Shock Raman spectra and structural transformation of powdered TKX-50 by the plate impact experiments combined with real-time Raman detection
Xue Yng , Qijun Liu ,*, Yundn Gn , Lei Yng , Zhengtng Liu , Fusheng Liu ,**
a Bond and Band Engineering Group,School of Physical Science and Technology,Southwest Jiaotong University,Chengdu 610031,People's Republic of China
b Xi'an Modern Chemistry Research Institute, Xi'an 710065, People's Republic of China
c State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi'an 710072, People's Republic of China
Keywords: Powdered TKX-50 Hugoniot Raman Decomposition Phase transition
ABSTRACT As an energetic material of great interest, the work capacity of dihydroxylammonium 5,5'-bistetrazole-1,1'-diolate (TKX-50) has been questioned recently.Although some research groups have explored the reasons for the low working ability of TKX-50, the plane impact experiment on powdered TKX-50 is obviously closer to the practical application, and the conclusions based on this are more guiding.Hence,we performed shock Hugoniot measurements of powdered TKX-50 between 5.65 and 16.29 GPa.The plane impact experiments of powdered TKX-50 were carried out and the shocked Raman spectra were collected.By Raman spectroscopy analysis, a new peak of powdered TKX-50 was found between 19.47 GPa and 24.96 GPa,which may be caused by decomposition/phase transition and was related with the low work capacity.
1.Introduction
As a nitrogen-rich energetic material with high attention,dihydroxylammonium 5,5'-bistetrazole-1,1'-diolate (TKX-50,C2H8N10O4,Fig.S1)which was reported and synthesized since 2012[1],is considered to be a high-performance energetic material and is expected to replace traditional energetic materials such as RDX and HMX [1-3].Compared with RDX, TKX-50 has good thermal stability, low toxicity and low sensitivity.So it is considered as a potential component of solid propellants and a better substitute for RDX in compound modified double base propellants[4].Because of these excellent characteristics,TKX-50 is also expected to be more widely used in the field of explosives, so it has been studied and tested in various aspects by research groups around the world[5-10].However, TKX-50 was proved to be an energetic material with high detonation speed, high detonation pressure and low work capability,not as outstanding as claimed previously[10-15].
The reason of TKX-50's low work capability has attracted much attention, and several groups have studied it.Zhao et al.believed that C2N2is generated during the decomposition process of TKX-50,and C2N2is liable to polymerize to form solid residue under high temperature and pressure, thus reducing the gas production of TKX-50 during the decomposition process [16].Xing and Liu et al.[11,13,14] considered that the proportion of molecules with larger molecular mass(CO2,CH2O2)in the detonation products of TKX-50 is small, and the detonation products are mainly products with small molecular mass.The light weight of small molecule products may be one of the reasons for the low work capacity of TKX-50.In short,the decomposition path and the product seem to be directly related to its work capacity.In the paper, we mainly study the decomposition process of TKX-50 under shock compression.
The thermal decomposition behavior of TKX-50 has been extensively studied, and it is widely accepted that TKX-50 undergoes hydrogen transfer [17-21] (that is, H is transferred from the O on the cation NH3OH+to the O on the anion)during thermal decomposition.It is confirmed that TKX-50 has good stability under hydrostatic pressure [6,9,22,23], and increasing pressure will inhibit hydrogen transfer during the thermal decomposition of TKX-50 [24].In the environment with high temperature and high pressure caused by shock compression, the decomposition mechanism of TKX-50 is still unclear,which is important to the practical use.Nevertheless, the very short impact time makes it extremely difficult to capture useful information in the decomposition process during the experiment.Hence, to understand the decomposition mechanism of TKX-50 under shock compression, the real-time measuring the molecular level response of shock-compressed TKX-50 must have a breakthrough.
In addition to good detection methods, the initial sample is extremely important to the decomposition process.For many years,single crystals of energetic materials have been used in Raman experiments of energetic materials [25-29].On the one hand, it avoids the inherent complexities in the mechanical response of inhomogeneous solid materials.On the other hand, the transmittance of single crystals is better.Even though it is difficult and costly to obtain single crystals of energetic materials that meet the experimental conditions,the homogeneity and good transmittance of single crystals are undoubtedly an important guarantee for collecting spectral signals, especially in shock wave experiments.However, in the practical use of energetic materials, the energetic materials powder is mixed with polymeric binders at a certain proportion, and then filled into the projectile.To investigate the reasons for the low work capacity of TKX-50,powdered samples are a more suitable carrier than single crystal samples.Using powdered samples to carry out impact experiments and collect Raman spectra is obviously closer to study the state of energetic materials in the actual use process,and is also conducive to provide a reference for the use, transportation and storage conditions of energetic materials.Nevertheless, since the powdered sample emits light under impact,the laser cannot enter the sample,let alone be scattered,so it is difficult to obtain the Raman spectrum of powdered TKX-50.Hence, how to capture the signal of powdered samples under shock compression is very important.
