Phlorizin alleviates deltamethrin-induced oxidative stress in brine shrimp Artemia*
Dandan MA, Qingli ZHOU, Liying SUI, Qingbin GUO, Huanhuan LIU,Honghe LIANG, Zhenjing LI,**, Zhongna SANG
1 School of Food Science and Engineering, Tianjin University of Science and Technology, Tianjin 300457, China
2 Asian Regional Artemia Reference Center, Key Laboratory of Marine Resource Chemistry and Food Technology (TUST),Ministry of Education, Tianjin University of Science and Technology, Tianjin 300457, China
3 Subtropical Crops Research Institute of Guangxi Zhuang Autonomous Region, Nanning 530001, China
4 Department of Nutrition and Food Hygiene, School of Public Health, Tianjin Medical University, Tianjin 300070, China
Abstract Deltamethrin (DEL), a commonly used pyrethroid pesticide, results in higher reactive oxygen species (ROS) levels in aquatic animals, which consequently unbalance the redox state.Phlorizin (PHL) is a flavonoid and a natural product promising to prevent or reduce pesticide-induced oxidative stress.Artemia is a micro-crustacean widely used in marine hatcheries and an experimental aquatic organism for environmental toxicology research.This research aimed to evaluate the toxicity of DEL on Artemia and the antioxidative effect of PHL against the toxicity.Results show that 0.08-mg/mL PHL exerted its antioxidative effects on hatching percentage of the cysts in 24-h incubation and on body length and survival rate of Artemia in 12-d culture.After 12-d culture, 12-, 24-, and 36-h DEL exposure showed significant drops in SOD, CAT, and GSH-Px enzyme activities, and significant increases in ROS and malondialdehyde (MDA) levels in Artemia (P<0.05).On the contrary, 0.08-mg/mL PHL application improved the enzyme activities and decreased the ROS and MDA levels (P<0.05).Moreover, 0.08-mg/mL PHL significantly increased mRNA expression levels of Cu/Zn SOD, CAT, GST, HO-1, NQO1, and Nrf2,and decreased mRNA expression level of Keap1 in the DEL-exposed Artemia (P<0.05).Therefore, DEL is toxic to Artemia, while PHL alleviates DEL-induced oxidative damage by possibly regulating the Nrf2 signaling pathway.This study provided a theoretical basis for PHL to reduce pesticide-induced toxicity in aquatic animals.
Keyword: Artemia; deltamethrin; phlorizin; oxidative stress
1 INTRODUCTION
In recent decades, pyrethroid pesticides have been widely utilized in agriculture because of their insecticidal effects against pests and parasites,accounting for approximately 25% of the insecticide market worldwide (Yavuz et al., 2015; Lu et al.,2019).However, deltamethrin (DEL), a member of the α-cyano class of pyrethroid pesticides, causes water pollution and harms the aquatic ecosystem and aquatic organisms (Yang et al., 2020).The metabolization of pyrethroid pesticides in aquatic animals is usually lower due to the lack of hydrolyzing enzymes (Zhang et al., 2019).It has been reported that DEL exposure induces various histopathological alterations in gills, liver, and brain of common carp (Cyprinuscarpio), rainbow trout(Oncorhynchusmykiss), gilthead seabream (Sparus aurata), and goldfish (Carassiusauratus) (Guardiola et al., 2014; Arslan et al., 2017; Zeng et al., 2021;Zhou et al., 2021).Some studies focused on the neurotoxicity of DEL on crustaceans, including prawns (Macrobrachiumrosenbergii), European lobster (Homarusgammarus), and Chinese mitten crab (Eriocheirsinensis) (Zhang et al., 2019;Parsons et al., 2020; Jiang et al., 2021).Human consumption of DEL-exposed aquaculture products may cause oxidative stress, threatening the individual’s health, such as cancers and neurobehavioral and reproductive disorders (Hossain et al., 2022).Studies indicate that DEL exposure induces oxidative stress by increasing the generation of reactive oxygen species (ROS) (Romero et al.,2015; Ding et al., 2017).Flavonoids are antioxidants that scavenge ROS (Liu et al., 2022).Isorhamnetin and luteolin, two common flavonoids, reduce ROS accumulation in human SH-SY5Y cells induced by H2O2or SIN-1 (Qu et al., 2010).Furthermore,flavonoids extracted fromBidensbipinnataL.decrease ROS generation and cell apoptosis in rat INS-1 cells (Yang et al., 2021).
Phlorizin (PHL) is a natural flavonoid compound extracted from various plants, including apples and sweet tea (Ehrenkranz et al., 2005; Wang et al.,2019a).PHL possesses some biological properties,including antioxidant, anti-diabetic, anti-inflammatory,and hepatoprotective activity (Muceniece et al.,2016; Anguita-Ruiz et al., 2020; David-Silva et al.,2020; El-Hawary et al., 2021; Zhang et al., 2021;Chen et al., 2022).Studies have revealed that PHL could lower ROS and malondialdehyde (MDA)levels and enhance the activity of antioxidant enzymes, eventually protecting against fatigueinduced oxidative stress in mice (Ma et al., 2022).PHL exhibits antioxidative activity by regulating theNrf2signaling pathway inDrosophilamelanogasterand rat liver (Wang et al., 2019b; Zhang et al.,2022).However, available information is limited on the effect of PHL on aquatic animals.
Artemiais a zooplanktonic crustacean that thrives in the hypersaline water body.Artemiahas been widely used in marine ecotoxicology research(Alishahi and Tulaby Dezfuly, 2019; Varó et al.,2019; Han et al., 2021) owing to its biological advantages, such as easy manipulation, shorter life cycle, and large offspring production (Matthews,1995; Libralato, 2014; Shekarchi et al., 2020).This research aims to determine whether DEL causes oxidative damage inArtemiaand whether PHL can prevent or alleviate the oxidative stress induced by DEL.The findings of this research may spotlight the role of PHL in ameliorating DEL-induced toxicity in aquatic animals.
