the trend GF >Bi-GF >N-GF >Bi/N-GF for the Fe2+/Fe3+redox reaction.Bi/N-GF possessed the smallestRctand can enable the fastest Fe2+/Fe3+reaction.Likewise,for the Cr3+/Cr2+redox reactions at the negative electrode,Bi/N-GF also had the lowestRct(Fig.4f).As shown in Fig.4g,h,the Bi/N-GF with a Bi/N ratio of 2.0 gave the lowestRctfor both Fe2+/Fe3+and Cr3+/Cr2+redox reactions in accordance with the afore-mentioned CV results.Reducing the size of Bi NPs led to smallerRct(Fig.S4c,d).
The rate cycle performance of the as-prepared samples for ICRFBs were tested with charge and discharge current densities between 60 and 120 mA cm−2.The EEs of different samples are shown in Fig.5a−d.The CEs and VEs are shown in Fig.S5.As illustrated in Fig.5a,the introduction of N and/or Bi improved the EE,with Bi/N-GF-2 being the best at various current densities.Bi/N-GF-2 afforded an EE as high as 85.8% at 60.0 mA cm−2,and 76.7% even at a large current density of 120.0 mA cm−2,substantially outperforming bare GF and many reported electrodes[23–24].A high CE of up to 97.7% was maintained for Bi/N-GF-2 at different current densities.The superior performance of Bi/N-GF can be attributed to the fact that Bi NPs form an intermediate with H+,thereby inhibiting the undesired HER and improving the CE and EE.Cycling tests at 60.0 mA cm−2showed that the EE of Bi/N-GF was retained above 80.0% even after 100 cycles (Fig.5c,d).Post characterization by SEM and TEM showed that the morphology of Bi/N-GF was retained but the size of Bi NPs increased to about 25 nm after the charge-discharge cycling at different current densities (Fig.S6).The performance degradation after the long-term chargedischarge cycling is hypothesized to result from the aggregation of Bi NPs.Other possible reasons include the formation of irreversible Cr ion species and also the accumulation of H2bubbles on the surface of the negative electrode during the charging process.Smaller Bi NPs were found to afford better battery performance in terms of rate cycle and long-term cycle (Figs.S7,S8).Notably,Bi/N-GF-2 displayed the lowest charging voltage overpotential and the highest discharge voltage overpotential (Fig.5e,g),indicating its excellent reaction kinetics.In addition,the capacities of Bi/N-GF-2 reached 862.7 mAh/L,which were remarkably higher than bare GF,N-GF,and Bi-GF after 100 cycles (Fig.5f,h).The outstanding battery performance is likely due to the synergistic impact of Bi NPs and N doping.On the one hand,Bi NPs react with protons to form BiHxduring charge,therefore thwarting the HER[21–22].On the other hand,the introduction of N species (especially pyridinic N)provides abundant adsorption sites for positively charged Cr ions,thus accelerating the Cr2+/Cr3+redox process.
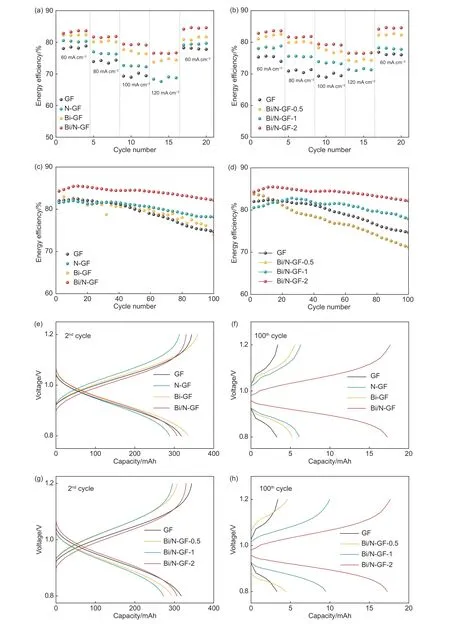
Fig.5 EEs at different current densities in ICRFB for (a) GF,N-GF,Bi-GF and Bi/N-GF,and (b) GF,Bi/N-GF-0.5,Bi/N-GF-1 and Bi/N-GF-2.EEs of (c)GF,N-GF,Bi-GF and Bi/N-GF and (d) GF,Bi/N-GF-0.5,Bi/N-GF-1 and Bi/N-GF-2 tested at 60.0 mA cm−2 and 100 cycles in ICRFB.Charge–discharge curves at the (e) 2nd and (f) 100th cycle in the voltage range of 0.8 to 1.2 V for GF,N-GF,Bi-GF and Bi/N-GF.Charge–discharge curves at the (g) 2nd and (h)100th cycle in the voltage range of 0.8 to 1.2 V for GF,Bi/N-GF-0.5,Bi/N-GF-1 and Bi/N-GF-2
4 Conclusion
We report a negative electrode consisting of amorphous Bi NPs immobilized on N-doped GF,for efficient and stable iron-chromium redox flow batteries (ICRFBs).The Bi/N-GF was synthesized by a combined self-polymerization with wet-chemistry reduction strategy followed by annealing.The resulting negative electrode showed substantially enhanced electrochemical performance for Fe2+/Fe3+and Cr3+/Cr2+redox reactions,as compared with bare GF.High CEs of up to 97.7% were preserved at different current densities.At 60.0 mA cm−2,the EE for Bi/NGF with an optimal Bi/N ratio of 2.0 reached 85.8%,and the capacity approached 862.7 mAh L−1after 100 cycles,which was approximately 5.3 times of the original GF.The remarkable capacity and cycle stability can be mainly attributed to the synergistic effect of Bi NPs and N species,which simultaneously suppresses the parasitic HER during charge and enhances the Cr2+/Cr3+redox reaction kinetics.In addition,the cointroduction of Bi and N species may reduces the charge transfer resistance and boosts the mass transfer rate,which is facilitating the ICRFBs.
Acknowledgements
This work was supported by the National Key Research and Development Program of China(2022YFC2105900),National Natural Science Foundation of China (22372007 and 21972010),and Fundamental Research Funds for the Central Universities(JD2310,ZY2317 and buctrc202226).
- 新型炭材料的其它文章
- Ir nanoclusters on ZIF-8-derived nitrogen-doped carbon frameworks to give a highly efficient hydrogen evolution reaction
- Cactus-like NC/CoxP electrode enables efficient and stable hydrogen evolution for saline water splitting
- A Co3O4/graphdiyne heterointerface for efficient ammonia production from nitrates
- 石墨烯基二氧化碳还原电催化材料研究进展
- MOF-derived nanocarbon materials for electrochemical catalysis and their advanced characterization
- A review of carbon-based catalysts and catalyst supports for simultaneous organic electro-oxidation and hydrogen evolution reactions

