Responses of nitrogen cycling and related microorganisms to brackish wetlands formed by evapotranspiration
Jiaohui FANG ,Tianshu LÜ ,Jian LIU ,Shangbin HE ,Xiufeng YANG ,Huashan DOU and Honghai ZHANG,*
1School of Life Sciences,Qufu Normal University,Qufu 273100(China)
2Environment Research Institute,Shandong University,Qingdao266237(China)
3Hulunbuir Academy of Inland Lakes in Northern Cold&Arid Areas,Hulunbuir 021000(China)
ABSTRACT Elevated evapotranspiration due to warmer air temperature could raise salinity and nutrient levels of some inland wetlands,potentially impacting nitrogen cycling.To characterize the impact of high evapotranspiration on soil microbial nitrogen cycling in inland wetlands,we compared freshwater and brackish marsh(or non-marsh)wetlands in terms of sediment ammonia-oxidizing rate(AOR),denitrifying rate(DR),and related microbial communities in a typical inland basin,the Hulun Lake basin,in China.Results showed that marsh ecosystems(ME)exhibited 31%higher AOR and 65%higher DR than non-marsh ecosystems(NE).For NE,freshwater non-marsh wetland exhibited 12%higher AOR than brackish non-marsh wetland.This was probably due to the inhibitory effects of highand salinity levels on ammonia-oxidizing archaea in brackish non-marsh wetland.Conversely,DR in brackish non-marsh wetland was 23%higher than that in freshwater non-marsh wetland,with total organic carbon(TOC)significantly influencing this difference,suggesting that the higher DR in brackish non-marsh wetland was mainly due to its higher TOC level.For ME,due to the direct and indirect interference of salinity,brackish marsh wetland displayed 26%lower AOR and 19%lower DR than freshwater marsh wetland.Besides,brackish wetlands harbored distinct ammonia-oxidizing and denitrifying microbial communities compared to freshwater wetlands.The assembly of these communities was dominated by stochastic processes,while brackish wetlands exhibited more prominent deterministic processes than freshwater wetlands.Overall,high evapotranspiration altered activities and community characteristics of ammonia oxidizers and denitrifiers in inland brackish wetlands by enhancing salinity and nutrient levels,while emergent plants occurring in ME could mitigate the adverse effects of salt stress of inland brackish wetlands on nitrogen cycling.
Key Words:ammonia-oxidizing archaea,ammonia-oxidizing bacteria,aquatic plants,denitrifying bacteria,nitrogen transformation,inland wetlands
INTRODUCTION
Nitrogen cycling,as a crucial component of the biogeochemical cycle,largely relies on the microorganism-driven nitrogen transformation (Nelsonet al.,2016).Within the context of nitrogen transformation in the soil,biological nitrification followed by denitrification are significant processes,as they serve as major pathways for the removal of nitrogen and are critical for the maintenance of water quality in wetlands(Wuet al.,2017;Deveautouret al.,2022).Ammonia oxidation to nitrite,the first and rate-limiting step of nitrification,is driven by ammonia-oxidizing archaea(AOA)and bacteria(AOB)(Morse and Bernhardt,2013;Hussainet al.,2023).ComammoxNitrospira,the complete ammonia oxidizers discovered in 2015,are capable of oxidizing ammonia to nitrate within a single organism,potentially also making a significant contribution to nitrification (Daimset al.,2015;van Kesselet al.,2015).Denitrification involves the use of nitrogen oxides(nitrate and nitrite)as terminal electron acceptors in the oxidation of organic compounds,ultimately reducing nitrate to gaseous dinitrogen or nitrous oxide(Groffman,2012;Seeleyet al.,2020).The variability of environmental conditions,such as hydrology and salinity,can easily alter microbial activity and community structure,impacting the nitrogen transformation and changing wetland nitrogen pools(Bernhardet al.,2005;Chiet al.,2021).
Inland wetlands,characterized by a lack of outflow,typically lose water through evaporation.Similar dynamics have been observed across various scales,from streams and marshes to rivers,lakes,and inland seas (Thorp and Covich,2010).The global climate is undergoing significant changes,with warming being the most prominent(Vicente-Serranoet al.,2014;IPCC,2021).The continuous increase of evapotranspiration due to warmer air temperature could substantially reduce the runoffin some inland wetlands,causing a rapid rise in their salinity and nutrient levels.Consequently,many inland wetlands may gradually transition from lotic freshwater to static brackish wetlands(Meyeret al.,2013;Ágredaet al.,2015).As this transition occurs,environmental factors such as salinity,organic matter,inorganic nitrogen,and hydrology might profoundly and variably influence microbial nitrogen transformations in static brackish and lotic freshwater wetlands.
Salinity might significantly impact biological processes responsible for nitrogen transformations in inland wetlands with a high evapotranspiration(Akhtaret al.,2012).Salinityinduced reductions in soil ammonia adsorption capacity might indirectly cause decreases in nitrification and denitrification activities(Rysgaardet al.,1999).Salinity can also restrict water availability,impacting cellular physiology and metabolic processes,thus directly inhibiting their nitrification and denitrification capabilities(Ghollarata and Raiesi,2007).Furthermore,organic carbon exerts a significant effect on the activity and composition of denitrifying and ammoniaoxidizing communities in soil(Guoet al.,2017;Wuet al.,2017).Specifically,most denitrifiers are chemoheterotrophs,and the increased organic carbon in inland wetlands due to high evapotranspiration could increase the availability of organic substrates necessary for denitrification,potentially stimulating denitrifying microorganism activity.However,the increasedin static brackish wetlands might inhibit AOA activity,since AOA prefer an oligotrophic environment with lowconcentrations(Verhammeet al.,2011).Overall,differences in the nitrification and denitrification microbial community composition and activities across different habitats(i.e.,brackish and freshwater wetlands,respectively)shaped by high evapotranspiration remain unclear.
Significant water level drops in inland wetlands due to high evapotranspiration may increase emergent plant distribution,eventually forming marsh wetlands,as water levels suitable for emergent plants are generally below 2 m(Fanget al.,2020).Previous studies have shown that emergent plant growth could amplify the soil nitrogen cycle of wetlands,including nitrification and denitrification(Wuet al.,2017;Fanget al.,2019;Zhanget al.,2020).However,current studies mostly focus on freshwater wetlands.It remains unclear whether the growth of emergent plants could alleviate the disturbance of salt stress and enhance the microbial nitrogen cycling in inland brackish wetlands.This knowledge gap hinders a comprehensive understanding of the impact of global warming-induced high evapotranspiration on wetland nitrogen cycling.
