Human brain organoid: trends,evolution,and remaining challenges
Minghui Li ,Yuhan Yuan ,Zongkun Hou ,Shilei Hao,*,Liang Jin,*,Bochu Wang,*
Abstract Advanced brain organoids provide promising platforms for deciphering the cellular and molecular processes of human neural development and diseases.Although various studies and reviews have described developments and advancements in brain organoids,few studies have comprehensively summarized and analyzed the global trends in this area of neuroscience.To identify and further facilitate the development of cerebral organoids,we utilized bibliometrics and visualization methods to analyze the global trends and evolution of brain organoids in the last 10 years.First,annual publications,countries/regions,organizations,journals,authors,co-citations,and keywords relating to brain organoids were identified.The hotspots in this field were also systematically identified.Subsequently,current applications for brain organoids in neuroscience,including human neural development,neural disorders,infectious diseases,regenerative medicine,drug discovery,and toxicity assessment studies,are comprehensively discussed.Towards that end,several considerations regarding the current challenges in brain organoid research and future strategies to advance neuroscience will be presented to further promote their application in neurological research.
Key Words: bibliometric analysis;brain organoids;cerebral organoids;global trends;neuroscience
Introduction
Rapid developments in human stem cell and threedimensional (3D)in vitroculture technologies pave the way for studies investigating human organogenesis and pathological mechanisms outside of the human body (Corsini and Knoblich,2022;Tang et al.,2022).Human organoids are 3D cell aggregates derived from human embryonic stem cells or induced pluripotent stem cells (iPSCs),which exhibit the intrinsic capacity to self-assemble into multi-layered structures (Yamanaka 2012).The cell aggregates,named embryoid bodies,contain three embryonic germ layers that mimic early human embryogenesis.So far,a huge diversity of organoids have been generated,including brain,retina,lung,liver,gastrointestinal,heart,and kidney organoids (Barkauskas et al.,2017;Nishinakamura,2019;Prior et al.,2019;Hofbauer et al.,2021;Cordella et al.,2022;Hou et al.,2022;Kelava et al.,2022;Scott and Huang,2022;Bouffi et al.,2023;Wahle et al.,2023;Figure 1).Stem-cell-derived organoids recapitulate the cellular and architectural complexity of developing organs,providing platforms with great potential for exploring organogenesis and diseases (Yamanaka,2012;Clevers,2016;Corsini and Knoblich,2022).Unlike other organs or tissues,understanding the processes involved in human neurogenesis and neural disorders is one of the most fascinating challenges in biology,as the brain is the body’s most intricate and complex organ (Koo et al.,2019;Qian et al.,2019).However,the largest challenge in revealing the cellular and molecular mechanisms of human brain organogenesis has been the inaccessibility of human embryonic/fetal tissues/organs at crucial gestational periods,which has impeded studies of human neural development and diseases (Kelley and Pasca,2022).Recent biotechnological advancements in brain organoids have already contributed enormously to deciphering the biological events occurring in the early stages of human neurogenesis and disease progression (Miura et al.,2022;Li et al.,2023a;Tang et al.,2022;Dixon and Muotri,2023).

Figure 1|Organoid generation from induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs).
Over the past 10 years,brain organoids have been used to reap plentiful and substantial rewards in neuroscience research aiming to better uncover human brain evolution and neural health.Accompanying the development of the technology,a significant number of papers have been published by worldwide institutions and laboratories.To gain deeper insights into the global trends in brain organoid research and further promote their development,we first conducted a bibliometric study of brain organoid-related articles.Bibliometric analysis,using methodologies to process large amounts of published literature,has been used to evaluate the scientific research activity in particular fields and further assess and predict research hotspots and frontier trends (Kamdem et al.,2019;Donthu et al.,2021;Xu et al.,2022;Zhu et al.,2022).With the aid of bibliometrics and visualization methods,it is easy to identify the annual trends in publications and citations,the most actively contributing countries/regions/journals/organizations/groups,and cocited references (Chen et al.,2012,2014;Zhu et al.2022;Li et al.,2023c).Α detailed overview of the research hotspots and trends can be presented by analyzing keyword co-occurrences and citation bursts.Therefore,in our study,bibliometric analysis was conducted based on data obtained from the Web of Science (WoS) Core Collection.Subsequently,the annual growth trends in brain organoid publications and citations and the countries/regions most actively contributing to brain organoid research were visualized based on WoS data.Then,the most contributing institutions,journals,and influential authors were analyzed using VOSviewer.Moreover,current brain organoid applications in neuroscience research,including human neural development,neural disorders,infectious diseases,regenerative medicine,drug discovery,and toxicity assessment,are comprehensively discussed in this paper.Finally,several considerations regarding the current challenges in brain organoid development and use and future advances in the neurological field will be also discussed.The aims and objectives of this study were to provide comprehensive a review of the use of brain organoids in neuroscience and to promote the development of neurological research.
Bibliometrics Analysis
Data source and methodology
The metadata used in this review were obtained from the WoS database (https://www.webofscience.com/wos/alldb/advanced-search).The process was limited to the WoS Core Collection from the Science Citation Index Expanded (SCIE)and Social Sciences Citation Index (SSCI).Advanced Search option: Topic (TS)=(“cerebral organoid”OR “brain organoid”OR “cerebral organoids”OR “brain organoids”OR “cerebral spheroid”OR “brain spheroid”OR “cerebral spheroids”OR“brain spheroids”),within the period from 2013 to June 30,2023.Α total of 2186 literature records (written in English)were obtained after the search.The annual publications and citations were extracted from the WoS data and managed using Microsoft Excel for 365 MSO (Redmond,WΑ,USΑ,Version 2206 Build 16.0.15330.20260) 64-bit.VOSviewer software (The Centre for Science and Technology Studies of Leiden University,The Netherlands,Version 6.1.R2,64-bit) was used to analyze the contributing organizations and journals,authors,co-citations,and keywords to form visualization maps.