2 MATERIAL AND METHOD
2.1 Artemia and experimental design
Artemiaparthenogeneticacysts were obtained from the Asian regionalArtemiaReference Center(AR-ARC) of Tianjin University of Science and Technology, China.First, toxicity test of DEL (CAS:52918-63-5, purity ≥98%, Sigma-Aldrich, Germany)(0, 0.001, 0.01, 0.1, 1, 5, and 10 μg/L) in nauplii (1-2 d old), juvenile (7 d old), and adult (12 d old) was conducted for 36 h to ensure the optimal exposure concentration toArtemia.Second, for hatching percentage, survival, and growth determination, we randomizedArtemiainto four main treatment groups: a control group, a DEL group (0.10 μg/L,10% adult LC50), PHL groups (0.03, 0.05, and 0.08 mg/mL, purity ≥98%, Aladdin, Shanghai, China),and DEL+PHL groups (0.03, 0.05, and 0.08 mg/mL),named Ctrl, DEL, 0.03 PHL, 0.05 PHL, 0.08 PHL,DEL+0.03 PHL, DEL+0.05 PHL, and DEL+0.08 PHL.Cysts hatching percentage was calculated after hatching for 24 h.Survival and growth ofArtemiawere recorded every 2 d during the 12-d culture from instar I nauplii to adultArtemia.
For antioxidant enzyme activities, and MDA and ROS level determination, adultArtemiawere randomized into the four main treatment groups mentioned above.Exposure duration ofArtemiawas 12, 24, and 36 h after 12-d general culture.For gene expression analysis, adultArtemiawere randomized into the four treatment groups, including Ctrl, DEL,0.08 PHL, and DEL+0.08 PHL.The exposure duration was only 24 h after the 12-d general culture.Each experiment or samples were triplicated at least if not specified elsewhere.
2.2 Median lethal concentration (LC50) of DEL on Artemia
Pre-experiment tests were conducted to narrow the range of treatment concentrations between 0 and 100% mortality.In different stages, including nauplii, juvenile, and adult,Artemiawere exposed to seven levels of DEL (0, 0.001, 0.01, 0.1, 1, 5, and 10 μg/L) with three replicates.Exposure to different stages lasted 36 h.Three replicates were used in each treatment, with 100Artemiaper replicate.Artemiawere not fed during experiment, and dead ones were removed immediately from the beaker.Mortality was calculated to determine the 36-h LC50using the regression probit analysis in the SPSS V20.0 statistical software (95% CI).
2.3 Hatching percentage determination
Approximately 0.1-g cysts were hatched in a conical tank containing 1-L brine water (salinity 30 artificial seawater (ASW), Instant Ocean, USA) at 27±1 ℃, with continuous aeration and 2 000-lx illumination.After hatching for 24 h, the number of nauplii, umbrella-stage nauplii, and unhatched cysts was recorded under the stereoscopic microscope(SZX12, China).The hatching percentage (H%) was calculated according to the following formula:
Hatching percentage (H%)=nauplii/(nauplii+umbrella-stage nauplii+unhatched cysts)×100%.
2.4 Survival and growth determination
One hundred newly-hatchedArtemianauplii were transferred to a 250-mL glass bottle containing 200-mL ASW (salinity 30) at a density of 1.5 inds./mL.Artemiawas fedChlorellatwice a day at 27±1 ℃.During the 12-d culture, the survival rate ofArtemiawas determined after 2-, 4-, 6-, 8-, 10-, and 12-d cultures.Artemiawas considered dead if its appendages stopped moving in 10 s.The average body length ofArtemiawas determined after 2-, 4-,6-, 8-, 10-, and 12-d cultures under a stereoscopic microscope (SZX12, China).AllArtemiawere returned quickly to the conical tank after determination.
2.5 Preparation of Artemia homogenates
After the end of the experiment,Artemiain each group were collected and homogenized in physiological saline or 0.1-mol/L phosphate buffer saline (1:9, w:v).The homogenate was centrifuged at 12 000×gfor 15 min at 4 °C.The fresh supernatants were used for assay kits and ROS determination, and frozen samples were stored at-80 ℃ for total RNA extraction.
2.6 Antioxidant parameters determination
Analysis kits (Nanjing, China) were used to measure the enzyme activities of SOD, CAT,GSH-Px, and MDA contents.The activities were expressed as units per milligram of protein, and the MDA contents were expressed as nanomole MDA per milligram of protein.The soluble protein content was determined according to Bradford’s study(1976).
2.7 ROS determination
The fluorescent dye 2′,7′-dichlorofluorescein diacetate (DCFH-DA) was used to determine ROS accumulation inArtemia(Kumar et al., 2017; Zhu et al., 2018) induced by DEL.The supernatants were transferred to 96-well plates and incubated with 10-μmol/L DCFH-DA at 37 °C for 30 min in dark.Fluorescence intensity was determined in a multimode plate reader (BioTek Instrument, Inc., USA) at 488-nm excitation and 525-nm emission wavelength.