The Hulun Lake is an inland lake in the hinterland of the Hulunbeir grassland,the largest grassland in China(Maet al.,2022).The evapotranspiration of the Hulun Lake basin is 6-7 times greater than its rainfall.The high evaporation results in the co-occurrence of lotic freshwater wetlands(i.e.,freshwater non-marsh wetlands and freshwater marsh wetlands)and static brackish wetlands(i.e.,brackish nonmarsh wetlands and brackish marsh wetlands)in the basin.Therefore,the Hulun Lake basin is an ideal area to explore the ecological differences of freshwater and brackish wetlands under the influence of global warming.
In this study,we examined the activity and community composition of ammonia oxidizers and denitrifiers in lotic freshwater wetlands and static brackish wetlands in the Hulun Lake basin in summer and autumn.Our study aimed to characterize the response of soil microbial nitrogen cycling to brackish inland wetlands formed by evapotranspiration.We proposed two hypotheses:i)influenced by high salinity levels,brackish wetlands exhibited significantly lower ammoniaoxidizing and denitrifying rates than freshwater wetlands and the related microbial communities also exhibited marked dissimilarities between the two wetland types and ii)due to the emergent plant growth alleviating salt stress,brackish marsh wetlands exhibited significantly higher ammonia-oxidizing and denitrifying rates than brackish non-marsh wetlands.Our study could contribute to a deeper understanding of the spatial variation of nitrogen cycling processes under global warming influence.
MATERIALS AND METHODS
Site description and sampling
The Hulun Lake basin,a typical inland lake basin,is situated in the western part of the Hulunbeir grassland in Inner Mongolia,China (115°31′7′′-120°55′11′′E,47°40′46′′-49°34′20′′N).This basin features a temperate,semi-arid continental climate with an annual precipitation of 240.5-383.6mm and an annual evaporation of 1 455.3-1 754.3 mm(Maet al.,2022).The Hulun Lake Basin hosts lotic freshwater wetlands(salinity<1‰)and static brackish wetlands(1‰≤salinity ≤3‰),with average salinities of 0.28‰and 1.24‰,respectively.Both freshwater and brackish wetlands in this basin include marsh wetlands with abundant plants growing at lower water levels and non-marsh wetlands with higher water levels without emergent plants(Fig.1a).Consequently,four types of wetlands are typically distributed in this basin:freshwater non-marsh wetland,freshwater marsh wetland,brackish non-marsh wetland,and brackish marsh wetland(Fig.1a).

Fig.1 Water system and distribution of brackish wetlands(brackish non-marsh wetland(BN)and brackish marsh wetland(BM))and freshwater wetlands(freshwater non-marsh wetland(FN)and freshwater marsh wetland(FM))in the Hulun Lake basin(a),remote sensing image and flow direction of BM and FM(b),and the vertical section of BM and FM(c).DOR=Dalan Orom River.
The Urxun River,a lotic freshwater river and one of the main tributaries of the Hulun Lake,belongs to the freshwater non-marsh wetland category.It has a length of 223.28 km and a depth of 2-3 m(Fig.1a).The wetland located on flat terrain on both sides of the Urxun River belongs to the freshwater marsh wetland category(Fig.1a,b),with a great abundance of emergent plants growing in its low-depth regions (<2 m).These plants includePhragmites australis(Cav.)Trin.ex Steud,Schoenoplectus tabernaemontani(C.C.Gmelin)Palla,andZizania latifolia(Griseb.) Stapf (Fig.1c).The Hulun Lake,belonging to the brackish non-marsh wetland category,is a throughput lake located on the Hulunbeir grassland,with an area of approximately 2 339 km2and a water depth of approximately 5.7 m.When the water volume of the lake is high,it flows outward along the Dalan Orom River;when the water volume is low,the lake becomes an inland lake.Since 2000,due to the continuous impact of the warm and dry climate in the basin,the temperature has shown a significant upward trend and the rainfall has decreased slowly,causing a decline in the storage capacity of the Hulun Lake.This has led to the gradual transformation of the Hulun Lake from an outflow lake into an inland lake.High evaporation promoted the transformation of the lake from a lotic freshwater lake to a static brackish lake.The Wulannuoer wetland,belonging to the brackish marsh wetland category,is formed by the convergence of the Urxun River tributaries on low and flat terrain,with a depth of less than 2 m (Fig.1a,b).Since 2000,due to the continuous impact of warm and dry climate,the evapotranspiration of this basin has increased,and the runoff of the Urxun River has significantly decreased,resulting in the relatively static flow of the Wulannuoer wetland and the gradually higher salinity of its water body.These environmental conditions have resulted in the salt-tolerant plant,Phragmites australis,being the main emergent plant species in this marsh wetland(Fig.1c).
To compare freshwater and brackish wetlands in terms of sediment ammonia-oxidizing and denitrifying processes,we took the Hulun Lake basin as the study area,sampling four types of wetlands in the summer (July) and autumn(September)of 2021.In each season,24 sampling points were randomly set in the four wetlands,freshwater non-marsh wetland,freshwater marsh wetland,brackish non-marsh wetland,and brackish marsh wetland,with setting of 6,9,6,and 3,respectively.At each sampling point,three parallel surface sediment samples were collected using a sediment core sampler with a diameter of 4 cm,and three parallel samples of surface water with approximately 0.5 L were taken into the sterile bottles,subsequently combined into a uniform sample each.In total,48 sediment samples and 48 water samples were collected.Each sediment sample was then divided into two subsamples.One subsample was stored at 4°C and subsequently used to determine the nitrogen cycling activity of microbial communities(i.e.,ammonia oxidation and denitrification)and soil physicochemical properties;the other subsample was stored at-80°C and subsequently used for DNA extraction.The water samples were used to analyze the physicochemical properties of the water.
Sediment and water physicochemical properties
We used the water test kit (Multi 3630 IDS,WTW,Munich,Germany)to measure the dissolved oxygen(DO),electrical conductivity(EC),and the temperature of the overlying waterin situ.To determine the chemical properties of sediment samples,we first used 2 mol L-1KCL to extract carbon and nitrogen from soil samples.Subsequently,the dissolved organic carbon content (DOC) of the sediment was determined using a TOC analyzer(TOC-L,Shimadzu,Kyoto,Japan).Sediment pH was determined using a water test kit (Multi 3630 IDS,WTW).Contents ofandwere determined using a continuous-flow autoanalyzer (AA3,Seal Analytical Ltd.,Southampton,UK).Finally,the total organic carbon(TOC)of sediment was measured using an elemental analyzer(Vario EL III,Elementar Analysensysteme,Langenselbold,Germany).