Results and discussion
Annual publications
A total of 2186 documents were identified based on the WoS database,including 875 articles,574 review articles,125 meeting abstracts,and 91 editorial materials,and we retrieved a total 52,545 of citations up to June 30,2023.The patterns of annual publication numbers,per-year citations,and per-year H-Indexes are shown inFigure 2A.The successful development of brain organoids was first reported in 2013 by Knoblich’s group (Lancaster et al.,2013).Since the pioneering study,publication and citation numbers exhibited an increasing trend year by year.As can be seen,there was a tremendous growth in publications since 2016,suggesting that brain organoid research was receiving increasing attention from scientists.Moreover,the annual number of citations exceeded 13,798 in 2022,indicating the growing interest in brain organoids.The decreased number of publications and citations in 2023 might have been caused by the incomplete statistics and/or the limited utility of brain organoids due to their limitations in heterogeneity,immaturity,and variability(Chiaradia and Lancaster,2020;Andrews and Kriegstein,2022).

Figure 2|Publication statistics for brain organoid research.
As with other advances in neuroscience,brain organoids have received widespread attention.Α total of 63 countries/regions are closely associated with brain organoid studies.The leading countries/regions (top 10) presented inFigure 2Bwere examined to determine their productivity and scientific influence on brain organoids.Αs indicated,the United States of Αmerica was the most actively contributing country to brain organoid science with 762 papers,followed by China (195 papers),Germany (192 papers),Italy (124 papers),England(116 papers),South Korea (90 papers),Canada (48 papers,3.89%),Japan (65 papers),the Netherlands (60),and France(58 papers).Meanwhile,in terms of the publications with H-Index,the USA was followed by Germany,England,China,etc.However,among these countries/regions,papers from England received the highest number of citations,with 28,617 citations (65.53 times per paper).
Most contributing institutions, authors, and publicationsΑ total of 1820 organizations worldwide contributed to brain organoid research.Organizations with publication numbers of more than 5 (204 organizations) were visualized using VOSviewer,as shown inFigure 3A.The overlay visualization maps of 204 organizations were constructed based on the average number of publications per year from 2013 to 2023.Αmong these organizations,the Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA),the University of Edinburgh,and the Max Planck Institute of Molecular Cell Biology and Genetics were most active in the earliest years of brain organoid-based research.This is consistent with the organizations that produced the earliest publications on the successful generation of brain organoids.Moreover,the publications from these organizations received higher numbers of citations compared to those of other organizations,as indicated by the size of the nodes in the network (a larger node represents higher citation numbers).The top 10 most influential organizations (in terms of publication numbers) are listed inFigure 3B.As indicated,the University of California,San Diego,contributed the largest number of publications (72 documents),followed by the University of California,San Francisco (48 papers);Chinese Academy of Sciences (42 papers);Harvard Medical School(42 papers);Harvard University (39 papers);Johns Hopkins University (38 papers accounting);University of Pennsylvania(34 papers);Stanford University (29 papers);the University of Milan (29 papers);and University of Chinese Academy of Sciences (28 papers).Over half of the organizations were from the USΑ.However,the publications from IMBΑ received the most citations,5135 times,even though only 14 papers were published (Additional Table 1).This could have been because IMBA published the first brain-organoid-related paper,which has been cited over 2700 times.These results further highlight the contributions of the pioneers in brain organoid studies.The visualization maps also show the broad range of collaborations among the organizations,suggesting that worldwide organizations enjoy close collaborative relationships with others in the brain organoid research field.

Table 1|The top 10 co-cited references and co-citations for brain organoid research

Figure 3|Contributions of world organizations to brain organoid research.