2.8 Real-time PCR analysis
The total RNA of adultArtemiawas extracted using the TRIzol method (TaKaRa, Japan), which was quantitatively and qualitatively determined using NanoDrop 2000 (Thermo Scientific, USA).Only employed high-quality RNA (OD260/OD280range 1.8-2.2 and RIN 8.0) were executed in the RTPCR analysis.PrimeScriptTMRT kit (TaKaRa, Japan)was used for cDNA synthesis.Theβ-actin gene was used as a housekeeping gene, and specific primer sequences were designed using Primer Premier 5.0(Table 1).RT-PCR was performed by the SYBR Green method.Reaction mixtures of 20 μL (0.8-μL forward primer, 0.8-μL reverse primer, 10-μL 2×TB Green Premic Ex Taq II, 2-μL cDNA, and 6.4-μL RNase-free water) were amplified for 30 s at 95 ℃followed by 40 cycles of 5 s at 95 ℃, annealing for 30 s, and finally, 30 s at 72 ℃.The tests were conducted three replicates per group.The relative expression of target genes was calculated using the 2-ΔΔCtmethod.
2.9 Statistical analysis
All data are presented as mean±standarddeviation.The significance was determined using a one-way analysis of variance followed by Duncan’s multiple range test.The level of significance was accepted atP<0.05 (SPSS 22.0, IBM, USA).
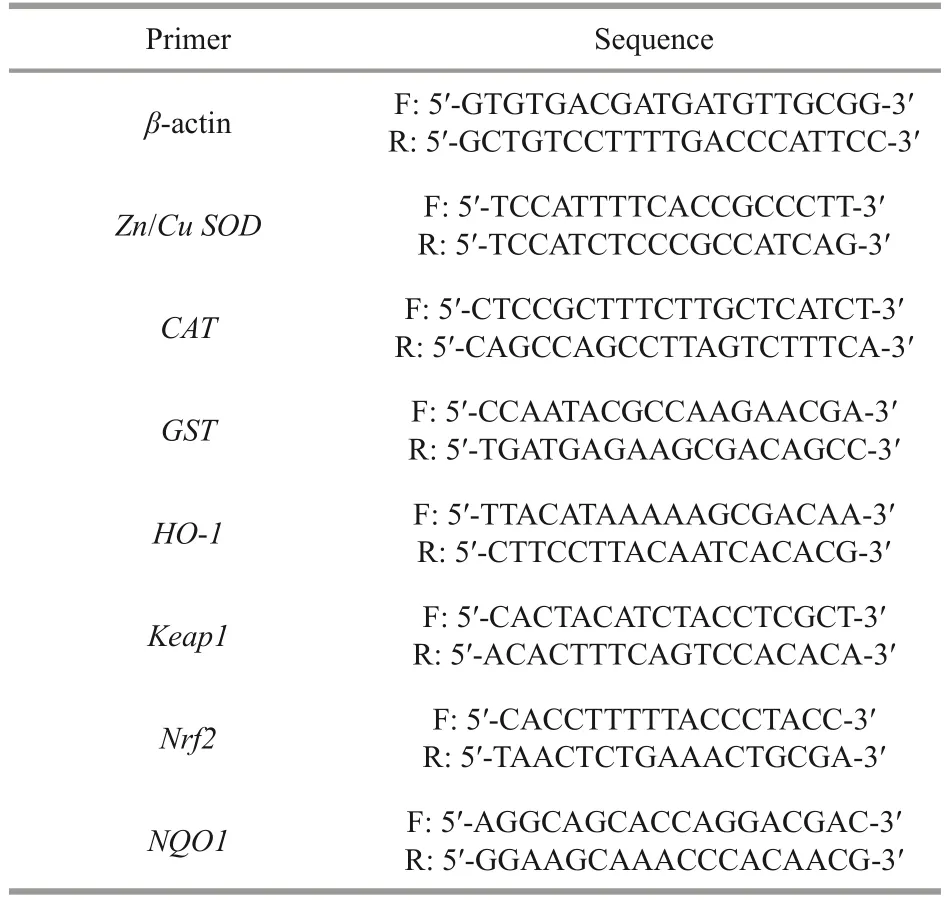
Table 1 Sequences and accessions of the primers used for RT-PCR
3 RESULT
3.1 LC50 of DEL on Artemia
The acute toxicity test of DEL exposure is shown in Table 2.The survival rate of nauplii, juvenile, and adultArtemiahad no significant difference at low concentrations (P>0.05); however, at high concentrations, the survival rate decreased remarkably(P<0.05).The LC50of DEL on nauplii, juvenile, and adultArtemiawas 190.459, 87.486, and 1.076 μg/L,respectively.No observed effective concentration(NOEC) of nauplii, juvenile, and adultArtemiawas 0.1, 0.01, and 0.001 μg/L, and the lowest effective concentration (LOEC) observed was 0.16, 0.08, and 0.007 μg/L.According to the sublethal concentration of 36 h, 0.1 μg/L-DEL (10% of LC50, adult) was selected in the follow-up experiments.
3.2 Effect of PHL on the hatching percentage,survival, and growth in DEL-exposed Artemia
As shown in Fig.1, after a 24 h incubation, the hatching percentage of the cysts significantly declined in the DEL group compared to the control group (P<0.05), while co-treatment with PHL caused a significant increase compared to the DEL group (P<0.05).Furthermore, cysts treated with PHL alone had an increased hatching percentage with increasing concentrations; the maximum value occurred in the PHL group at 0.08 mg/mL (P<0.05).
The survival rate ofArtemiais shown in Fig.2a.The DEL group showed a reduced trend after a 4-d exposure, while co-treatment with PHL caused a significant increase compared to the DEL group (P<0.05); significantly higher survival rates were observed in the PHL-treated group at 0.08 mg/mL compared to the control group (P<0.05).Furthermore, the survival rate ofArtemiatreated with 0.03-, 0.05- and 0.08-mg/mL PHL alone was significantly increased compared to the control group (P<0.05).