Ammonia-oxidizing and denitrifying rates
Acetylene(Giguereet al.,2015)and 1-octyne(Tayloret al.,2013)inhibition methods were employed to measure the total ammonia-oxidizing rates of AOA and AOB in soil.The addition of acetylene during the culture process inhibits all autotrophic ammoxidation processes,and the addition of 4 μmol L-11-octyne only inhibits the activity of AOB,allowing the ammonia-oxidizing process of AOA to continue uninterrupted.The specific determination method of ammonia-oxidizing rate was described in Supplementary Material.
An acetylene inhibition method was used to determine the potential denitrifying rate in soil(Groffman and Tiedje,1989;Groffmanet al.,1993).Acetylene at a concentration of 10%inhibits N2O reductase activity in the soil,preventing the reduction of N2O to N2,thus N2O becomes the main product of denitrification.The specific determination method of potential denitrifying rate was also described in Supplementary Material.
DNA extraction,sequencing,and sequence analysis
Microbial communities involved in the ammonia oxidation and denitrification in sediment samples were analyzed through profiling of ammonia oxidation-related genes(amoAfrom AOA and AOB)and denitrification-related genes(nirSandnirK) using Illumina high-throughput sequencing in Tiny Gene Biotechnology Co.(Shanghai,China).The total genomic DNA for each sample was extracted using a PowerSoil DNA separation kit(MO BIO Laboratory,Carlsbad,USA).Primers ArchamoAF/ArchamoAR,amoA1F/amoA2R(Zhanget al.,2015),Cd3aF/R3cd(Throbäcket al.,2004),andnirK876/nirK1040(Henryet al.,2004)were used for polymerase chain reaction(PCR)amplification of the AOAamoA,AOBamoA,nirS,andnirKgenes,respectively.The PCR amplification was conducted using ABI GeneAmp 9700 PCR thermocycler(Applied Biosystems,Foster City,USA).Prior to library pooling,all amplification products were purified and quantified using a DNA gel extraction kit(Axygen,Hangzhou,China)and FTC-3000 real-time quantitative thermal cycler(Funglyn Biotech,Shanghai,China),respectively.Finally,the DNA library was sequenced on Illumina NovaSeq PE250 platform (Illumina,San Diego,USA)by Tiny Gene Biotechnology Co.(Shanghai,China).
Paired raw sequences for AOBamoA,nirS,andnirKgenes were joined by FLASH(version 1.2.11,https://ccb.jhu.edu/software/FLASH/)except AOAamoAgene.The raw sequences for AOAamoAgene were analyzed in singleend since the long length of the amplicon of AOAamoAgene (635 bp).Low-quality contigs were quality filtered using Mothur(v.1.31,https://www.mothur.org/).The clustering of reads into operational taxonomic units (OTUs)was based on a 97% similarity threshold using UPARSE(https://www.drive5.com/uparse/).The OTU representative sequences were taxonomically assigned against the National Center for Biotechnology Information(NCBI)non-redundant protein database(nr)(https://ftp.ncbi.nlm.nih.gov/blast/db/).A phylogenetic tree was then generated based on the neighbor-joining method using MEGA software (v.7.0,https://www.megasoftware.net/).The nucleotide sequences in this study were deposited in the Sequence Read Archive of the NCBI under the accession numbers PRJNA906187 and PRJNA906263.
Processes underlying the assembly of microbial communities involved in ammonia oxidation and denitrification
The assembly processes of ammonia oxidation and denitrification microbial communities in freshwater and brackish wetlands were investigated by habitat niche breadth,neutral community model,and null model.To apply Levins’niche breadth method to our samples,we used the“spaa”software package in R(v.4.0.3)to calculate the habitat niche breadth of microbial communities involved in ammonia oxidation and denitrification(Liet al.,2021).The habitat niche breadth value reflects the potential of ammonia oxidizers and denitrifiers to adapt to an array of environmental conditions.To assess the contribution of stochastic processes to the assembly process of these microbial communities,a neutral community model was employed to predict the relationship between the relative abundance change of OTUs and the frequency of their occurrence(Sloanet al.,2016).TheR2value in this model determines the overall goodness of fit of the neutral community model.The higher theR2value,the greater the impact of stochastic processes on community structure.The null model was used to calculate theβ-nearest taxon index(βNTI);this was done to assess the relative importance of deterministic and stochastic processes in the assembly of microbial communities involved in ammonia oxidation and denitrification.The deviation of the observedβ-nearest taxon distance(βMNTD)from the meanβMNTD was compared to an estimate ofβNTI using the“picante”package of R(v.4.1.3).An|βNTI|value less than 2 is indicative of the predominance of stochastic processes,and conversely,a value greater than 2 is indicative of the predominance of deterministic processes.
Statistical analyses
SPSS software(v.23.0)was used to perform a two-way analysis of variance to determine the significant differences of ammonia-oxidizing and denitrifying rates between brackish and freshwater wetlands and between seasons.Stepwise regression was used to eliminate factors that cause multicollinearity,with the aim of analyzing the main environmental factors that drive changes in ammonia-oxidizing and denitrifying rates(SPSS software v.23.0 was used to perform this step).A random forest model was also used to rank factors underlying ammonia-oxidizing and denitrifying rates based on their importance,and determine the main influencing factors in the“randomForest”package of R software(v.4.1.3).Permutational multivariate analysis of variance(PERMANOVA)was used to examine the statistical significance of the variation in microbial community structure within the context of wetland types(i.e.,brackish and freshwater wetlands)and marsh formation(i.e.,marsh ecosystem and non-marsh ecosystem) using the“vegan”package of R (v.4.1.3).A Mantel test was conducted to analyze the relationship between environmental factors and the microbial communities involved in the ammonia oxidation(i.e.,those possessing AOAamoAand AOBamoA)and denitrification(i.e.,those possessingnirSandnirK)in freshwater and brackish wetlands;the“vegan”package in R(v.4.1.3)was used to achieve this aim.Variance partitioning analysis(VPA) was used to quantify the relative contributions of wetland types(brackish and freshwater wetlands)and seasons and of wetland types and important physicochemical properties on ammonia-oxidizing and denitrifying microbial communities,hence determining the main driving factors that cause changes in microbial communities between brackish and freshwater wetlands;the“vegan”package in R(v.4.0.3)was used to achieve this aim.
