Total flavonoids of Astragalus membranaceus protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice by inhibiting ferroptosis through SLC7A11/GPX-4 signaling pathway
Zitin Go,Gorui Wng,Yujie Chen,Wuke Yun,Jun Ci,Aiping Feng,Jie Fng,Qi Xu,*,Xiojun Wu*
a School of Public Health,Shanghai University of Traditional Chinese Medicine,Shanghai 201203,China
b Shanghai Key Laboratory of Compound Chinese Medicines,The Ministry of Education (MOE) Key Laboratory for Standardization of Chinese Medicine,Institute of Chinese Materia Medica,Shanghai University of Traditional Chinese Medicine,Shanghai 201203,China
c Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine,Shanghai University of Traditional Chinese Medicine,Shanghai 201203,China
Keywords:Parkinson’s disease Total flavonoids of Astragalus membranaceus Ferroptosis SLC7A11
ABSTRACT Parkinson’s disease (PD) is a common neurodegenerative disorder with no cure.Astragalus membranaceus is used in Chinese culture as a food supplement to boost immunity.The present study aimed to explore the neuroprotective effects of total flavonoids extracted from A.membranaceus (TFA) and their protective mechanisms.TFA offered neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse model of Parkinsonism,by improving behavior performance in the gait analysis and pole test,and inhibiting the decline of tyrosine hydroxylase (TH) positive neurons and TH protein expression in substantia nigra of mice.TFA also prevented 1-methyl-4-phenylpyridinium (MPP+) induced neurotoxicity in SHSY5Y cells,by increasing GSH and GSH/GSSG ratio,and reducing reactive oxygen species.In addition,the neuroprotective effects of TFA were associated with its ability to restore MPTP/MPP+ induced downregulation of SLC7A11 and glutathione peroxidase 4 (GPX-4).In conclusion,we demonstrated that TFA exerted significant neuroprotection against MPTP/MPP+ induced neurodegeneration by inhibiting ferroptosis through the regulation of SLC7A11/GPX-4 axis,suggesting the use of TFA as a possible food supplement in the prevention of PD.
1. Introduction
Parkinson’s disease (PD) is one of the common neurodegenerative illnesses that involves in motor and non-motor symptoms[1].The etiology causing degeneration and death of the neurons in the substantia nigra (SN) is a multifactorial process in PD that remains not well understood.Moreover,current available treatments have little effects on disease progression.Thus,precise molecular mechanisms need to be understood to help devise strategies to prevent or delay the progression of PD.
Ferroptosis is a novel type of regulated cell death that is characterized by the accumulation of intracellular lipid reactive oxygen species (ROS) accompanied with dysregulated iron metabolism and glutathione depletion[2].Accumulating evidence has demonstrated that ferroptosis is closely associated with the occurrence and development of many diseases including PD.Iron accumulation is considered as one of the major factors in the induction of ferroptosis.Elevated iron levels have long been confirmed in the brains of PD patients[3].Dysregulated expressions of genes or proteins associated with iron homeostasis were also reported in brains of PD patients[4].Moreover,iron chelators or ferroptosis inhibitors were observed to attenuate neurodegeneration through inhibiting lipid peroxidation in experimental models of PD[5,6],which further strengthens the correlation between ferroptosis and the pathogenesis of PD.
Several signaling molecules associated with ferroptosis,such as glutathione peroxidase 4 (GPX-4) and nuclear factor erythroid 2-related factor 2 (NFE2L2/Nrf2),were reported to be involved in PD pathophysiology[7].Among those,GPX-4 is the key endogenous factor for cellular resistance to ferroptosis.The intracellular GSH level is controlled by an amino acid transporter,system xC-,which transfers extracellular cysteine for intracellular glutamate.Dysfunction of system xC-or its major functional subunit SLC7A11 results in the consumption of GSH and decreased expression of GPX-4,leading to ferroptosis[8].SLC7A11/GPX-4 signaling axis plays a vital role in PD pathogenesis.Inactivation of SLC7A11/GPX-4 signaling axis can promote the degradation of dopaminergic neurons in PD[7,9].Altered expression of system xC-or GPX-4,as well as decreased GPX-4 expression and reduced GSH levels were found in experimental models of PD and PD patients[10,11],respectively,providing the groundwork for SLC7A11/GPX-4 to be taken as a new therapeutic target for PD therapy.