A total of 8191 authors were associated with brain organoid research.Additional Table 2shows the top 10 most highly productive authors according to the publication citation numbers.The 25 papers from Juergen Knoblich’s group have been cited 6898 times in total (average 275.92 times per paper),followed by those by Madeline Lancaster (6071 citations,average 242.84 times per paper) up until June 30,2023.Interestingly,Prof.Lancaster used to work in Prof.Knoblich’s lab at IMBA,Austria.Knoblich is best known for his work in genes,genetics,and cellular differentiation.It is worth mentioning that Lancaster’s lab was the first to establish a protocol to create cerebral organoids in 2013.They generated a cerebral organoid that was applied to model human brain developmental events and neural diseases such as microcephaly (Lancaster et al.,2013).The unguided brain organoid methodologies developed by Knoblich’s group could be used to generate whole-brain organoids that contained the forebrain,midbrain,and hindbrain,and provide a platform for exploring cell-type diversity (Lancaster et al.2013).Αlthough diverse brain regions have been identified within the same organoids,their development hindered by their high variability and heterogeneity (Qian et al.,2019;Chiaradia and Lancaster,2020).Thus,protocols have been developed and adapted to instruct stem cells to establish brain-region-specific organoid models (Lancaster et al.,2017;Qian et al.,2018;Sloan et al.,2018).Guided brain organoid differentiation also highlights the critical role of growth factors during brain development.The third and fourth positions in terms of the number of publication citations were Hongjun Song and Guo-Li Ming (23 papers and 24 papers,respectively).It is also worth noting that both Hongjun Song and Guo-Li Ming worked in the Institute for Cell Engineering,departments of Neurology and Neuroscience,Johns Hopkins University School of Medicine,and they published several cooperative publications on brain organoid research.For instance,“Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure”(Qian et al.,2016) was published in Cell in 2016.They focused on the production of human brain-region-specific organoids,such as forebrain,midbrain,and hypothalamic organoids,and used the organoid systems to model neurological disorders as well as virus infections,such as Zika virus (ZIKV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2;Qian et al.,2016;Jacob et al.,2020;Huang et al.,2021;Zhang et al.,2021).Brain development modeling continued to advance,as for example,Pasca’s group optimized neural assembly technologies to explore neuronal interactions and migration between different brain regions (Miura et al.,2020;Birey et al.,2022;Birey and Pașca,2022;Kelley and Pasca,2022;Miura et al.,2022;Pașca et al.,2022).Αlthough Sergiu Pasca (15 papers) is in the ninth position based on document citation numbers,his brilliant contributions to optimizing brain assembloid methodologies are very apparent.Interestingly,in terms of publication numbers,Dr.Alysson R.Muotri,a professor in the departments of Pediatrics and Cellular and Molecular Medicine at the University of California,San Diego,has published the most papers (27 publications) in brainorganoid-based research (Additional Table 3).Muotri’s lab created the first “archealized”human brain organoid platform to study human brain evolution (Muotri,2021).Using brain organoids as evolutionary tools and integrating cutting-edge genome-editing technologies will likely illuminate the origins of human neural disorders (Adams et al.,2019;Muotri,2019,2021;Trujillo et al.,2021).
Co-citation analysis has been used to highlight documents with highly frequent citations in the research field and guide researchers about future developments (Yang et al.,2019).Therefore,co-citation analysis of brain-organoid-based documents was conducted using VOSviewer.Table 1shows the top 10 most co-cited references.Lancaster et al.’s article“Cerebral organoids model human brain development and microcephaly”(Lancaster et al.,2013) published inNaturein 2013 had the highest number of co-citations (992 times).From the same group,the paper “Generation of cerebral organoids from human pluripotent stem cells”(Lancaster and Knoblich 2014) published inNature Protocolsin 2014 also received a high number of co-citations,evidencing the outstanding contributions of Knoblich’s team to brain organoid research.Both papers focused on remodeling human brain developmentin vitro,as well as disease models such as microcephaly.Similarly,a study by Qian’s group showed that brain-region-specific organoids formed in a miniature spinning bioreactor can be used to model ZIKV infections(Qian et al.,2016).In addition,brain organoids derived from iPSCs from autism spectrum disorder (ASD) patients were used to investigate neurodevelopmental alterations (Mariani et al.,2015).Based on the successful generation of human brain organoid model systems,functional cortical neurons and astrocytes,neuron diversity and network dynamics,axial polarity and the inside-out layer pattern,and the gene expression programs of neurons have been recapitulated inin vitro(Camp et al.,2015b;Kadoshima et al.,2013;Paşca et al.,2015;Quadrato et al.,2017).Moreover,next-generation brain organoid systems in the form of assembloids and vascularized brain organoids have been generated by Birey et al.,2017 and Mansour et al.,2018,respectively.
Most influential journals and co-occurrence of keywords
Α total of 540 journals have published documents on brain organoids.Of these,91 journals with five or more publications are visualized inFigure 4Abased on citation numbers.The number of publications and total citations are listed inAdditional Table 4.As shown inFigure 4B,the journal with the most cited papers wasNature(15 papers with 5554 citations),followed byCell Stem Cell(41 papers with 4532 citations),Cell(15 papers with 2253 citations),Cell Reports(19 papers with 1574 citations),etc.In terms of average citations,the top five journals wereNature,Nature Protocols,Cell,Nature Biotechnology,andScience.Obviously,papers from these journals have played central roles in brain organoid research.Keywords are critical parts of the article that directly represent its main concepts.A study of keyword co-occurrence using VOSviewer can help to identify the core topics of publications(Zhu et al.,2021).Α density map visualization of keywords cooccurring at a frequency of no fewer than five was generated through the VOSviewer application and is shown inFigure 5.The frequently emerging keywords were organoids,brain or cerebral organoids,induced pluripotent stem cells,brain organoid,disease modeling,and stem cells.The colors signal differences among the hot topics,and different clusters are identified in brain organoid research.For instance,the green cluster represents microcephaly,ZIKV,and autism.Brain organoids have been successfully generated to investigate human brain development and disorders such as microcephaly,ASD,Parkinson’s disease,and Rett syndrome.Keywords such as ZIKV,flavivirus,SΑRS-CoV-2,and HIV (human immunodeficiency virus) show that brain organoids have been widely applied for modeling virus infection.Moreover,the cluster in orange represents the application of brain organoids in regenerative medicine,precision medicine,personalized medicine,drug discovery,and neurotoxicity.The overlay visualization map (Additional Figure 1) displays the evolution of keywords over time from 2013 to 2023.The node color shows the average number of papers year (blue shows that keywords appeared frequently earlier,and red indicates the keywords were more popular recently).For instance,the keywords microcephaly,ZIKV,autism,drug screening,induced pluripotential stem cells,and development are more frequent at an earlier time in brain organoid research,suggesting that early brain organoid models were used to investigate neural development,as disease models,and in drug discovery.As organoid technology is continuously innovating,advanced bioengineering strategies have been utilized to promote the development of brain organoids,as illustrated by organ-on-achip,microfluidic,and biomaterial studies.Since the outbreak of coronavirus disease 2019 (COVID-19),brain organoids have been widely applied to investigate infection by SARS-CoV-2.Thus,keywords,such as COVID-19 and SARS-CoV-2,are more common recently.Therefore,this map presents a simple but helpful way to reveal the scientific trends relating to the use of brain organoids in the neuroscience field.