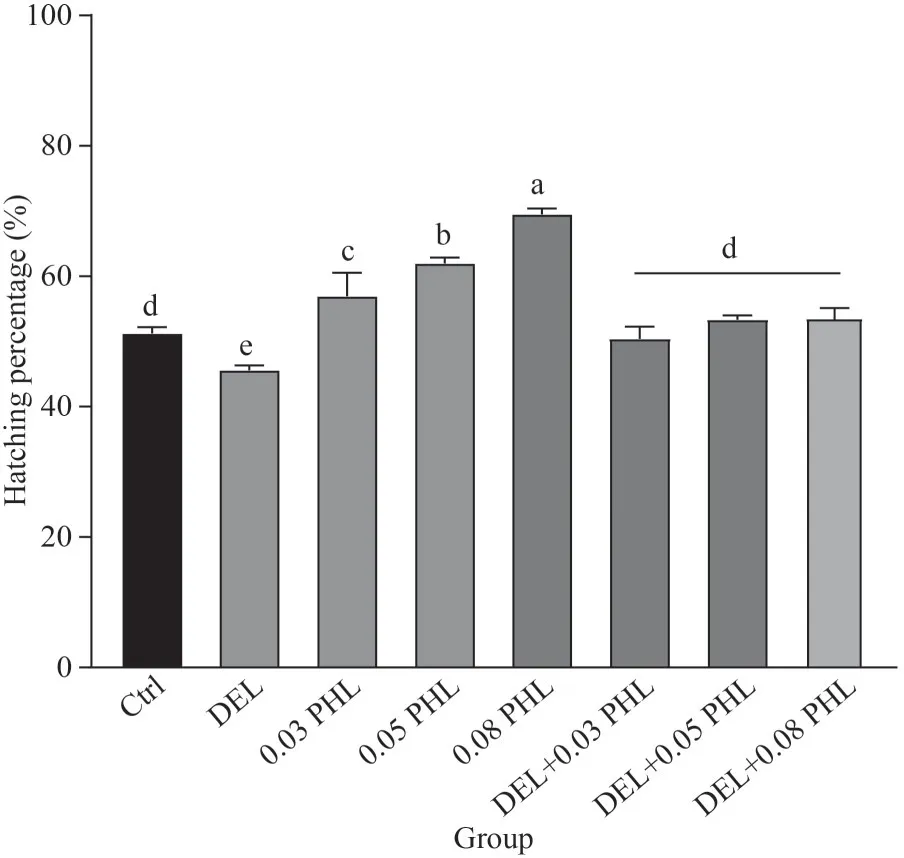
Fig.1 Hatching percentage of Artemia when exposed to DEL, 0.03-, 0.05-, 0.08-mg/mL PHL, and their combinations
The body length ofArtemiais shown in Fig.2b.DEL decreased the body length ofArtemiaafter an 8-d exposure (P<0.05).In contrast, 0.08-mg/mL PHL and combined DEL with 0.08-mg/mL PHL significantly increased the body length ofArtemia(P<0.05).
3.3 Effect of PHL on antioxidant enzymes and lipid peroxidation in DEL-exposed Artemia
As shown in Fig.3, significantly reduced activities of the enzymes (SOD, CAT, and GSH-Px)were seen in DEL-treated adultArtemiaafter 24-and 36-h exposure compared with the control group(P<0.05).Co-administration of PHL (0.03, 0.05, and 0.08 mg/mL) with DEL significantly increased the abovementioned parameters in a dose-dependent manner compared to the DEL group after 24-h exposure (P<0.05).The MDA levels in adultArtemiatreated with DEL alone were significantly increased after 24- and 36-h exposure compared to the control group (P<0.05).This increase was attenuated by the treatment with 0.08-mg/mL PHLafter 24-h exposure.However, enzymatic activities and MDA content didn’t have significant difference between DHL group and DEL+PHL groups after 12-and 36-h exposure (P>0.05).
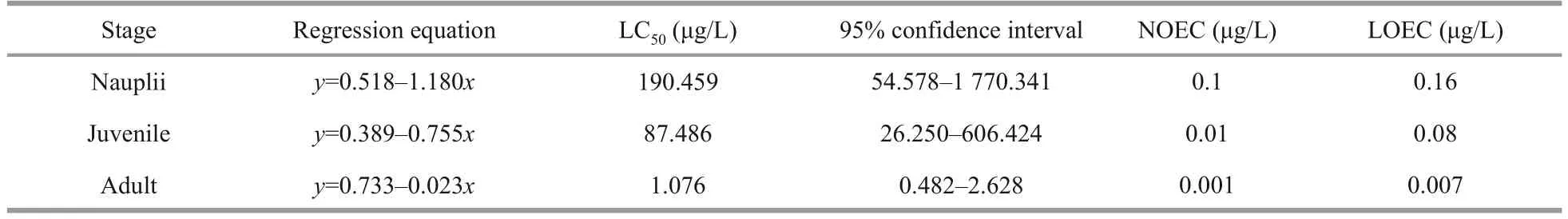
Table 2 LC50, NOEC, and LOEC of DEL for nauplii, juvenile, and adult Artemia