Astragalus membranaceusis used as a traditional herb in Asian countries for centuries.In Chinese culture,it is also used as a healthy food supplement to boost immunity[12,13].A.membranaceushas a wide range of biochemical and pharmacological activities such as immunomodulatory,anti-hyperglycemic and anti-viral activities[14].Among more than 200 compounds that have been isolated fromA.membranaceus,flavonoids are considered to be one of the major bioactive components responsible for its pharmacological activities[13].Although studies have reported that total flavonoids extracted fromA.membranaceus(TFA) have a variety of health benefits[15],its therapeutic potential in PD has not been elucidated.The objective of the current study is to explore the protective effects of TFA in animal and cell models of PD and to investigate whether its neuroprotection is exerted through the inhibition of ferroptosis.
2. Materials and methods
2.1 Materials
Antibodies against GPX-4 and SLC7A11 were purchased from Bioss Biotech (Beijing,China).Antibodies against tyrosine hydroxylase (TH) andβ-actin were purchased from Cell Signaling Technology (Massachusetts,US).CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit was bought from Promega (Wisconsin,US).5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) was purchased from Meilunbio(Dalian,China).Bicinchoninic acid (BCA) protein assay kit was purchased from Yeasen Biotech (Shanghai,China).1-methyl-4-phenylpyridinium (MPP+) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) were purchased from Sigma Aldrich(Shanghai,China).High-glucose Dulbecco’s Modified Eagle medium,fetal bovine serum (FBS),and penicillin and streptomycin (P/S)mixture were purchased from Haling Biotech(Shanghai,China).All the other chemicals used were of analytical grade.
2.2 TFA extraction
TFA was obtained from Chengdu Desite Biotech Co.,Ltd.(Chengdu,China).As described by the manufacture’s protocol,Astragalus was extracted with 90% methanol for 2 h for three cycles,then the extracts were concentrated at 60 °C and purified by polyamide column chromatography.The major composition of TFA was identified as calycoflavone glucoside (17.8%),ononin (19.8%),formononetin (33.5%) and calycosin (27.9%) by high-performance liquid chromatography.
2.3 Animal treatments
All animal experiments were performed in accordance with the approved animal protocols and guidelines established by the Animal Research Center at Shanghai University of Traditional Chinese Medicine (PZSHTCM201030013).Male C57BL/6 mice (8 weeks old,25–30 g) were purchased from Lingchang Biotech Co.,Ltd (Shanghai,China).The animals were fed and housed in a pathogen-free facility with 12 h of light and dark per day at an ambient temperature of 25 °C.
Thirty mice were divided into 3 groups including control,MPTP,and MPTP+TFA.The animals in MPTP+TFA group were treated with TFA (100 mg/kg) by oral gavage for 3 weeks.From day 17 to day 21,the animals in MPTP group and MPTP+TFA group were given MPTP (20 mg/kg) by daily intraperitoneal injection.The control mice were injected with equal volumes of saline.All the animals were sacrificed 3 days after the last injection of MPTP or saline.
2.4 Gait analysis
Gait analysis was performed using the Catwalk gait analysis system (CatWalk XT,Noldus Information Technology,Netherlands)as described in the manufacturer’s protocol.It is a simple and effective measure of behavior deficiency and gait abnormalities.Briefly,the system enables mice to walk voluntarily at preferred speeds across the glass walkway,below which a video camera is recording.A run is considered as successful if the animal walks through the walkway without hesitation.All mice were trained and subjected to three consecutive runs one day before the measurement.On the day of the test,animals were allowed to cross the walkway once or twice.The gait parameters,including average speed,cadence,duration to travel a particular distance,and maximum-variation obtained from the successful run,were analyzed by the CatWalk XT software automatically.
2.5 Pole test
The pole test was conducted to evaluate the motor dysfunction as described in previous reference[16].This apparatus includes a 50-cmlong wooden pole wrapped in gauze and a 0.5 cm diameter ball on the top.The mice were held head up on the top of the pole apparatus and the time spent from the top to the bottom was recorded to reflect bradykinesia.
2.6 Western blot analysis
The experiment was performed as previously described in our publication[17].After treatments,the cells or tissues were lysed with RIPA buffer containing protease and phosphatase inhibitor tablets(Meilun,Dalian,China).The protein samples were separated by 10%or 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis(SDS-PAGE) and then transferred onto a polyvinylidene fluoride membrane.After blocking with 5% nonfat milk,proteins on membranes were separately labeled using primary rabbit antibodies against TH,GPX-4,and SLC7A11 for 12 h at 4 °C.Membranes were then incubated with secondary antibody against rabbit for 1 h at room temperature.The reaction band was amplified through electrochemiluminescence and the densitometry was analyzed by an image system (Tanon Image software,Shanghai,China).Protein signals were normalized toβ-actin signal.