In a word, detection technique is decisive for the study of decomposition process of TKX-50.In this work, we develop a sample preparation method to obtain Raman spectra of TKX-50 powder samples of good quality under shock.The plate impact experiments combined with real-time Raman detection are used to collect Raman spectra to explore the structural transformation and chemical decomposition of powdered TKX-50 under different shock states, hoping to give the reason of low work capability.
2.Experimental methods
After TKX-50 was evenly ground, the powdered TKX-50 with adhesive added(the amount of adhesive added is less than 3 wt%)was pressed into φ13mm sheets of a certain thickness by tablet press.The TKX-50 sheets were bonded in the test target using epoxy,and the structure diagram of the test target is shown in Fig.1.All experiments in this work were done using 25 mm light gas gun.And Al, Cu or Ta flyers with polished surfaces were impacted onto the target assemblies.The flyer velocity could be recorded by the magnetic velocity measurement system.And the Raman spectrum collection of TKX-50 was completed using the High-Spectral-Resolution Laser Raman System [30].The Nd-YAG laser was used as the light source, of which operating at 532 nm, pulse energy at the sample was 50-300 mJ and pulse width was 10 ns.
3.Analysis and discussion
3.1.Hugoniot states
Shock-wave impedance-match(IM)measurement is a common method to generate shock equation-of-state data for different materials.By measuring the shock or particle velocities in the known materials and in the samples, using the Rankine-Hugoniot conservation relations, the pressure (P) and density (ρ) in the shocked sample can be deduced.The Rankine-Hugoniot equations are as follows [31,32]:
whereP0and ρ0stand for the pressure and density of material ahead of the shock wave,usis the shock velocity,upis the particle velocity.
The Hugoniot data of TKX-50 is obtained by combining the known Hugoniot curves of the impactor and base plate and using IM technology.The particle velocity(up)and shock velocities(us)of TKX-50 are presented in Table 1 and are plotted in Fig.S2.From Fig.S2, the relationship betweenupandusof TKX-50 is basically linear.Fitting these data yields a linear relationship ofus= 3.106 +1.778up(5.65-16.29 GPa).Thus, the impact Hugoniot parameter (C0= 3.106, λ = 1.778) of TKX-50 is obtained, which is the necessary parameter for calculating the pressure in part 3.2.The linear relationship betweenupandusof TKX-50 also can indicate that TKX-50 did not undergo phase transition in the range of 5.65-16.29 GPa.
3.2.Raman spectra
In this work, six peaks can be clearly seen in the Raman spectrum of TKX-50 under ambient pressure in the range of 800-1800 cm-1.And for further discussion, the six peaks were marked as v1 to v6 from left to right (as shown at the bottom of Fig.2,and a clearer ambient Raman spectrum of TKX-50 was shown in Fig.S3).The specific vibration assignments of the six peaks are listed in Table S1.The Raman spectra of TKX-50 at different shocked pressures were investigated by 9 plate impact experiments combined with real-time Raman detection.The relevant experimental parameters are listed in Table 2, and Fig.2 shows the Raman spectra.
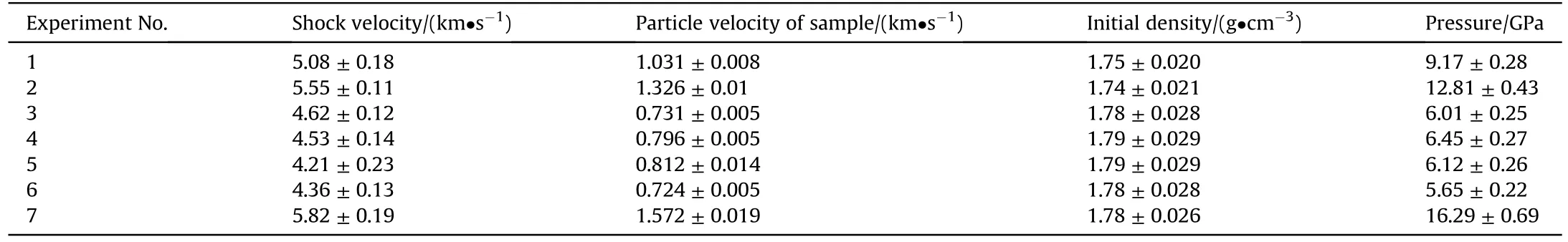
Table 1The Hugoniot data of TKX-50.