Figure 4|Network of the top contributing journals.

Figure 5|Network of keywords co-occurring with a frequency of no fewer than 5 in brain organoid research.
Application of Brain Organoids in the Neuroscience Field
The rapid development of brain organoid technologies has promoted their application in multiple fields within neuroscience (Zhang et al.,2023b).To further illustrate the diversity and development of brain organoids,their previous application in human neural development,neural disorders,infectious diseases,regenerative medicine,drug discovery,and toxicity evaluation (Figure 6) studies is reviewed in this chapter.
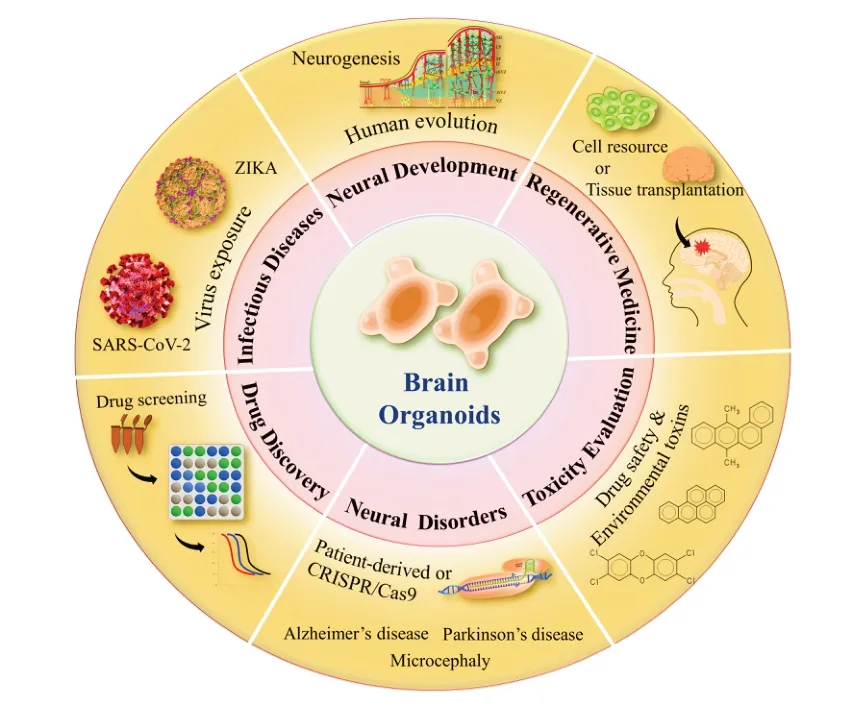
Figure 6|Overview of brain organoid technology applications
Brain organoids in human neural development
The most striking difference between the brains of rodents and humans is their cortical development (Lui et al.,2011).Unlike rodents,the specific outer subventricular zone (oSVZ) in the developing human cerebral cortex contains a tremendously large population of outer radial glia cells (oRGCs),which contribute to human cortex size and complexity (Hansen et al.,2010;Lui et al.,2011).oRGCs were found in human organoid models that present a well-organized progenitor zone with an oSVZ layer (Qian et al.,2016;Rosebrock et al.,2022).The organoid also expressed specific markers that are found in the human cortical layer,and cells exhibited the functional characteristics of mature neurons.It is worth mentioning that the genetic features of the human cortex can be accurately recapitulated in cortical organoids,as illustrated by single-cell RNΑ sequencing (Camp et al.,2015).Moreover,the recently developed organoid fusion technique provides a unique tool to investigate neural events,such as interneuron migration and neuronal long distance-projections (Bagley et al.,2017;Xiang et al.,2019;Andersen et al.,2020;Miura et al.,2020,2022;Kelley and Pasca,2022).For instance,by fusing human thalamus-like brain organoids and cortical-like brain organoids,Xiang et al.(2019) established a 3D model that recapitulates the reciprocal projections between the thalamus and cortex.A highly ordered and correctly arranged neural network can help researchers study electrical activity in the developing human brain.Similarly,human cortico-striatal assembloids assembled from cortical spheroids and striatal spheroids were shown to contain cortical neurons that projected long-range axons into striatal organoids and formed functional synaptic connections (Miura et al.,2020).More recently,Lancaster’s lab found that androgens specifically increased the neurogenic output of excitatory neuronal progenitors in human brain organoids (Kelava et al.,2022).From these findings,we foresee an unprecedented opportunity to recapitulate humanspecific neurodevelopmental events that cannot be studied in experimentally intractable species.