2.7 Immunohistochemistry
Immunohistochemical staining was performed as previously described[18].The brains were fixed in 4% paraformaldehyde for 24 h,and sequentially transferred to 15 g/100 mL and 30 g/100 mL sucrose solutions for dehydration.The fixed brains,including the SN,were cut into 8 μm sections by a cryostat and blocked with 5%bovine serum albumin for 30 min followed by incubation overnight with primary antibody against TH at 4 °C.After incubation with fluorescence-conjugated secondary antibody against rabbit,the sections were visualized and the images were analyzed by a fluorescent microscope (Olympus IX81,Japan).
2.8 Cell cultures and treatments
SH-SY5Y neuroblastoma cells (National Collection of Authenticated Cell Cultures,Shanghai,China) were maintained in T25 flasks with high-glucose Dulbecco’s Modified Eagle Medium supplemented with 10 g/100 mL FBS and 1 g/100 mL P/S in a humidified atmosphere containing 5% CO2at 37 °C.To evaluate the neuroprotective role of TFA against MPP+induced neurotoxicity,cells were seeded in 96-well plates at a density of 1 × 105cells/well and then treated with 50 or 100 μg/mL TFA for 2 h.The medium was replaced with fresh medium or 2 mmol/L MPP+for the subsequent 24 h.To determine the role of ferroptosis in TFA mediated neuroprotection,cells were treated with 20 μmol/L of erastin for 2 h before the treatment of TFA and MPP+.
2.9 Cell viability measurement
Cell viability was assessed using MTS assay as described in our previous publication[19].Briefly,cells were incubated with MTS (1:20 dilution) and incubated for 2 h at 37 °C in a 5% CO2incubator.Cell viability was analyzed based on the conversion of MTS tetrazolium salt using the microplate reader at the absorbance of 490 nm.
2.10 Statistical analysis
One-way ANOVA was used to estimate the overall significance determined by Tukey’s tests corrected for multiple comparisons.Data are presented as mean ± standard error of mean (SEM).Statistical analysis was performed using Graphpad Prism 7.0 software (GraphPad Software,USA).A probability level of 5% (P<0.05) was considered to be significant.
3. Results and discussion
3.1 TFA improved the behavioral performance in gait analysis and pole test in MPTP treated mice
The average speed and cadence in unit time were dropped by 25.7% and 21.8% (P <0.01) in MPTP treated mice (Fig.1A,B).TFA improved the gait performance by increasing average speed and cadence by 26.3% (P <0.01) and 22.8% (P <0.05),respectively.Moreover,the walking duration and maximum variation were increased by 23.2% (P <0.05) and 106.8% (P <0.01) in MPTP treated mice,which were significantly ameliorated (P <0.01) by TFA pretreatment (Fig.1C,D).Motor coordination and bradykinesia were also measured using the pole test.As shown in Fig.1E,the MPTP treated mice exhibited a 38.2% (P <0.001) increase in total time to descend the pole compared with the control mice.However,TFA counteracted the neurotoxic effects induced by MPTP and reduced total descent time near to the control level (P <0.001).These results demonstrated that TFA could improve mice spontaneous activity and rescue the impaired motor function,suggesting TFA might be a potential candidate to mitigate PD motor symptoms.
3.2 TFA prevented loss of TH positive neurons in the SN of MPTP treated mice
We investigated the protective effects of TFA against MPTP induced loss of TH positive neurons (Fig.2).We found that administration of MPTP induced 51.7% decrease of TH positive neurons (P <0.01) in the SN compared with the control mice(Fig.2A and B).TFA pretreatment prevented the decrease and restored TH positive neurons to 77.2% of the control (P <0.05).Consistent with the reduced number of TH positive neurons induced by MPTP,the protein expression of TH in SN was also decreased by 39.8%(P <0.001) in MPTP treated mice,compared with the control mice(Fig.2C and D).TFA pretreatment ameliorated the reduction and increased the protein expression of TH by 35.3% (P <0.01).

Fig.2 The protective effects of TFA against MPTP induced loss of TH positive neurons (A-B) and TH protein expression estimated by western blot analysis (C-D).Values are mean ± SEM (n =3).Means compared with MPTP group,*P <0.05,**P <0.01,***P <0.001.