RESULTS
Variations of ammonia-oxidizing and denitrifying rates between brackish and freshwater wetlands and their main underlying factors
No significant difference in the total ammonia-oxidizing rate was observed between seasons,but significant differences were found between brackish wetlands and freshwater wetlands (Fig.2a).The total ammonia-oxidizing rate in freshwater non-marsh wetland(1.85±0.25 mg N kg-1d-1)was higher than that in brackish non-marsh wetland(1.65±0.12 mg N kg-1d-1).The freshwater marsh wetland(2.62±0.29 mg N kg-1d-1)was characterized by a significantly higher level (P <0.05) of total ammonia-oxidizing rate than the brackish marsh wetland(1.95±0.22 mg N kg-1d-1).Furthermore,the total ammonia-oxidizing rates of freshwater and brackish marsh wetlands were significantly higher (P <0.05) than those of freshwater and brackish non-marsh wetlands with equal salinity levels (salinity<1‰or 1‰≤salinity ≤3‰).
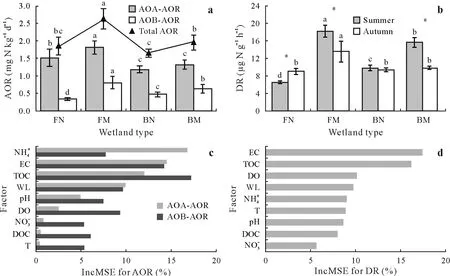
Fig.2 Sediment ammonia-oxidizing rate(AOR,a)and denitrifying rate(DR,b)of freshwater non-marsh wetland(FN),freshwater marsh wetland(FM),brackish non-marsh wetland (BN),and brackish marsh wetland (BM) as well as the rank of their influencing environmental factors according to their importance by random forest model(c-d).In a and b,values are means with standard deviations shown by the vertical bars(n=6),and different letters on the bars represent significant differences among different wetland types at P <0.05.The asterisk*indicates significant differences between seasons for each wetland type at P <0.05.IncMSE=the increase in mean square error,with a larger value indicating a greater importance of the factor;AOA-AOR=AOR of ammonia-oxidizing archaea;AOB-AOR=AOR of ammonia-oxidizing bacteria;EC=water electrical conductivity;WL=water level;DO=water dissolved O;TOC=sediment total organic C;DOC=sediment dissolved organic C;T=water temperature;and =sediment and contents,respectively;pH=sediment pH.
The ammonia-oxidizing rates of AOA in freshwater nonmarsh (1.52±0.25 mg N kg-1d-1) and marsh (1.82±0.19 mg N kg-1d-1) wetlands were significantly higher(P <0.05)than those of brackish non-marsh(1.18±0.11 mg N kg-1d-1) and marsh (1.32±0.13 mg N kg-1d-1)wetlands,consistent with change patterns of total ammoniaoxidizing rate(Fig.2a).The ammonia-oxidizing rate of AOA accounted for most of the total ammonia-oxidizing rate(i.e.,averaging 70%)and displayed a high degree of linear fitting with the total ammonia-oxidizing rate (R2=0.91,P <0.05).Similar to patterns observed in AOA activity,the ammonia-oxidizing rate of AOB was significantly higher(P <0.05)in freshwater marsh wetland(0.81±0.18 mg N kg-1d-1)than in brackish marsh wetland(0.62±0.13 mg N kg-1d-1)(Fig.2a).In contrast,the ammonia-oxidizing rate of AOB was significantly lower(P <0.05)in freshwater non-marsh wetland (0.34±0.03 mg N kg-1d-1) than in brackish non-marsh wetland (0.47±0.07 mg N kg-1d-1).Moreover,freshwater and brackish marsh wetlands were characterized by significantly higher levels(P <0.05)of ammonia-oxidizing rates of both AOA and AOB than freshwater and brackish non-marsh wetlands with an equal salinity(salinity<1‰or 1‰≤salinity ≤3‰).
Significant differences in denitrifying rate were observed between brackish and freshwater wetlands (Fig.2b).The freshwater non-marsh wetland(0.78±0.14 μg N g-1h-1)was characterized by a lower denitrifying rate than the brackish non-marsh wetland(0.96±0.06μg N g-1h-1),with the difference being significant(P <0.05)in summer.However,denitrifying rate was significantly lower (P <0.05) in brackish marsh wetland (1.28±0.30 μg N g-1h-1)than in freshwater marsh wetland(1.59±0.30 μg N g-1h-1).Furthermore,the denitrifying rates of freshwater and brackish marsh wetlands were higher(P <0.05)than those in freshwater and brackish non-marsh wetlands with equal salinity(salinity<1‰or 1‰≤salinity ≤3‰).
The physicochemical properties of soil fluctuated greatly across the four different wetland types(Table I).Specifically,brackish non-marsh and marsh wetlands had 322%higher EC,51%higher,259%higher TOC than freshwater non-marsh and marsh wetlands(P <0.05).The TOC contents in freshwater and brackish marsh wetlands were 131%and 37%higher(P <0.05)than in freshwater and brackish non-marsh wetlands,respectively.Furthermore,levels in freshwater non-marsh wetland were 23%higher than those in freshwater marsh wetland,whilelevels in brackish non-marsh wetland were 8%lower(P <0.05)than those in brackish marsh wetland.
All physicochemical properties were individually used as an input in a regression model,and the factors that less explained ammonia-oxidizing and denitrifying rates and were highly correlated with other factors were eliminated to obtain the optimal set of explanatory variables for the two rates.In addition,a random forest model was employed to rank the importance of the various factors on the ammoniaoxidizing and denitrifying rates(Fig.2c,d).Based on the two methods mentioned above,we found thatand EC were the main influencing factors for ammonia-oxidizing rate of AOA,EC and TOC were the main influencing factors for ammonia-oxidizing rate of AOB,and EC had the greatest influence on denitrifying rate,followed by TOC.
Differences in microbial community structures associated with ammonia oxidation and denitrification between brackish and freshwater wetlands
A large proportion of OTU-representative sequences of theamoAgene-encoding AOA(9%-84%)and AOB(6%-78%)microorganisms,which were taxonomically annotated against nr in NCBI,could not be classified into any known group.To investigate the community structure of ammonia oxidation-related microorganisms,amoA-encoding AOA and AOB OTUs with a relative abundance>0.3% were used as input for a phylogenetic analysis(Fig.S1,see Supplementary Material for Fig.S1).The phylogenetic trees showed that the AOA sequences were primarily affiliated with“Group I.1b”(Nitrososphaera)and“Group I.1a”(Nitrosopumilus),with“Group I.1b”being the most dominant of the two,especially in non-marsh wetlands(Figs.S1a and 3).Additionally,freshwater non-marsh(84%)and marsh(69%)wetlands exhibited a higher relative abundance of“Group I.1b”than brackish non-marsh(74%)and marsh(48%)wetlands.ForamoA-encoding AOB sequences,NitrosospiraandNitrosomonaswere the two typical AOB clusters found in wetland soils(Figs.S1b and 3b).The relative abundance ofNitrosospirawas greater in brackish wetlands (66%),while the relative abundance ofNitrosomonaswas higher in freshwater wetlands(67%).Furthermore,as illustrated in Fig.3,Proteobacteria was the dominant phylum of both thenirSandnirKgene-encoding bacteria,representing,on average,a vast majority (90% and 88%,respectively) of species.