Fig.2 Survival rate (a) and body length (b) of Artemia when exposed to DEL, 0.03-, 0.05-, 0.08-mg/mL PHL, and their combinations
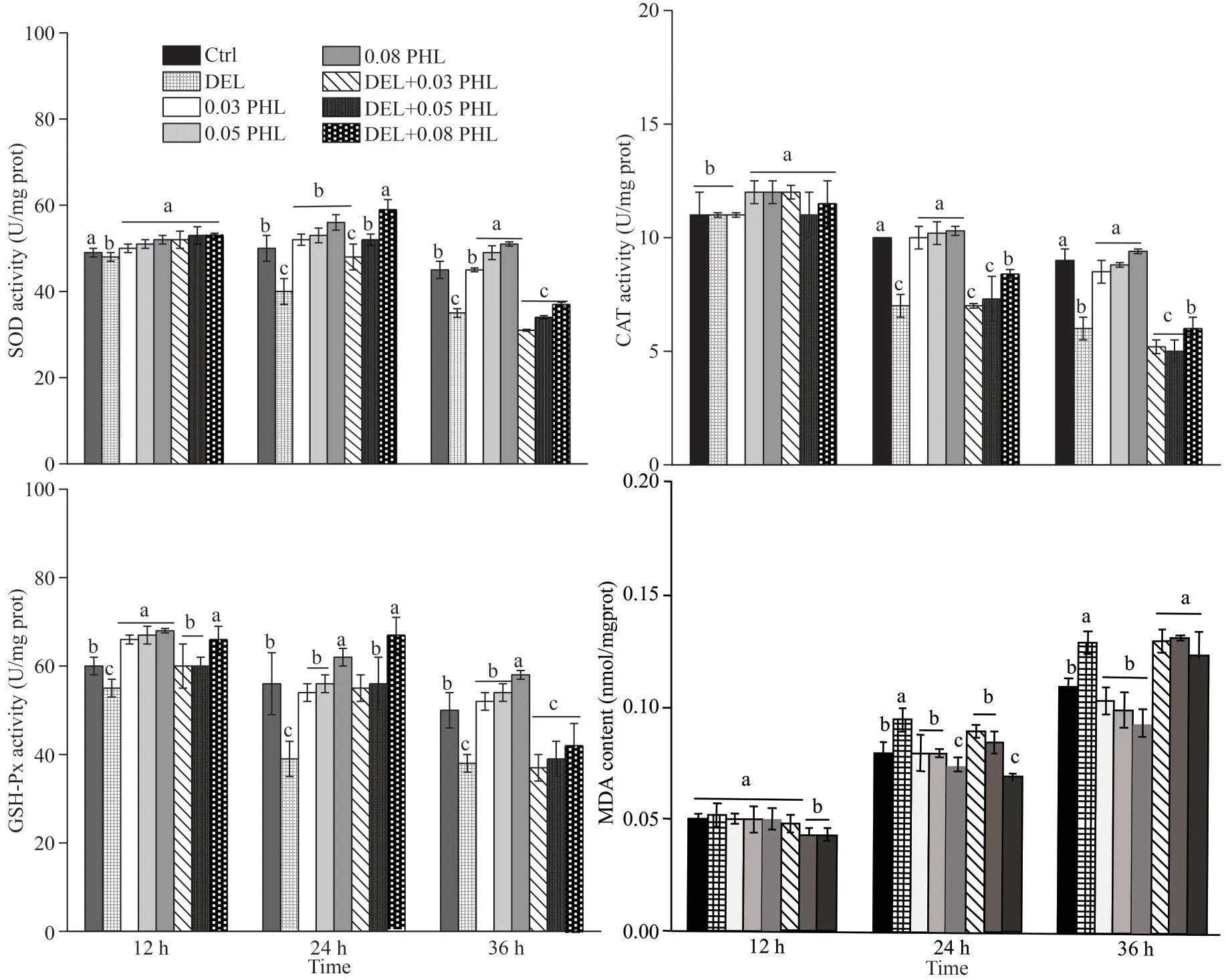
Fig.3 Activity of SOD, CAT, GSH-Px, and MDA contents in adult Artemia when exposed to DEL, 0.03-, 0.05-, 0.08-mg/mL PHL, and their combinations at 12, 24, and 36 h after 12-d general culture
3.4 Effect of PHL on ROS production in DELexposed Artemia
Exposure to DEL significantly increased fluorescence intensity compared with the control group after 12-, 24-, and 36-h exposure (P<0.05) (Fig.4).However, co-administration of PHL (0.08 mg/mL)with DEL only significantly reduced ROS levels at 24 and 36 h (P<0.05) compared with the DEL group, with the maximum decrease occurring after 24-h exposure (P<0.05).There were no significant differences between the control group and PHL groups after 12-, 24-, and 36-h exposure (P>0.05).
3.5 Effect of PHL on Nrf2 signaling pathway in DEL-exposed Artemia
As shown in Fig.5, compared with the control group, DEL dramatically decreased the mRNA levels ofSOD,CAT,GST,NOQ1, andHO-1, while the mRNA levels ofKeap1were notably increased(P<0.05).Compared with the DEL group, coadministration of PHL (0.08 mg/mL) with DEL in adultArtemiasignificantly enhanced the mRNA levels ofSOD,CAT,GST,NOQ1,Nrf2, andHO-1and significantly decreased the mRNA levels ofKeap1(P<0.05).However, there were no significant differences between the control and PHL groups(P>0.05).