Brain organoids in human neural disorders
In addition to revealing the mechanisms underlying neurodevelopmental processes,brain organoids provide a promising approach to studying neural diseases,such as microcephaly,Alzheimer’s disease,Parkinson’s disease,ASD,schizophrenia,and bipolar disorder (Quadrato et al.,2016;Αmin and Pasca,2018;Cerneckis et al.,2023;Dixon and Muotri,2023;Zhang et al.,2023c).iPSC technology,i.e.,converting adult human somatic cells into iPSCs by the introduction of reprogramming factors (Takahashi et al.,2007;Yu et al.,2007),provides a unique opportunity to investigate neuropsychiatric diseases by generating patient iPSC-derived brain organoids.Previous studies in the neuropsychiatric field have been mostly hindered by human tissue/organ inaccessibility and the lack of appropriate models because of the remarkable structural and functional differences between human and animal brains (Wang et al.,2020a).iPSCs can capture the genetic diversity of patients and help model the pathogenesis of diseases caused by genetic variants.In 2013,patient-specific iPSCs were first used to create a human brain organoid model of a neurodevelopmental disorder,microcephaly (Lancaster et al.,2013).This work in microcephaly patient-derived brain organoids discovered that radial glia progenitors in progenitor zones fail to properly expand during premature neural differentiation,resulting in a small brain.Both genetic factors and environmental stressors,such as infections and toxic chemical exposure,can result in microcephaly during pregnancy.For instance,an infection of developing forebrain organoids with ZIKV induced cell death and reduced cell proliferation,causing neuronal loss and volume change that reassembled microcephaly (Qian et al.,2016).To investigate neuropsychiatric disease,idiopathic ASD-patient-derived forebrain (telencephalic) organoids from patients were generated by Mariani et al.(2015).The brain organoids showed the overproduction of GABAergic neurons,which might have been caused by an early increase in forkhead box G1 (FOXG1) expression.Recently,more ΑSD risk genes,such as SUV420H1 (or KMT5B),ΑRID1B,and CHD8,have been found to contribute to ASD pathology using organoid models (Paulsen et al.2022).However,the challenges involved in brain organoid modelling of neuropsychiatric disorders need to be addressed.For instance,current brain organoids cannot fully characterize the latestage human brain,and neuropsychiatric diseases typically manifest in later fetal or postnatal development (Quadrato et al.,2016).Appropriate strategies to improve brain organoid maturation would significantly promote their usefulness in neural disorder studies.
Patient-derived brain organoids provide advantageous platforms to model hereditary neurodevelopmental diseases and offer remarkable flexibility for studying gene therapeutic strategies.For example,Lancaster et al.(2013) used patientderived brain organoids to recapitulate the CDK5RΑP2 mutation-dependent pathogenesis of microcephaly;however,the phenotype of the disease was reduced by reintroducing the CDK5RΑP2 protein.Similarly,iPSCs from patients with Pitt-Hopkins Syndrome (with the TCF4 mutation) were employed to create brain organoids,which showed a decreased number of cortical neurons and impaired electrical activity (Papes et al.,2022).More importantly,a reversal of phenotypic abnormalities was found after genetic correction with a CRISPR-based trans-epigenetic strategy.Recently,CRISPR-associated protein 9 (Cas9) technologies have been extensively used for gene therapy owing to their highly efficient gene editing ability,providing great potential for personalized treatments with genetically corrected iPSCs (Maeder and Gersbach,2016;Liao et al.,2017).This evidence highlights the ability of brain organoid models to offer unique opportunities to develop individualized treatments for patients with neural diseases and the formulation of personalized therapies.
Brain organoids in human neural infections
Brain organoids have been extensively applied to conduct studies into diverse virus infections,such as ZIKV,SΑRS-CoV-2,human cytomegalovirus,and HIV (Su et al.,2021;Fan et al.,2022b;Priyathilaka et al.,2022;Ostermann and Schaal,2023).ZIKV can be passed from an infectious mother to the fetus,leading to congenital Zika syndrome,which includes microcephaly and fetal growth restriction (Rasmussen et al.,2016).Hongjun Song and Guo-Li Ming first successfully utilized brain-region-specific organoids to explore ZIKV-mediated pathogenesis (Qian et al.,2016).Since this pioneering study,ZIKV has been found to preferentially target neural progenitor cells (NPCs),oligodendrocyte progenitor cells,and glial precursors (Cugola et al.,2016;Gabriel et al.,2017;Li et al.,2018;Priyathilaka et al.,2022).Moreover,ZIKV was shown to hijack host cells to facilitate their replication and assembly,resulting in increased viral copy numbers in infected brain organoids (Dang et al.,2016;Priyathilaka et al.,2022).ZIKVinfected macrophage or microglia-like cells acted as vectors to transmit the virus to vulnerable neurons in human organoids(Mesci et al.,2018;Muffat et al.,2018).Given the neurological complications of SΑRS-CoV-2 infections during the COVID-19 epidemic,brain organoids,such as cortical,hypothalamic,hippocampal,and midbrain organoids,have been applied to investigate the neurotropism of SARS-CoV-2 (Jacob et al.,2020;Pellegrini et al.,2020;Ramani et al.,2020;Zhang et al.,2020;Wang et al.,2021b;Ostermann and Schaal,2023).Αngiotensinogen 2 has been identified as a key host receptor for SARS-CoV-2 and is highly and specifically expressed in choroid plexus epithelial cells,leading to their relatively high and productive infection by SARS-CoV-2 compared with the sparse infection of neurons and glial cells in brain organoids(Jacob et al.,2020;Pellegrini et al.,2020).This evidence is consistent with the dysregulation of the choroid plexus found in postmortem human adult brain tissue (Yang et al.,2021).These data demonstrated that brain organoids can be used to model central nervous system pathologies of viral infection and provide new insights into the potential neurotoxic effects of viruses.Considering the critical roles of immune cells in SARS-CoV-2 infection,Samudyata and colleagues revealed that SARS-CoV-2 promotes microglia synapse elimination in microglia-containing brain organoids (Samudyata et al.,2022).Moreover,a brain organoid model with microglia has been applied to study infections with other virus,such as HIV,ZIKV,Rubella virus,and herpes simplex virus (Gumbs et al.,2022;Qiao et al.,2023;Retallack et al.,2023;Rybak-Wolf et al.,2023;Xu et al.,2021).Thus,advanced brain organoids with immune cells can be used to help decipher the pathogenic mechanisms underlying virus-induced neurological diseases.