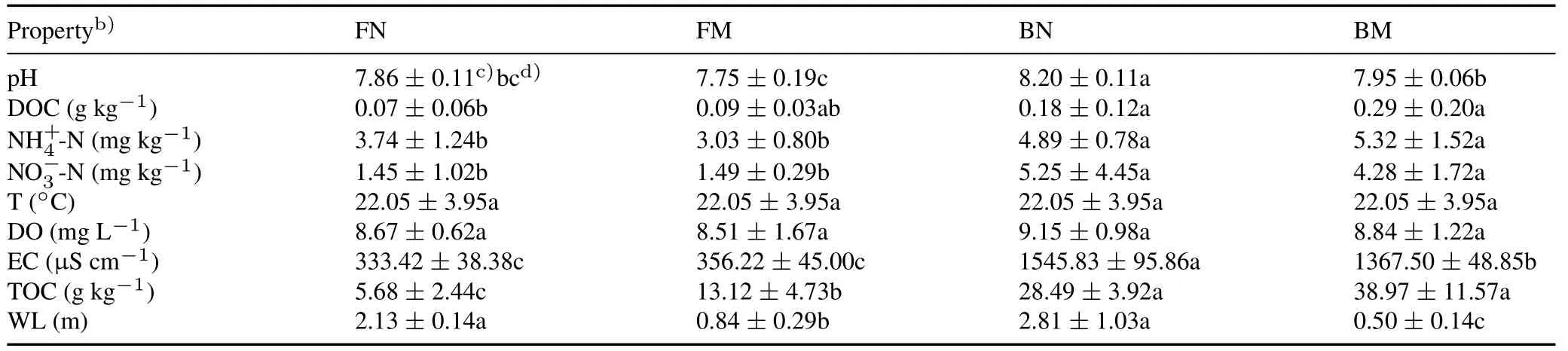
TABLE IPhysicochemical properties of water and sediments of the four wetland typesa) in the Hulun Lake basin

Fig.3 Community composition of amoA,nirS,and nirK-containing microorganisms in freshwater non-marsh wetland(FN),freshwater marsh wetland(FM),brackish non-marsh wetland(BN),and brackish marsh wetland(BM).The summer and autumn samples are represented by-s and-a,respectively.AOA=ammonia-oxidizing archaea;AOB=ammonia-oxidizing bacteria.
Through a PERMANOVA analysis,we found that soil ammonia-oxidizing and denitrifying microbial communities varied significantly between brackish and freshwater wetland types (Fig.4).The AOB and denitrifier (nirSandnirK)communities varied significantly between marsh and non-marsh formation,whereas AOA community had no significant variation between them(Fig.4).Moreover,when the composition of microbial communities related to nitrogen cycling were concerned,wetland types(17%-36%)had a greater explanatory power than marsh formation(5%-14%),suggesting that the variations of wetland types caused by high evapotranspiration was more likely to cause the change of soil nitrogen cycling-related microbial communities than the formation of marsh wetlands.
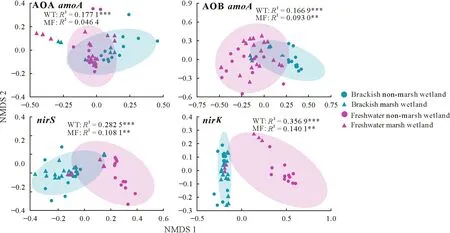
Fig.4 Non-metric multidimensional scaling(NMDS)of amoA,nirS,and nirK-containing microbial communities as well as the variations in the community structure between wetland types(WT)and between marsh formation(MF)using permutational multivariate analysis of variance(PERMANOVA).The 95%confidence ellipses are shown around the samples grouped based on WT.The asterisks**and***indicate significant differences at P <0.01 and P <0.001,respectively.AOA=ammonia-oxidizing archaea;AOB=ammonia-oxidizing bacteria.
Assembly processes of microbial communities associated with ammonia oxidation and denitrification in brackish and freshwater wetlands
The assembly processes of ammonia-oxidizing and denitrifying microbial communities in brackish and freshwater wetlands were investigated using habitat niche breadth,neutral community model,and null model(Fig.5).Firstly,the habitat niche breadths of AOB and denitrifier (nirS-andnirK-containing) communities in brackish wetlands were lower than those in freshwater wetlands,regardless of the marsh or non-marsh environment(Fig.5).In contrast,AOA communities showed no significant difference in habitat niche breadth between brackish and freshwater wetlands(Fig.5).Secondly,except for the AOB community in brackish non-marsh and marsh wetlands(29%),more than 56%of variations in the communities of AOA and denitrifiers(nirS-andnirK-containing)were determined based on the neutral community model,indicating that stochastic process played a vital role in shaping the ammonia-oxidizing and denitrifying microbial communities in wetlands(Fig.5).TheR2value of neutral model for AOB and denitrifier (nirSandnirK-containing)communities indicated that stochastic processes played a smaller role in the microbial communities associated with the nitrogen cycle in brackish wetlands than in freshwater wetlands(Fig.5).Furthermore,the null model revealed that both stochastic(47%)and deterministic(53%)processes were important in shaping the AOB community in brackish marsh wetland(Fig.S2,see Supplementary Material for Fig.S2).However,except for AOB in brackish marsh wetland,stochasticity was dominant in the assemblage of nitrogen-cycling microbial communities in freshwater and brackish wetlands,since most(>75%)absoluteβNTI values were<2.In contrast,deterministic factors had a greater effect on the community structure of ammonia-oxidizing and denitrifying microorganisms in brackish wetlands (13%-53%)compared with freshwater wetlands(0%-10%)(Fig.S2).