3.3 TFA exerted neuroprotective effects via SLC7A11/GPX-4 axis in the SN of MPTP treated mice
Next,we explored the molecular mechanisms underlying the neuroprotective effects of TFA by assessing ferroptosis related proteins SLC7A11 and GPX-4 (Fig.3).We observed that the protein expressions of SLC7A11 and GPX-4 were decreased by 37.0% and 37.3% (P<0.01),respectively,in the SN of MPTP treated mice(Fig.3B and C).TFA pretreatment prevented the reduction and restored the expression of SLC7A11 and GPX-4 near to the control level(P<0.001).The results indicated SLC7A11/GPX-4 axis may play an important role in TFA mediated neuroprotection.
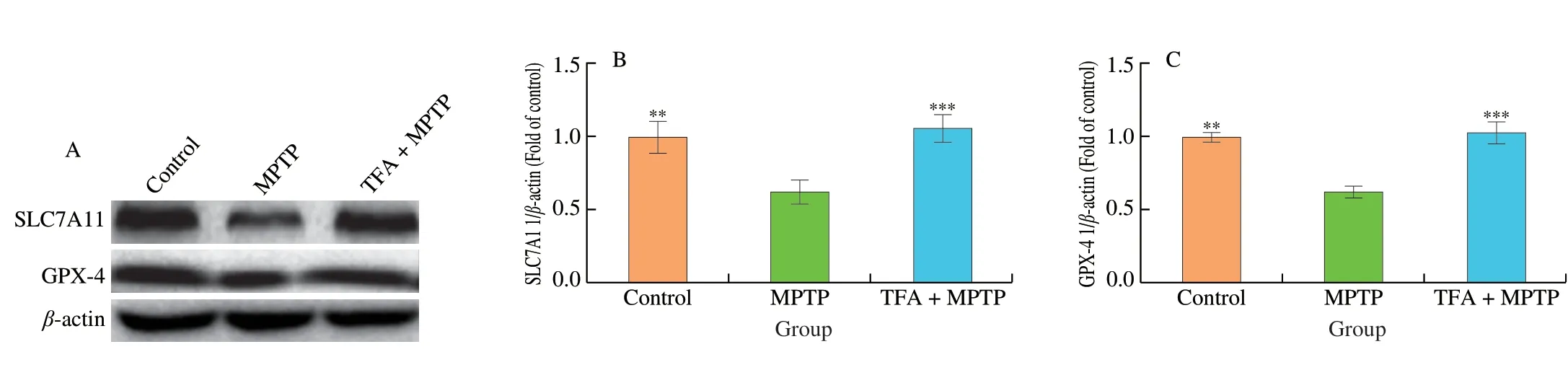
Fig.3 The protective effects of TFA against MPTP induced downregulation of SLC7A11 (A-B) and GPX-4 (A-C) estimated by western blot analysis.Values are mean ± SEM (n =3).Means compared with MPTP group,**P <0.01,***P <0.001.
3.4 TFA prevented MPP+ induced neurotoxicity by reducing ROS and increasing GSH in SH-SY5Y cells
To examine the effects of TFA on dopaminergic neurons,SH-SY5Y cells were treated with various concentrations of TFA for 24 h.As shown in Fig.4A,TFA did not exhibit cytotoxicity at concentrations less than 200 μg/mL.Based on these results,we chose TFA concentrations at 50 and 100 μg/mL to evaluate the protective effects against MPP+induced neurotoxicity.Cell viability was decreased by 75.1% (P <0.001) after 2 mmol/L MPP+treatment,which was significantly relieved by 50 and 100 μg/mL TFA pretreatment (P <0.001) (Fig.4B).Since TFA at 100 μg/mL showed more protective effects,we chose the 100 μg/mL dose to further investigate its neuroprotective mechanisms in the subsequent experiments.Intracellular ROS level was increased by 39.8%(P <0.01) after MPP+treatment,while TFA pretreatment blocked the increases of ROS levels (P <0.01,Fig.4C).In addition,MPP+treatment reduced intracellular GSH and GSH/GSSG ratio to 63.2%and 62.1% of the control cells,respectively (P <0.001,Fig.4D and E),while TFA showed significant protection through elevating GSH by 30.8% and 53.9%,respectively (P <0.001).