Brain organoids in drug discovery
Based on disease modeling,brain organoids have been used for screening potential therapeutic drugs (Nowogrodzki,2018;Salick et al.,2021;Zhou et al.,2023).For instance,Xu et al.(2016) identified two small-molecule compounds,emricasan (a pan-caspase inhibitor) and niclosamide (an FDAapproved anthelmintic drug),that effectively protect NPCs from ZIKV-induced cell death.The two-drug combination treatment was found to cause a reduction in ZIKV replication.Another study conducted by Li et al.(2017a) demonstrated the protective role of cholesterol-25-hydroxylase against ZIKV infection in human cortical organoids.The enzymatic product of cholesterol-25-hydroxylase,25-hydroxycholesterol,was found to inhibit ZIKV infection and prevent tissue damage in human cortical organoids,as well as mice and macaques.Moreover,enoxacin exerts anti-ZIKV activity and circumvents ZIKV-induced microcephalic phenotypes in brain organoids (Xu et al.,2019).These data underscore the efficacy of brain organoid models in compound screening anti-ZIKV drugs.Brain organoids are also applied in preclinical AD drug discovery.Treatment of AD-like organoids with β-and γ-secretase inhibitors was found to significantly reduce amyloid and tau pathology (Raja et al.,2016).Via their application in SARS-CoV-2 infection studies,brain organoids have promoted the discovery of drugs that prevent and treat brain-related COVID-19 symptoms.Although there is evidence that sofosbuvir (an FDA-approved nucleotide polymerase inhibitor) treatment can rescue the neurological impairments in infected brain organoids,further clinical studies are urgently needed (Mesci et al.,2020).Recently,Song et al.(2021) showed that neuronal infection by SARS-CoV-2 is inhibited by blocking angiotensinogen 2 with IgG antibodies or COVID-19 patient-derived cerebrospinal fluid.However,more efforts are required to identify drugs effective against SARSCoV-2 infection that can mediate virus-induced neurological complications.Given the high cost and complex process of drug discovery,high-content screening systems,such as disease-related brain organoids combined with mathematical modeling,have been established to accelerate drug discovery and testing for neurological disorders (Park et al.,2021a;Pasteuning-Vuhman et al.,2021).
Brain organoids in toxicity assessments
The central nervous system is extremely sensitive and vulnerable to exogenous substances.Disturbances caused by chemicals may induce abnormal developmental processes in the brain,ultimately leading to neural disorders (Fan et al.,2022a).With brain organoids that recapitulate key events in the developing brain,brain organoid models have opened up new avenues for drug-and chemical-related neurodevelopmental toxicity assessments (Schwartz et al.,2015;Caporale et al.,2022;Seo et al.,2022;Wang et al.,2023a;Yang et al.,2023).For instance,neocortical organoids were exposed to cocaine to mimic prenatal cocaine exposure(Lee et al.,2017).The results showed that CYP3Α5 mediated the adverse influence of cocaine on neocorticogenesis,including reactive oxygen species production,inhibition of neocortical progenitor cell proliferation,premature neuronal differentiation,and neurodevelopmental disruptions (Lee et al.,2017).Similarly,brain organoids were applied to probe the toxicity of prenatal alcohol exposure in early neurodevelopment (Zhu et al.,2017;Arzua et al.,2020).With ethanol exposure,brain organoids exhibited disrupted neurogenesis,as indicated by abnormal neural differentiation and attenuated neurite outgrowth (Zhu et al.,2017).Moreover,a human brain organoid-on-a-chip system was used to mimic prenatal nicotine exposure (Wang et al.,2018).Abnormal neuronal differentiation and migration and disordered regionalization were found in nicotineexposed brain organoids.These data might facilitate a better understanding of the neurodevelopmental toxicity of active or passive exposure to toxic substances during pregnancy.Given that microplastic (MP) pollution is believed to pose a threat to human beings,diverse human organoids have been generated to evaluate the potential toxicity of MP exposure to humans(Bredeck et al.,2022;Cheng et al.,2022;Li et al.,2023b).For example,Hua and colleagues investigated the toxicity of polystyrene (PS)-MPs to human forebrain development (Hua et al.,2022).The study elucidated that short-term PS-MP exposure induced cell proliferation,while long-term exposure decreased cell viability in forebrain cerebral spheroids.The toxicity of PS-MPs to neural development exhibited sizeand concentration-dependent effects (Hua et al.,2022).In addition to toxic chemicals,brain organoids provide a valuable tool for toxicity assessment in drug discovery (Chhibber et al.,2020).Recently,the brain physiome concept has emerged that bridges brain organoids with in silico modeling to predict the safety and toxicity of unknown drugs (Seo et al.,2022).This high-throughput test platform is advocated as a way to promote new drug discoveries.Thus,studies indicate that human brain organoids provide invaluablein vitrosystems that are superior for assessing chemical and drug toxicity compared with animal models.