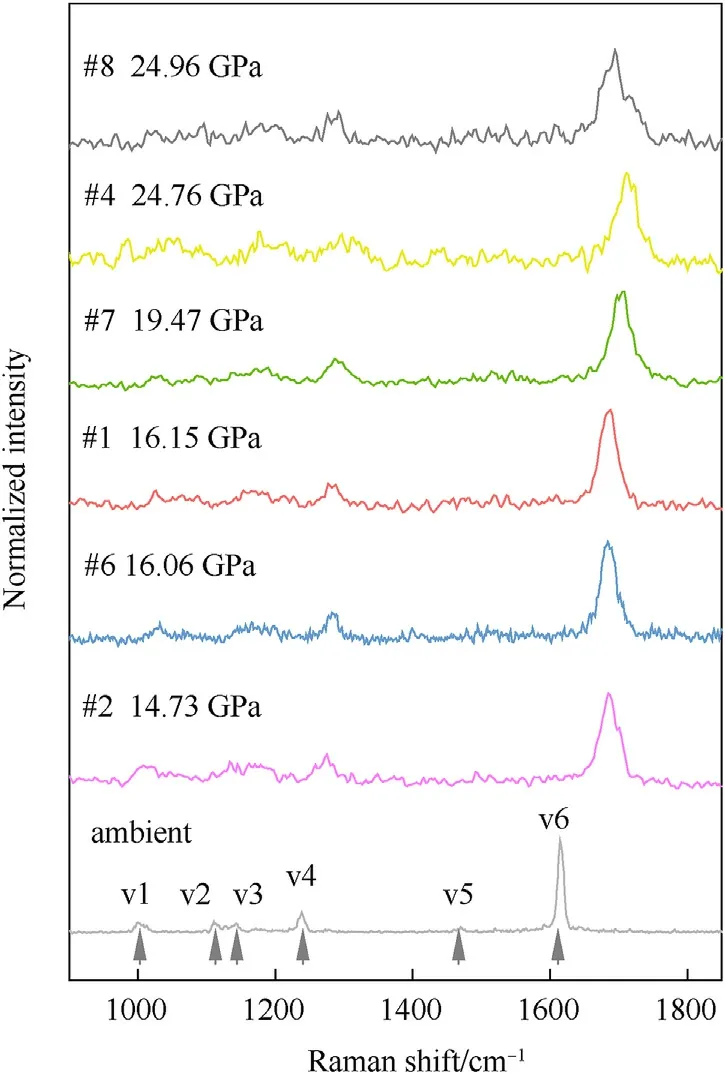
Fig.2.Raman spectra of TKX-50 at different impact pressures.

Fig.3.Pressure effects on v6 in TKX-50.The error is within the solid circle.
In order to better distinguish the peaks in Raman spectra,all the Raman spectra of TKX-50 gained in this work were normalized.As can be seen from Fig.2, all peaks of the spectra after shocked are broadened.Coupled with the poor signal to noise ratio of shocked samples,v2 and v3 in all post-impact spectra are indistinguishable.v1 is also difficult to distinguish from the background and is completely indistinguishable after 19.47 GPa (see #7).It is easy to see the blue shift of v4 under pressure.However,with the increase of pressure, v4 cannot be resolved at 24.76 GPa, while it can be reluctantly resolved at 24.96 GPa, perhaps because of the poor signal to noise ratio.Contact Tables S1, v1 and v4 all represent vibrations on the cation ring of TKX-50.Then it can be guessed that the cation ring structure was present up to 19.47 GPa.