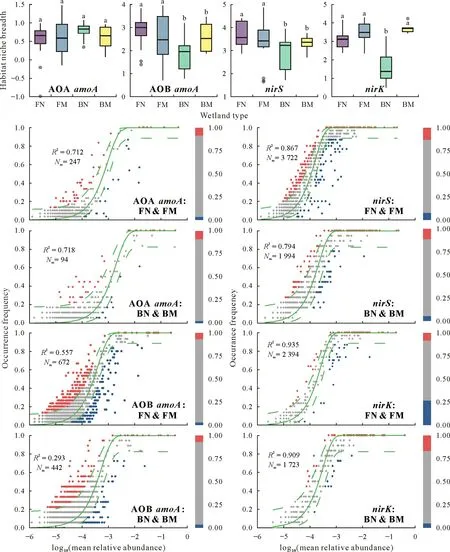
Fig.5 Stochastic and deterministic processes in the assembly of the amoA,nirS,and nirK-containing microbial communities in freshwater non-marsh wetland(FN),freshwater marsh wetland(FM),brackish non-marsh wetland(BN),and brackish marsh wetland(BM)using habitat niche breadth and neutral community model.For the above four boxplot subfigures,different letters on the bars indicate significant differences between wetland types at P <0.05.For the below eight subfigures,the bar plots refer to the relative abundances of operational taxonomic units(OTUs)that occur more frequently(red)or less frequently(blue)than that predicted by the model;the dotted green line is the 95%confidence intervals predicted by the neutral model and R2 is the overall goodness of fit of the model.Nm=metacommunity size(N)×immigration(m);AOA=ammonia-oxidizing archaea;AOB=ammonia-oxidizing bacteria.
Major factors influencing variations of microbial communities associated with ammonia oxidation and denitrification between brackish and freshwater wetlands
To explore the environmental driving factors shaping the variations of ammonia-oxidizing and denitrifying microorganisms between freshwater and brackish wetlands,we performed a Mantel test between the microbial communities and the environmental factors(Fig.6).The results showed that,for ammonia oxidation,AOB community was significantly related to EC,TOC,pH,and temperature,and AOA community was also significantly correlated within addition to the above factors(Fig.6a).For denitrification,nirS-containing microbial community was mainly related to EC,pH,TOC,and water level.ThenirK-containing microbial community was also significantly correlated with DOC,,andin addition to the above factors(Fig.6b).After removing the physiochemical properties that were not statistically different across the different wetlands,we found that EC and TOC were the top two driving factors with high correlation coefficients with ammonia-oxidizing and denitrifying microbial communities.
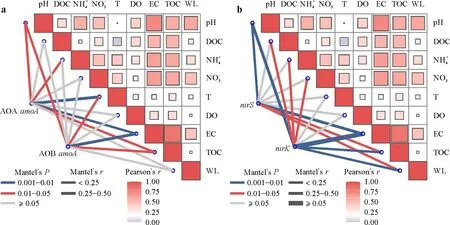
Fig.6 Relationships between environmental factors and the amoA(a),nirS,and nirK-containing(b)microbial communities by Mantel analysis.AOA=ammonia-oxidizing archaea;AOB=ammonia-oxidizing bacteria;EC=water electrical conductivity;WL=water level;DO=water dissolved O;T=water temperature;TOC=sediment total organic C;DOC=sediment dissolved organic C;and =sediment and contents,respectively;pH=sediment pH.
From VPA,we found that wetland types(freshwater and brackish wetlands)had a greater influence on the microorganisms involved in the ammonia oxidation and denitrificationthan seasons(Fig.7).Further analysis of wetland types,EC,and TOC within the context of microbial community shifts revealed that,regardless of combining EC and TOC,wetland types explained 17%,16%,27%,and 35%of the variations of AOA,AOB,nirS-containing,andnirK-containing microbial communities,respectively.However,wetland types independently accounted for only 6%,2%,2%,and 2%of respective changes.The impact of wetland types on the variation of microbial communities related to ammonia oxidation and denitrification decreased sharply without considering the combining contribution of physiochemical properties,revealing that EC and TOC were important factors underlying the differences of microbial communities related to the nitrogen cycle in brackish and freshwater wetlands.
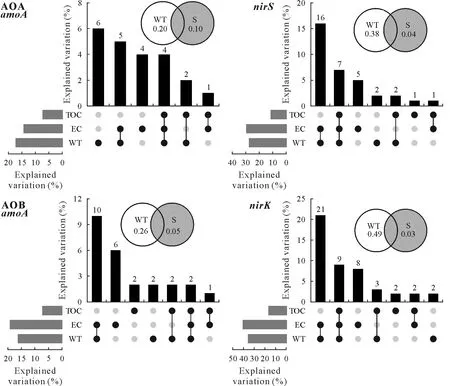
Fig.7 Variation partitioning models showing the effects of wetland types(WT)and seasons(S)and the effects of WT and important sediment physicochemical properties(electrical conductivity(EC)and total organic C(TOC))on the amoA,nirS,and nirK-containing microbial communities.The numbers in the circles represent the contributions of WT and S to the changes in N cycling-related microbial communities.The dot matrix and the corresponding column bar above it show the shared and exclusive contributions of WT,EC,and TOC to the variations of the N cycling-related microbial communities.AOA=ammonia-oxidizing archaea;AOB=ammonia-oxidizing bacteria.
DISCUSSION
Mechanisms underlying the variation of ammonia-oxidizing and denitrifying rates between brackish and freshwater wetlands
In our study,the ammonia-oxidizing rate of AOA was significantly lower in brackish non-marsh wetland than in freshwater non-marsh wetland,while the ammonia-oxidizing rate of AOB between these two wetlands showed opposite variation patterns(Fig.2a).The niche differentiation of AOA and AOB,caused by their physiological differences,might explain this phenomenon.Soil TOC,,and salinity play vital roles in the niche differentiation of AOA and AOB(Xiaet al.,2011;Guoet al.,2017).Specifically,AOA prefers an oligotrophic environment with lowconcentrations(Gubry-Ranginet al.,2010).The inhibition of ammoniaoxidizing rate of AOA could occur in a brackish wetland environment with highconcentrations (Verhammeet al.,2011).Similarly,the relatively higher salinity in brackish wetlands could also inhibit ammonia-oxidizing rate of AOA.Previous studies found that the abundance of AOA was negatively correlated with the salinity of salt marsh and estuarine wetlands(Mosier and Francis,2008;Moinet al.,2009).Thus,the relatively highand salinity levels could explain the lower ammonia-oxidizing rate of AOA in brackish wetlands than in freshwater wetlands,whether in marsh or non-marsh ecosystems.The elevated soil CO2supports more AOB than AOA,although both groups are autotrophic microorganisms (Xiaet al.,2011).In brackish wetlands,organic matter deposition and concentration caused by poor drainage and high evapotranspiration were more suitable for the proliferation of the AOB community,since the high soil TOC level could enhance the respiration rate of heterotrophic microorganisms(Enwallet al.,2007).This might promote the ammonia-oxidizing rate of AOB in brackish non-marsh wetland,making it higher than in freshwater non-marsh wetland.However,contrary to trends observed in non-marsh environments,brackish marsh wetland was characterized by a lower ammonia-oxidizing rate of AOB than freshwater marsh wetland(Fig.2a).Emergent plants widely distributed in marsh wetlands can import root-derived organic carbon,vulnerable to decomposition by soil microorganisms,into the rhizosphere through root exudates and root litter(Fanget al.,2019,2021).This process would enhance the decomposition rate of soil organic matter by 3 to 5 times(Yinet al.,2020),which could potentially increase the CO2content in the soil,whether in brackish or freshwater marsh wetlands.Thus,in this case,compared with freshwater marsh wetland,the adverse effects of salt stress in brackish marsh wetland on the ammonia-oxidizing rate of AOB emerged.