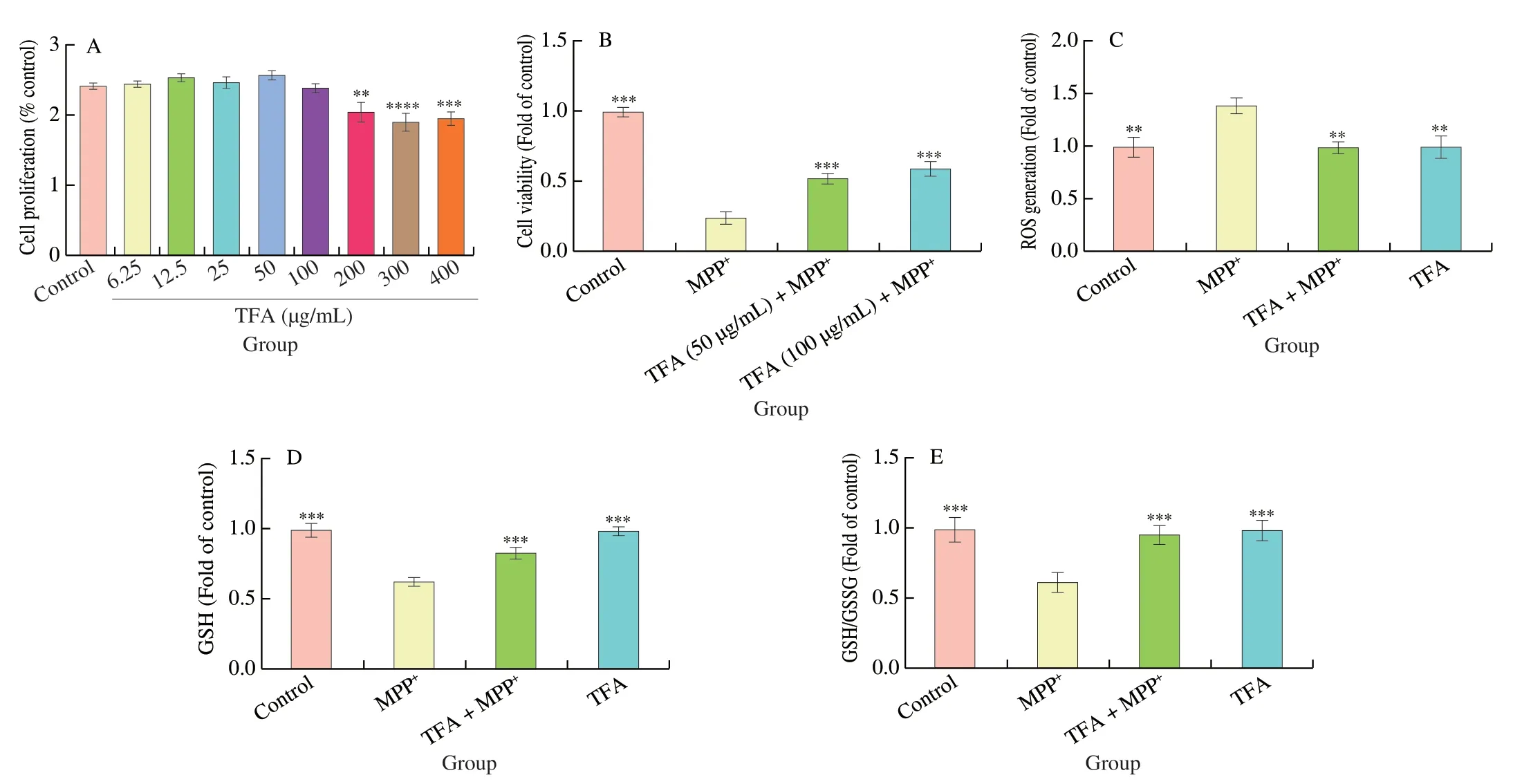
Fig.4 The cytotoxicity of TFA (A) and the protective effect of TFA against MPP+ induced cell death (B),ROS generation (C),reduced GSH (D) and GSH/GSSG ratio (E).Values are mean ± SEM (n =6 for A,B and C,n =3 for D and E).Means compared with MPP+ **P <0.01,***P <0.001.