Whereas,v6 can be clearly distinguished from 0 to 24.96 GPa.v6 shows obvious blue shift and the impact pressure effects on it are presented in Fig.3.Known from Table S1, v6 is caused by C-C stretching and cation rocking (about 1615 cm-1at atmospheric pressure,hereafter called the mode as C-C mode).Compared with the variation curve of C-C mode with pressure obtained by Dreger et al.[23]under static compression,the curve obtained in this work significantly moves down.That is, the blue shift of C-C mode obtained by impact test is less than that under static compression with the same pressure.The shock state contains both high temperature and high pressure.Thus, it was tentatively believed that the less blue shift could be caused by the high temperature accompanying the shock state [33].The influence of temperature and pressure on the material can be reflected in its volume change.In order to explore whether it is the effect of temperature, the specific volume of the samples under impact is calculated and compared with the results of density functional theory (DFT)method and static compression experiments[7,22].The results are shown in Fig.4.The specific volume of the primary impact samples is calculated from Eq.(1), and the specific volume of the re-shock samples is extrapolated from the Hugoniot data.The extrapolation process is provided in supporting information.From Fig.4,the impact results are basically consistent with the static compression and theoretical calculation results, only slightly offset when the specific volume is less than 0.4 cm3/g.Namely before the impact pressure reaches 24.76 GPa, the combination of temperature and pressure results in fewer blue shift in C-C mode,compared to static pressure.And the volume expansion alone cannot well explain the lower blue shift of C-C mode,perhaps more variation occurs in the sample.
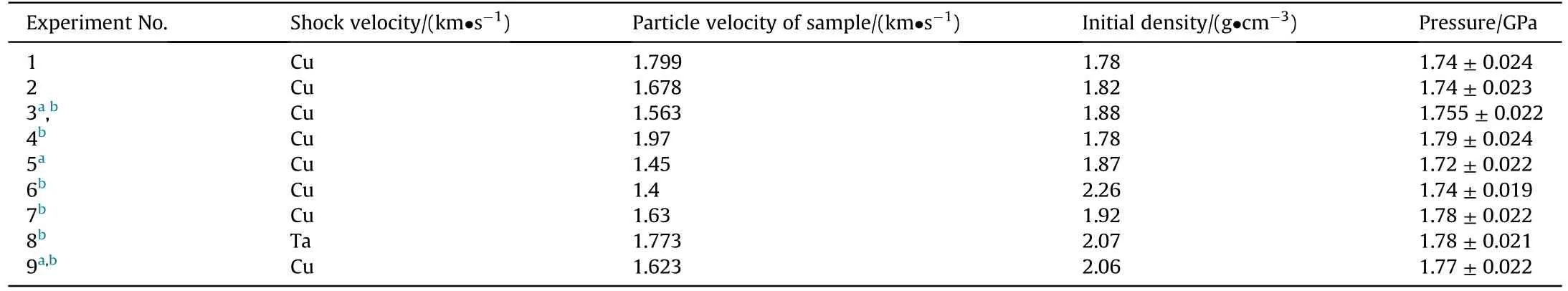
Table 2Parameters for plate impact experiments on TKX-50.
Dreger et al.proved experimentally that with static compression until 50 GPa[22],there was no other change in C-C mode,except the blue shift.Noticing that Lu et al.[8]kept TKX-50 at 3.1 GPa and 593 K for 15 min,the C-C mode split into two peaks,although they did not point it out.Muravyev and Zhao et al.indicated that diammonium 5,5'-bistetrazole-1,1'-diolate(ABTOX)is an intermediate in the decomposition process of TKX-50[16,19,35].Dreger et al.[34]also showed the Raman spectra of the two intermediates of TKX-50 prior to complete melting or decomposition, which is mainly ABTOX at the lower temperature, and diammonium 5,5'-bistetrazolate and/or dihydroxylammonium 5,5'-bistetrazolate at the higher temperature.Both intermediates have a strong vibration mode caused by C-C stretching and its cation rocking, whose frequency is slightly lower than that of TKX-50 under ambient pressure and higher pressure[34,36].