Brain organoids in regenerative medicine
Brain organoids have emerged as potential sources of cells for cell-replacement therapies and transplantable tissues for regenerative medicine treatment of injured or diseasedtissues (Shirai et al.,2016;Chen et al.,2019;Dong et al.,2020;Tang et al.,2022;Jgamadze et al.,2023).Organoid-derived NPCs can not only differentiate into target cells that can replace damaged neural cells but also promote endogenous neurogenesis and stimulate endogenous repair mechanisms(De Feo et al.,2012).The transplantation of human retinal organoid-derived retinal progenitors (C-Kit+/SSEA4-) into a degenerative rodent retina ameliorated the visual function and protected the retinal structure of the rodents (Zou et al.,2019).It has been shown that cerebral organoid transplantation has more advantages than cell suspension transplantation (Wang et al.,2020b),and successful transplants of brain organoids have been performed.For instance,Mansour et al.(2018) successfully established a method for transplanting whole-brain organoids into the adult mouse brain.The organoid graft displayed axonal outgrowth into the host brain with synaptic connectivity and developed functional neuronal networks and blood vessels.Similarly,Revah et al.(2022) engrafted intact human cortical organoids into the S1 of early-postnatal immunocompromised rats.Neurons from human cortical organoid grafts matured and engaged with host circuits associated with rat rewardseeking behaviors.Furthermore,Wang et al.(2020b) found that cerebral organoid transplantation in rats ameliorated neurological motor function and reduced traumatic brain injury.Recently,a cortical organoid graft was found to integrate structurally and functionally with the injured adult rat visual system,showing a translation strategy for restoring cortical function (Jgamadze et al.,2023).These cases indicate that brain organoids could provide novel therapeutic strategies for neural disorders and diseases.Despite these potential advantages,the cross-species limitations and ethical concerns over the feasibility of cell therapy and transplantation in clinical applications are still unclear.Substantial efforts are needed to fully describe human brain evolution and the pathomechanisms of human neural diseases.It is also uncertain whether the lack of lamination in transplanted brain organoids will affect the function of host circuits.
Challenges and Perspectives in the Brain Organoid Research Field
Despite the rapid development of brain organoids and their promising prospects as tools in neuroscience,brain organoid technology has some significant limitations.Although microphysiological systems can be used to optimize the microenvironment of brain organoids,it is infeasible to build human-brain-relevant culture conditions (Trujillo and Muotri,2018;Tan et al.,2021).Moreover,the induction of brain organoids is mostly based on empirical protocols or previous studies;thus,essential factors could be missing or over-represented,leading to abnormal neural development and maturation and a failure to faithfully produce human brain structures.For example,immune cells (e.g.,microglia and astrocytes) and endothelial cells do not appear during ectoderm induction,leading brain organoids to lack an immune system and vasculature.Therefore,the optimization of cell cultures with selected factors and media is vital in the generation of advanced brain organoids.There is no doubt that brain-resident microglia play critical roles in brain development and microenvironmental maintenance.Recently,neuroimmune organoids have been generated by co-culturing brain organoids with primary or iPSC-derived microglia (Popova et al.,2021;Figure 7A–C).The presence of the microglia was found to protect against double-stranded DNA breaks and promote neural network synchronization in brain organoids by modulating synaptic density.Αlternatively,gene editing,such as PU.1 (myeloid-specific transcription factor) overexpression,can be used to produce microgliacontaining human brain organoids,which has facilitated studies of brain development and diseases (Cakir et al.,2022;Zhang et al.,2023a).Blood vessels are independent structures that provide adequate oxygen and nutrients and a structure for oriented neuron growth.Advanced biotechnologies and methodologies,such as genome editing,coculture,multidifferentiation,microfluidic chips,transplantationin vivo,and assembly methods,have been applied to promote neural organoid vascularization (Mansour et al.,2018;Cakir et al.,2019;Ham et al.,2020;Kaushik et al.,2020;Shi et al.,2020;Worsdorfer et al.,2020;Yue et al.,2020;Li et al.,2023a;Sun et al.,2022;Wang et al.,2023b).However,the vascular networks generated within organoids are disordered and fail to recapitulate the function of blood vessels.The principle of angiogenesis during neurogenesis needs to be considered for brain organoid vascularization.

Figure 7|Strategies to promote the development of brain organoids.