4 DISCUSSION
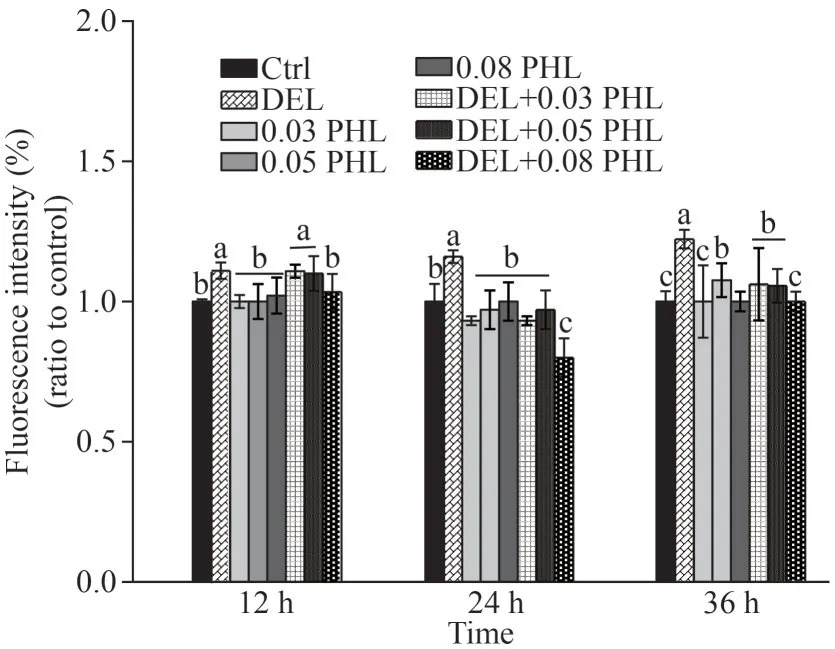
Fig.4 ROS level in adult Artemia when exposed to DEL,0.03-, 0.05-, 0.08-mg/mL PHL, and their combinations at 12, 24, and 36 h after 12-d general culture
DEL is widely employed in agriculture and forestry due to its effective insecticidal function against pests and parasites (Radovanović et al.,2017).However, continuous DEL exposure results in several negative effects, being especially harm to aquatic species (Li et al., 2022).The current study determined the LC50of DEL in nauplii, juvenile, and adultArtemia.According to LC50data, DEL is more toxic to adultArtemiathan nauplii and juvenileArtemia.It can be deduced that adultArtemiacould increase DEL ingestion and metabolism, and thus aggravating the toxic effect of DEL.Therefore, this study focuses on the toxicity of DEL to adultArtemia.For the DEL concentration exposure toArtemia, 0.1 μg/L (10% of LC50) was selected considering a severe scenario where DEL pollution occurs.Cysts hatching and growth are the main indicators for evaluating the developmental status ofArtemia, which were determined to select the optimal concentration of PHL against toxicity induced by DEL.Our results show that the hatching percentage, survival rate, and body length in DELexposedArtemiasignificantly reduced compared with the control group, and 0.08-mg/mL PHL and co-administration of PHL (0.08 mg/mL) with DEL significantly improved these indicators (P<0.05).This suggests DEL inhibited growth, survival and hatching percentage ofArtemia; PHL exerted its antioxidative effect and improved these indicators mentioned above.Similarly, the survival rate and body length decreased in DEL-inducedDaphnia magna(Toumi et al., 2013), zebrafish embryo/larvae(Daniorerio) (Parlak, 2018), and Nile tilapia(Oreochromisniloticus) (Dawood et al., 2020).Dietary supplementation with PHL also improved the growth of obese mice (Mei et al., 2016) and significantly extended the lifespans of nematode(Caenorhabditiselegans) (Park and Park, 2022).
DEL exposure induces excessive ROS production and oxidative stress in aquatic animals(Awoyemi et al., 2019; Hong et al., 2019; Yonar et al., 2019).Antioxidant enzymes, including SOD,CAT, and GSH-Px, are important indicators exposed to various environmental conditions in aquatic invertebrates (Viciano et al., 2017), serving to prevent or reduce tissue damage (Kong et al., 2021).SOD metabolizes the superoxide anion radical (·O2-)and H+into O2and H2O2, which is subsequently transformed into H2O by CAT and GSH-PX(Halliwell, 2012).Furthermore, MDA is the main product of lipid peroxidation, and its contents reflect the degree of lipid peroxidation (Zhang et al.,2020a).ROS is an indicator of antioxidants, where a decrease in ROS content indicates an increase in antioxidant capacity and a reduction in oxidative damage (Sayeed et al., 2003; Chakraborty et al.,2013).The present study demonstrated that DEL administration was associated with a significant increase in MDA and ROS levels and a decrease in the activity of SOD, CAT, and GSH-Px in adultArtemiaafter 24- and 36-h exposure.PHL acts as a scavenger of ROS because of its two phenolic hydroxyl groups and can donate hydrogen atoms to superoxide anions, hydroxyl radicals, and peroxyl radicals (Shang et al., 2022).PHL treatment (0.03,0.05, and 0.08 mg/mL) reduced DEL-induced damages by increasing antioxidant enzyme activities and decreasing MDA and ROS levels that DEL had increased after 24-h exposure.A dose of 0.08-mg/mL PHL combined with DEL is more effective.Furthermore, when the exposure time of DEL is 24 h,the protection effect of PHL is more significant.The possible reason is that the longer the exposure time of DEL, the more serious the oxidative damage inArtemia.Therefore, exposure toArtemiafor 12 h have not inducedArtemia’s oxidative damage and exposure toArtemiafor 36-h results in the inability of PHL to reverse this damage.Similarly, a previous study reported that DEL exposure elicited a persistent reduction in the activity of SOD, CAT,and GST in rainbow trout (Oncorhynchusmykiss)(Elia et al., 2017).PHL enhanced the antioxidant capacity by increasing the enzyme activity inDrosophilamelanogaster, rat brain, and human HepG2 cells (Tian et al., 2018; Wang et al., 2019a,b).DEL exposure induced excessive ROS and MDA levels in zebrafish (Daniorerio) and rats (Hong et al., 2019; Zhang et al., 2020b), and PHL significantly decreased MDA levels in rats (El-Hawary et al., 2021).PHL pretreatments prevented UVB-induced ROS overproduction and death in HaCaT cells (Zhai et al., 2015).
There is increasing evidence that the Keap1/Nrf2/ARE signaling pathway modulates the antioxidant defense system to affect oxidative equilibrium and other cellular processes (Wang et al., 2003).A previous study has shown that the Nrf2 signaling pathway is concerned with cypermethrin-induced apoptosis of the cortical neurons (Zhou et al., 2020).Meanwhile, DEL causes cardiomyocyte injury by downregulatingNrf2and its downstream protein,HO-1(Yang et al., 2022).Furthermore, PHL promotedNrf2translocated from the cytoplasm to the nucleus and upregulated its downstream antioxidative transcription factor, including heme oxygenase-1 and NADPH quinone oxidoreductase-1(Ma et al., 2022).In this study, DEL exposure decreased the mRNA expressions ofCu/ZnSOD,Nrf2,GST,NQO1,HO-1, andCATand increased the mRNA expression of Keap1 inArtemia.There was no significant difference inArtemiatreated with PHL alone and controls, which may be attributed to the fact that theNrf2signaling pathway is activated only when oxidative stress occurs.Compared to the DEL-exposed group, co-administration of PHL(0.08 mg/mL) and DEL significantly upregulated the mRNA expression levels ofCu/ZnSOD,Nrf2,GST,NQO1,HO-1, andCATand decreased the mRNA expression level of Keap1 gene.These results support the opinion that DEL induces oxidative stress inArtemia, while PHL reduces oxidative damage by activating the Nrf2 signaling pathway (Shi et al., 2019; Li et al., 2021; Yang et al.,2022).As shown in Fig.5, exposure to oxidative stress breaks the complex betweenKeap1andNrf2,leading to the translocation ofNrf2to the nucleus(Zhou et al., 2019).Then, Nrf2 binds the antioxidant-responsive element (ARE) consensus sequence, activates the transcription ofHO-1, and increases phase II antioxidant enzyme activity,includingSOD,CAT, andGSH-Px(Magesh et al.,2012).

Fig.5 Gene expression included Cu/Zn SOD, CAT, GST, HO-1, NQO1, Keap1, and Nrf2 in adult Artemia when exposed to DEL, 0.08-mg/mL PHL, and the combinations at 24 h after 12-d general culture