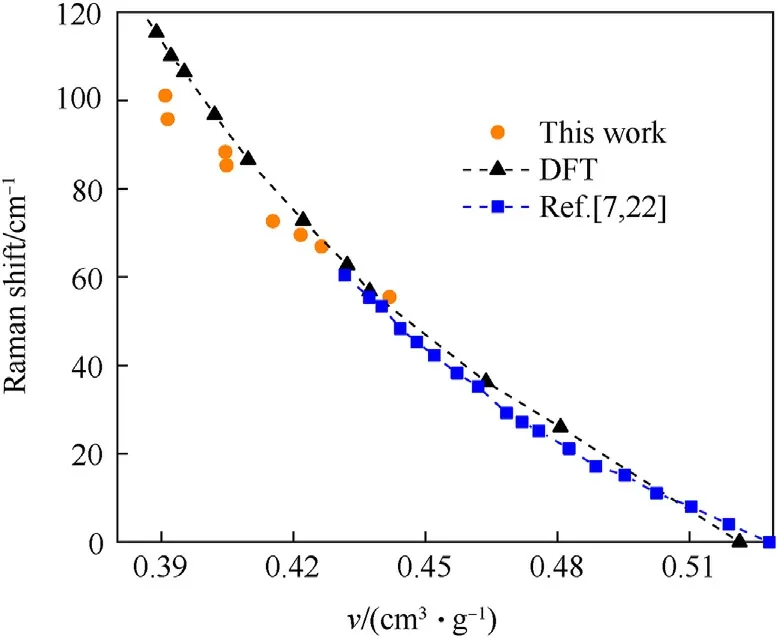
Fig.4.Raman shift of TKX-50 at different specific volumes.The results of static compression experiments were obtained by fitting the experimental results in Refs.[7,22].

Fig.5.The enlarge figure of C-C mode under pressure.The blue dashed line represents the fit peak for v6 and the orange dashed line represents the fit peak for v7.
In this work,it is obvious that a new peak v7 appears when the impact pressure reaches 24.76 GPa (see #8), whose frequency is slightly less than v6.For clarity,the enlarge figure of C-C mode in#4, #7 and #8 is presented in Fig.5.The peaks are fitted and the result is also shown in Fig.5.The blue dashed line represents the fit peak for v6 and the orange dashed line represents the fit peak for v7.Fig.5 shows that a Lorentz peak can be used to fit the peak curve well at 19.47 GPa.At 24.76 GPa,two Lorentz peaks are needed to fit the Raman peak well.At 24.96 GPa, there are clearly two distinct peaks.The new peak v7 firstly appears at 24.76 GPa.And the relative intensity of the v7 to v6 is enhanced with the increasing pressure.It is reasonable to believe that the emergence of v7 is due to decomposition of TKX-50.While Lu et al.[8] reported the temperature-induced TKX-50 phase transition before TKX-50 decomposition.They argued that the temperature-induced phase transition reduces the symmetry of the crystal, and induces the broadening of Raman peaks and emergence of new peak.Therefore,it cannot be ruled out that TKX-50 underwent a phase transition under these shock states.In other words,a part of TKX-50 is likely to decompose or phase transition before it exploded.In this case,either a slower decomposition process relative to the explosion resulting in a lag in energy release, or the reduction in gas production due to the generation of C2N2from the decomposition process [16], or the energy consumption of the phase transition would reduce the work ability of TKX-50.As the structure changes,the Hugoniot state of the sample would change, and the specific volume derived based on the Hugoniot data would be problematic.This can also explain the slightly more deviation between the impact experimental results and the calculated results when the specific volume is less than 0.4 cm3/g in Fig.4.
In conclusion,it is possible that TKX-50 still maintains the same structure as at ambient pressure until the impact pressure reaches 19.47 GPa in our experimental test precision.It's sure that, from impact pressure 19.47 GPa-24.96 GPa, a structural transformation has taken place,possibly due to decomposition or phase transition.However, more and higher-precision experiments are needed to measure what the new peak is and whether there are other changes from 19.47 GPa to 24.76 GPa.
4.Conclusions
In this work, the Hugoniot curve of powdered TKX-50 is firstly obtained,which provides important parameters for the subsequent powdered TKX-50 impact experiments[37].Then,the high-spectral resolution laser Raman system is used to collect the Raman spectra of powdered TKX-50 samples under different impact pressures.And it turns out experimentally that there is a new Raman peak at the impact pressure from 19.47 GPa to 24.96 GPa,which is probably caused by decomposition or phase transition of TKX-50.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request and available within the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No.12072299), the Fundamental Research Funds for the Central Universities (Grant No.2682020ZT102).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.05.023.
- Defence Technology的其它文章
- The interaction between a shaped charge jet and a single moving plate
- Machine learning for predicting the outcome of terminal ballistics events
- Fabrication and characterization of multi-scale coated boron powders with improved combustion performance: A brief review
- Experimental research on the launching system of auxiliary charge with filter cartridge structure
- Dependence of impact regime boundaries on the initial temperatures of projectiles and targets
- Experimental and numerical study of hypervelocity impact damage on composite overwrapped pressure vessels