Our study found that total ammonia-oxidizing rate was significantly related to the ammonia-oxidizing rate of AOA,which was lower in brackish wetlands than in freshwater wetlands(Fig.2a).Additionally,the ammonia-oxidizing rate of AOA accounted for the majority of total ammonia-oxidizing rate,indicating that AOA might dominate the ammonia oxidation of wetlands in the Hulun Lake basin.Thus,the lower total ammonia-oxidizing rate in brackish wetlands,similar to the ammonia-oxidizing rate of AOA,might be mainly due to the inhibitory effects of high salinity andconcentration.The increasing salinity in brackish wetlands could decrease nitrification rate directly by limiting the physiological fitness of nitrifying microorganisms and indirectly by affecting the availability of soil oxygen (Zhouet al.,2017).However,there are some shortcomings in exploring the response of wetland nitrification to high evapotranspiration in this study:the nitrifiers involved in this study only included the typital ammonia oxidiziers AOA and AOB,while comammoxNitrospira,the complete ammonia oxidizers that might also play an important role in the nitrification process of wetland ecosystems (Yuanet al.,2021),were not covered in this study.Moreover,Liet al.(2019)found that 1-octyne has no inhibition on the activities of comammoxNitrospiraclade A in pasture and arable soils,while Linet al.(2023)found that 1-octyne inhibits the growth of comammoxNitrospirain acidic soil.There is currently no clear conclusion on whether 1-octyne has an inhibitory effect on the comammoxNitrospirain wetland soil.Regardless of whether 1-octyne has inhibitory effect on the comammoxNitrospira,the presence of comammoxNitrospirawas not considered in this study when 1-octyne was used as an inhibitor to exclusively inhibit the AOB,which might lead to an overestimation of AOA or AOB activity in this study.Thus,the response of comammoxNitrospirato high evaporation in inland wetlands should be considered in the further studies.
In our study,the denitrification rate was significantly greater in brackish non-marsh wetland than in freshwater non-marsh wetland in summer (Fig.2b).This could be attributed to the elevated TOC in brackish wetlands,providing organic substrates for denitrification microorganisms.This observation aligns with previous studies demonstrating that increased organic carbon availability can enhance soil denitrification rates (Panet al.,2022).However,in contrast to non-marsh environments,brackish marsh wetland exhibited lower denitrification rates than freshwater marsh wetland(Fig.2b).This indicated that despite the higher TOC levels in brackish marsh wetland,the lower denitrification rate might be primarily disturbed by salt stress.Salinity is a major catalyst within the context of low soil denitrification rates(Wanget al.,2018).Salt could directly interfere with microbial activity;salinity above a critical threshold can decrease soil enzyme activity and microbial respiration(Tripathiet al.,2006).Additionally,salt stress in brackish marsh wetland soils could cause growth inhibition,physiological drought,and root damage of emergent plants (Xieet al.,2020),potentially leading to decreased plant productivity and reduced input of root-derived carbon(Ghollarata and Raiesi,2007).This would then indirectly affect the denitrification activity in brackish marsh wetland to a lesser extent than that in freshwater marsh wetland.In line with this,Wanget al.(2018)found that the soil denitrification rates in the freshwater marsh wetland were significantly higher than those in the brackish marsh wetland.
Differences in ammonia-oxidizing and denitrifying microbial communities between brackish and freshwater wetlands
Previous studies have reported shifts in ammoniaoxidizing and denitrifying microbial communities in wetlands along salinity gradients (Franklinet al.,2017).We observed distinct microbiota involved in ammonia oxidation and denitrification in both marsh and non-marsh environments between brackish and freshwater wetlands(Fig.4).The primary reason for the significant difference may be due to the distinct environmental factors that characterize brackish and freshwater wetlands.We found that,similar to the change mechanism of ammonia-oxidizing rate,TOC and EC accounted for the differences of AOA and AOB communities between brackish and freshwater wetlands (Figs.6a and 7).Previous studies found that salinity and organic carbon could significantly influence ammonia-oxidizing microbial community structure(Santoset al.,2020;Tanget al.,2023).In our study,for the AOA community,we observed a lower relative abundance of ”Group I.1b”in brackish wetlands than in freshwater wetlands,suggesting that ‘Group I.1b’may be more sensitive to the salinity of brackish wetlands(Fig.3).For the AOB community,we found thatNitrosomonasclusters dominated in freshwater wetlands,whileNitrosospiraclusters prevailed in brackish wetlands(Fig.3),which may be primarily attributed to the salinity differences of the two wetland types.Previous studies have reported thatNitrosospiratypically predominates in high-salinity environments such as marine or sediment systems(Wanget al.,2018).
Furthermore,we found that,similar to the mechanisms underlying denitrification rate changes,the variation in denitrifying microbial communities was affected by TOC and EC between brackish and freshwater wetlands(Figs.6b and 7).Our findings were consistent with those of previous studies,which reported that soil organic carbon and EC were significantly related to the community structure of microbial communities involved in denitrification(Wanget al.,2018;Moulton-Brownet al.,2022).
The second reason for the difference in microbial community composition between freshwater and brackish wetlands may be that flowing freshwater in freshwater wetlands can acquire microorganisms from surrounding ecosystems.Rivers are dynamic wetland ecosystems with surface runoff flowing through villages,farmed lands,and grasslands,which would undoubtedly contribute to the deposition of microorganisms into rivers(Liuet al.,2022).However,the brackish wetlands are less affected by other ecosystems due to its relatively static water flow.