Cortical organoids attract significant interest in brain organoid research,as the human cortex is the most complex and evolutionarily expansive part of the brain compared to that of animals (Qian et al.,2019;Rakic,2009).However,current cortical organoids are still not close to recapitulating the complicated human cerebral cortex.A major limitation of these systems is that the cortical wrinkling seen in the human brain does not occur in cortical organoids.One of the most prominent characteristics of the cortex is the cortical expansion and folding that lead to the size and complexity of the human brain (Fernandez et al.,2016;Borrell,2018;Llinares-Benadero and Borrell,2019).Previous evidence has shown that genetic,cell biological,and biomechanical factors are critical in the complex and multifaceted process of cerebral cortical folding (Αlbert and Huttner,2015;Florio et al.,2015;Del Toro et al.,2017;Borrell,2018;Llinares-Benadero and Borrell,2019).For instance,Jaenisch’s group showed that key features of the developing human cortex,including its expansion in size and surface folding,can be modeled in brain organoids by activating the PTEN-AKT signaling pathway (Li et al.,2017b).However,this evidence inadequately reflects the gene expression associated with normal human fetal brain development.Moreover,Karzbrun et al.(2018) reported the appearance of surface wrinkles in developing human cerebral organoids on a chip (Figure 7DandE).The physical mechanism modeled cortex folding extremely well.Nevertheless,the physical cues involved in human brain development are still poorly understood and need to be comprehensively assessed in follow-up studies(Budday et al.,2015).Previous studies provided evidence that oRGCs were largely absent in lissencephalic rodents,despite being important for human cortical expansion,suggesting that brain gyrification could be closely associated with oRGCs(Bershteyn et al.,2017;Rash et al.,2019;Zarzor et al.,2023).Furthermore,it is worth mentioning that cortical organoid maturation resembles the developmental stages of the human brain and plays a vital role in the occurrence of gyrification because the folding process takes place during gestational weeks 16–40 (Sun and Hevner,2014;Garcia et al.,2018a,b;Urresti et al.,2021).Therefore,more novel,sophisticated,and appropriate designs and methodologies should be implemented to create human brain organoids with gyri and to recapitulate the properties of native brainsin vitro.
Although previous studies have detected neuronal action potentials,excitatory and inhibitory postsynaptic currents,and spontaneous network activity within neural organoids,singleneuron action potentials have not be recorded (Mansour et al.,2018;Trujillo et al.,2019;Tasnim and Liu,2022).Recently,Le Floch et al.(2022) designed stretchable mesh nanoelectrodes that,when distributed across brain organoids,created a cyborg brain organoid platform (Figure 7F).The integrated,stretchable electrode arrays enabled long-term stable,continuous recording and captured the emergence of single-cell action potentials within brain organoids during early development.Semiconductor devices can be used to not only provide promising platforms for evaluating the functional development of brain organoids but also to introduce potential stimulators,such as electronic,optoelectronic,thermal,mechanical,and biochemical interfaces,to promote brain organoid development (Park et al.,2021b;Le Floch et al.,2022;Li et al.,2022e;Figure 7G–I).Given the critical roles of electrical stimulation in neurogenesis and neurodevelopment,exogenous electrical stimulation from biocompatible meshes was used to monitor and modulate the electrical activity of neurons with cortical organoids.Moreover,the rapid development of advanced biomaterials,such as conductive hydrogel polymers,opens new avenues for the building of 3D electroconductive scaffolds for brain organoids (Xu et al.,2020;Wang et al.,2021a;Song et al.,2022).
This study had some limitations that should be noted.For instance,the database used in this study included data from 2013 to June 30,2023,and thus lacked the latest publications that may report further novel methods and technologies in brain organoid-based studies.Currently,no single article database includes all the documents ever published.Therefore,comparative studies are needed to distinguish the similarities and differences among the different databases using bibliometric analysis.Although VOSviewer can handle abundant documents and create excellent data visualizations,it cannot conduct temporal bibliometric analyses like CiteSpace (Tay,2022).Therefore,integrating VOSviewer with other platforms is warranted to comprehensively reveal global trends in brain organoid-based research.
Concluding Remarks
Brain organoid technologies have been gaining momentum in neuroscience in the last decade and have provided promising models for the study of diverse neurological disorders and for deciphering the mechanisms underlying human brain development.In this study,bibliometric analyses were conducted with the VOSviewer application.We systematically summarized the brain organoid research trends in terms of publication numbers over the years,research-productive countries/regions,organizations,journals,authors,and influential documents,as well as hot topics.It is worth noting that many research organizations have close collaborative relationships with each other.Though there has been rapid growth in the number of publications on brain organoids since 2013,as well as their widespread application in human neural development,neural disorders,infectious diseases,regenerative medicine,drug discovery,and toxicity assessment,this research field is still in the infancy stage,and several limitations and issues need to be addressed.Therefore,research innovations embracing fresh thinking and novel technology and methods,such as gene editing and bioengineering,are needed to continuously update brain organoid research.Despite the considerable challenges ahead,such as how to reproduce blood vessels and immune cells in brain organoids,the brain organoid is the model that most closely recapitulates human brain development and disorders,both in terms of cellular diversity and neuronal organization,to date.Taken together,the findings of our study show that brain organoid research has yielded groundbreaking results in deciphering human brain developmental events and neural diseases during the past 10 years.The present paper should help researchers gain in-depth knowledge of brain organoid research developments and trends.
Author contributions:Methodology,data curation,visualization,supervision,writing,and review:ML.Data curation,review,and visualization:YY and ZH.Conceptualization,supervision,and review:SH,LJ,and BW.All authors approved the final manuscript.
Conflicts of interest:The authors declare that they have no conflicts of interest.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Santiago Ramirez,McGovern Medical School,University of Texas Health Science at Houston,USA.
Additional files:
Additional file 1:Open peer review report 1.
Additional Table 1:The most actively contributing organizations in brain organoid research according to publication citations.
Additional Table 2:The top 10 most highly productive authors according to the publication citations.
Additional Table 3:The top 10 most highly productive authors according to the publication numbers.
Additional Table 4:The most influential journals in brain organoid research according to publications and citations.
Additional Figure 1:The overlay visualization of keyword co-occurrence in brain organoid research.
- 中国神经再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments