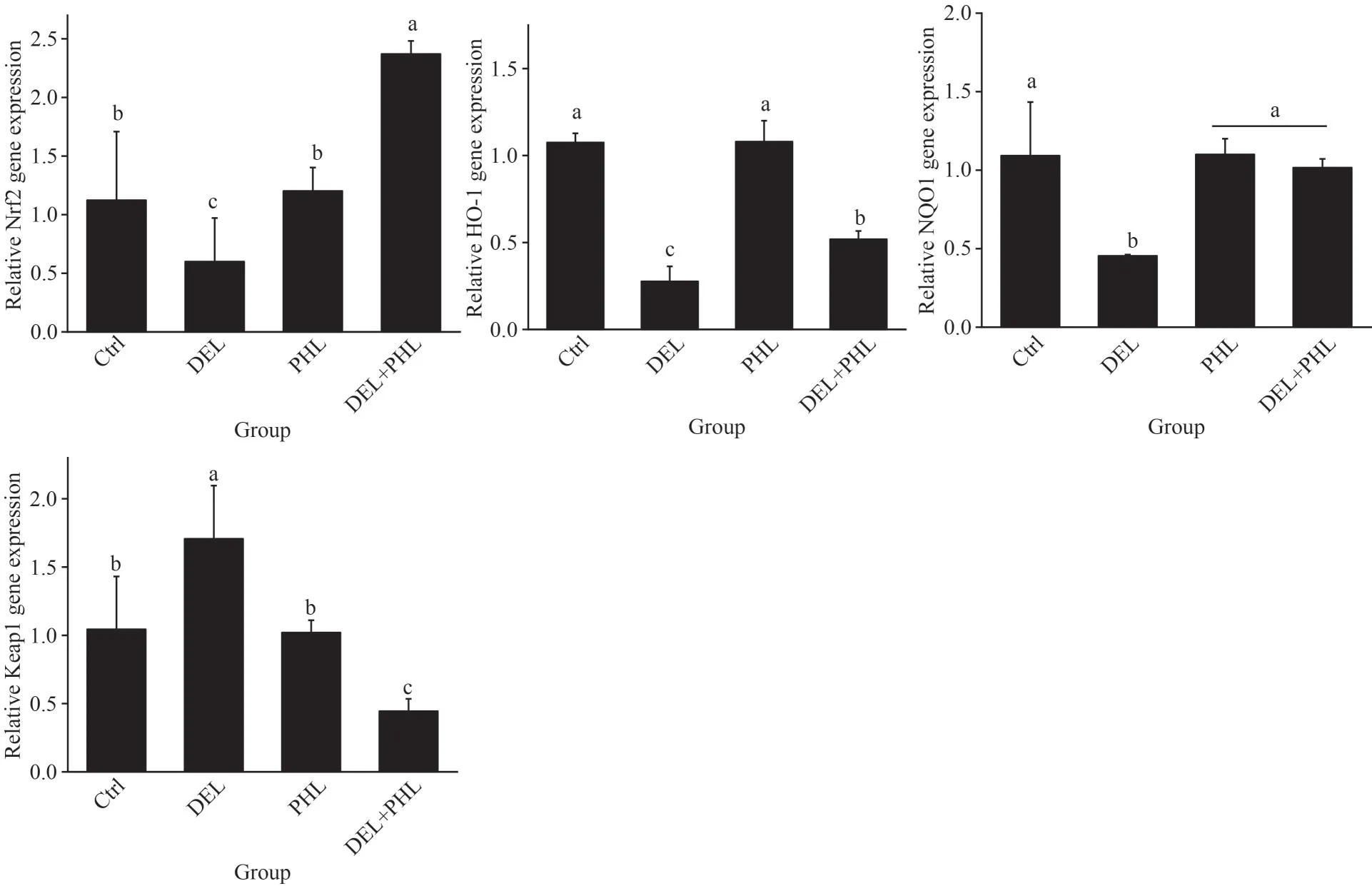
Fig.5 Continued
5 CONCLUSION
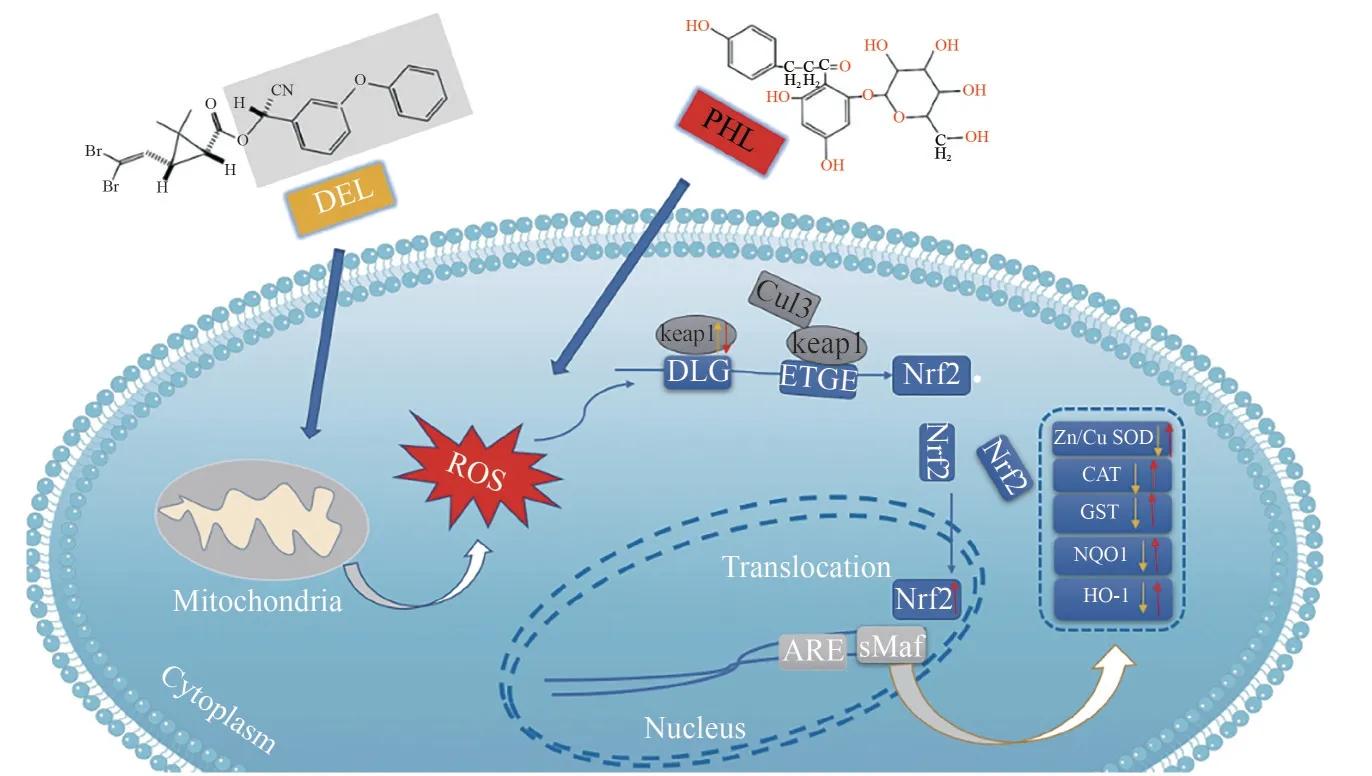
Fig.6 Oxidative stress-mediated mechanism proposed for DEL and PHL via Nrf2 signaling pathway
PHL promoted the hatching percentage, survival,and growth ofArtemiaand improved the activity of CAT, GSH-Px, and SOD enzymes, while it decreased MDA and ROS levels inArtemiainduced by DEL.Furthermore, PHL increased the mRNA expressions ofCu/Zn SOD,Nrf2,GST,NQO1,HO-1, andCATand decreased the mRNA expression ofKeap1in theNrf2signaling pathway.This study indicated that PHL could promote the growth and antioxidant stress ability ofArtemiainduced by DEL, which expanded the application of PHL and provided a theoretical basis for PHL as a functional feed additive for the healthy culture of aquatic animals.
6 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during this study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
The authors thank Asian Regional Artemia Reference Center (AR-ARC) of TUST for providing all the necessary facilities for conducting the experiments.
 Journal of Oceanology and Limnology2024年1期
Journal of Oceanology and Limnology2024年1期
- Journal of Oceanology and Limnology的其它文章
- Contrasts of bimodal tropical instability waves (TIWs)-induced wind stress perturbations in the Pacific Ocean among observations, ocean models, and coupled climate models*
- Variability of the Pacific subtropical cells under global warming in CMIP6 models*
- Identification of thermal front dynamics in the northern Malacca Strait using ROMS 3D-model*
- Magmatic-tectonic response of the South China Craton to the Paleo-Pacific subduction during the Triassic: a new viewpoint based on Well NK-1*
- An improved positioning model of deep-seafloor datum point at large incidence angle*
- Microplastics in sediment of the Three Gorges Reservoir:abundance and characteristics under different environmental conditions*