Mechanisms controlling the assembly of ammonia-oxidizing and denitrifying microbial communities in brackish and freshwater wetlands
We found that the neutral community model explained more than half of the variation in microbial communities involved in ammonia oxidation(AOA)and denitrification(nirS-andnirK-contianing)in both freshwater and brackish wetlands(Fig.5).This indicated that stochastic processes dominated the assembly of AOA and denitrifier communities in the wetlands of the Hulun Lake basin.Consistent with the finding of the neutral community model,the null model revealed that stochastic processes had the greatest contribution for the assembly of AOA and denitrifier communities in the two wetland types(Fig.S2),further confirming the profound influence of stochastic processes.The main reason behind the predominance of stochastic processes may be due to the fluidity of wetlands.Overlying water in wetlands can increase stochastic changes in the relative abundance of sediment microorganisms through the water-sediment interface,which may cause strong ecological drift and enhance the dominance of stochastic processes (Meyerhofet al.,2016;Zenget al.,2019).Additionally,the relatively high nutrient levels in the Hulun Lake basin could be resulting in weakened environmental filtration and release of microbial communities from environmental pressures tied to the nutrients acquisition(Maet al.,2022),which may increase the contribution of stochastic processes in wetland nitrogen cycling-related microbial communities.
In our study,although the assembly processes of ammonia-oxidizing and denitrifying microbial communities in freshwater and brackish wetlands were dominated by stochastic processes,deterministic processes played a more prominent role in brackish wetlands than in freshwater wetlands,as revealed by the null and neutral models(Figs.5 and S2).The habitat filtration hypothesis can explain this phenomenon,stating that the species able to enter and remain in a habitat from the regional species pool are determined by environmental factors,and specific habitats only accommodate species suited(i.e.,morphologically,physiologically,etc.)for living in that particular environment(Kraftet al.,2015).Salinity is the key factor influencing the microbial community structure,and only microorganisms with suitable functions and phenotypes for salt stress can survive in high-salt environments(Oren,2011;Zhanget al.,2019).Therefore,the elevated soil salinity in wetlands would enhance environmental pressure and exert stronger selection pressures on the soil microbial community,making deterministic processes more important in brackish wetlands than in freshwater wetlands(Zhanget al.,2019).Furthermore,we found that the habitat niche breadth of AOB and denitrifier microbial communities was narrower in brackish wetlands than in freshwater wetlands(Fig.6a,b),which further confirmed that ammonia-oxidizing and denitrifying microbial communities in brackish wetlands were more impacted by environmental filtration.
Mechanism of emergent plant growth on soil ammonia oxidation and denitrification in brackish marsh wetlands
In our study,although the ammonia-oxidizing and denitrifying rates of brackish marsh wetland were significantly lower than those of freshwater marsh wetland,both rates increased in brackish marsh wetland compared to brackish non-marsh wetland.This indicated that emergent plants played a crucial important role in mitigating the adverse effects of salinity on ammonia oxidation and denitrification in brackish wetlands.Boyrahmadi and Raiesi(2018) demonstrated the positive impact of plant roots and their secretions on the microbial activity and biomass of saline soil.Thus,the primary mechanism,by which the emergent plants in brackish wetlands alleviate the negative effects of high salinity on microbial communities,may involve the input of plant-derived carbon substrates (Boyrahmadi and Raiesi,2018).Specifically,the organic carbon fixed by wetland plantsviaphotosynthesis is finally transferred to rhizosphere by root exudates and plant litter,increasing labile forms of carbon substrates in the rhizosphere,which could enhance microbial activityviathe roots(Fanget al.,2019,2021;Herreet al.,2022).Our research supports this hypothesis,as we observed higher organic carbon content in brackish marsh wetland compared to brackish non-marsh wetland(Table I).Furthermore,halophytic plants,such asPhragmites australis,have the ability to accumulate salt concentrations in their leaves,which can equal or surpass those found in seawater.This adaptation helps reduce salinity and prevent marsh salinization(Boyrahmadi and Raiesi,2018).Our study provides evidence for this phenomenon,as we observed significantly lower EC in brackish marsh wetland compared to brackish non-marsh wetland(Table I).Consequently,emergent plants in brackish marsh wetland might alleviate the salt stress by creating more favorable soil nutrient conditions and microhabitats.This,in turn,promotes the activity and growth of ammonia-oxidizing and denitrifying microorganisms(Iwaokaet al.,2018).
CONCLUSIONS
In summary,this study investigated the characteristics of changes in soil nitrogen cycling-related microbial communities in inland wetlands under the backdrop of increased evapotranspiration due to global warming.Our findings indicate that heightened evapotranspiration under global warming may alter the activities and community characteristics of microorganisms involved in ammonia oxidation and denitrification in certain inland wetlands by increasing salinity and nutrient levels and altering hydrology,ultimately affecting the global wetland nitrogen cycle.Moreover,the growth of emergent plants in brackish marsh wetland can mitigate the adverse effect of salinity stress on ammonia oxidizers and denitrifiers.To more accurately assess the impact of global warming on nitrification processrelated microorganisms in inland wetlands,future studies should focus on elucidating the response of comammoxNitrospirain wetlands to increased evapotranspiration.Our results underscore the severity of global warming impact on nitrogen cycling processes in inland wetlands and the necessity of promoting emergent plant growth in these ecosystems to maintain wetland nitrogen cycling functions in the context of global climate change.
ACKNOWLEDGEMENTS
This work was supported by the Natural Science Foundation of Shandong Province,China(Nos.ZR2022QC146 and ZR2021YQ22),the National Natural Science Foundation of China(Nos.31872242,32070405,32270444,and 32200349),and the Colleges and Universities Youth Innovation Science and Technology Teams Support Program of Shandong Province,China(No.2021KJ015).We thank staffat the Management Station of the Inner Mongolia Hulun Lake National Nature Reserve for helping with sample collection.We would like to thank Editage for English language editing.
SUPPLEMENTARY MATERIAL
Supplementary material for this article can be found in the online version.
CONTRIBUTION OFAUTHORS
Jiaohui FANG and Tianshu LÜ contribute equally to this work.
- Pedosphere的其它文章
- Removal of atmospheric methane by soil ecosystems and its controlling variables from microbial to global scales
- Preface:Special issue on soil ecology and sustainability for celebrating the 70thanniversary of ISSCAS
- Assessment of soil total phosphorus storage in a complex topography along China’s southeast coast based on multiple mapping scales
- Application of controlled-release urea increases maize N uptake,environmental benefits and economic returns via optimizing temporal and spatial distributions of soil mineral N
- Effects of herbicide butachlor application on the growth of periphytic biofilms and nitrogen loss in paddy systems
- Global patterns of soil phosphatase responses to nitrogen and phosphorus fertilization