3.5 TFA attenuated MPP+ induced neurotoxicity via SLC7A11/GPX-4 axis in SH-SY5Y cells
In addition,we also examined the role of SLC7A11/GPX-4 axis in TFA mediated neuroprotection in SH-SY5Y cells (Fig.5).We observed that MPP+reduced protein expression of SLC7A11 and GPX-4 to 67.6% (P <0.05) and 30.3% (P <0.01) of the control cells (Fig.5A-C).Consistent within vivoresults,TFA pretreatment upregulated protein expression of SLC7A11 and GPX-4 by 35.6%and 81.5% (P <0.05),compared with MPP+treatment.To further confirm whether TFA exerted neuroprotection through the inhibition of ferroptosis,we used erastin to inhibit SLC7A11 and induce ferroptosis in SH-SY5Y cells.The combination of erastin and MPP+further decreased cell viability by 32.9% compared with MPP+alone(P <0.01).Moreover,TFA did not prevent the neurotoxicity induced by erastin and MPP+,indicating that TFA prevented MPP+induced neurotoxicity likely through the inhibition of ferroptosis.
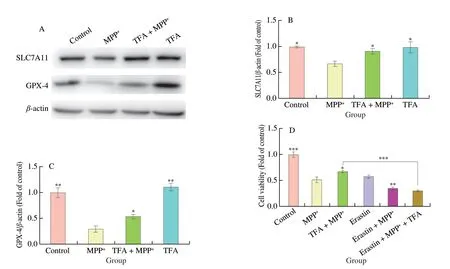
Fig.5 The protective effects of TFA against MPP+ induced downregulation of SLC7A11 (A-B) and GPX-4 (A-C) and cell death (D).Values are mean ± SEM (n =3 for B and C,n =6 for D).Means compared with MPP+ group,*P <0.05,**P <0.01,***P <0.001.
4. Discussion
Flavonoids are water soluble polyphenols synthesized by plants that are common components in the human diet.They are widely present in various kinds of foods such as fruits,nuts,teas grains and vegetables and contribute to the colorfulness of fruits,leaves and flowers[20,21].There are more than 4 000 kinds of flavonoids identified to date,and their biological functions such as antioxidant and antiinflammatory activities are extensively reported.It was also reported that flavonoids can significantly improve cognitive capabilities and prevent age-related neurodegenerative disorders such as Alzheimer’s disease and PD[22-24].However,different types of flavonoids possess distinct biochemical activities based on their configuration,combination of hydroxyl groups,and specific functional group substituents.Although various flavonoids were demonstrated to exert neuroprotective action in PD[24],the neuroprotective role of TFA in PD is seldom studied.In our current study,we demonstrated that TFA protected against MPTP and MPP+induced neurodegeneration in experimental models of PD.
Neurotoxin MPTP and its active oxidative metabolite MPP+have been well employed for the establishment ofin vivoandin vitromodels of PD.MPTP is converted to MPP+after crossing the blood-brain barrier,and destroys the dopaminergic neurons by inhibiting mitochondrial function in primates and lower animals.Although MPTP rarely induces the formation of Lewy bodies,the neurochemical,behavioral,and histopathological alterations in mice closely resemble the clinical symptoms of PD patients.A recent study found that MPP+induces cell death accompanied with decreased GPX-4 and GSH levels,which could be mitigated by a selective ferroptosis inhibitor ferrostatin-1[25].Consistent with this study,our study found that MPP+induced neuronal death by reducing GPX-4 expression and its upstream regulator SLC7A11,and by increasing ROS production.Since GPX-4 and SLC7A11 have been well accepted as powerful indices to evaluate ferroptosis[26],our results demonstrated the role of ferroptosis in MPP+induced cytotoxicity.Moreover,ourin vivoresults also demonstrated the decreased expression of SLC7A11 and GPX-4 in the SN of MPTP treated mice,confirming that dysfunction of SLC7A11/GPX-4 axis is involved in MPTP induced neurodegeneration.
A.membranaceusis widely applied in Chinese Medicine to treat neurological diseases[24].A handful of studies have demonstrated that Astragalus polysaccharides could attenuate neurodegeneration in experimental models of PD[27-29],However,the neuroprotective effects of TFA in PD are seldom investigated.Our previous study demonstrated that TFA inhibited microglia mediated neuroinflammation by regulating JNK/AKT/NFkB signaling pathway in experimental autoimmune encephalomyelitis,an animal model of multiple sclerosis[30].In our current study,we demonstrated that TFA attenuated MPTP/MPP+induced neurodegeneration in bothin vivoandin vitromodels of PD through inhibition of ferroptosis.We found that TFA increased cellular antioxidant capacity by upregulating SLC7A11 and GPX-4 expression,and elevating intracellular GSH level and GSH/GSSG ratio.In addition,ferroptosis inducer erastin aggravated MPP+induced neurotoxicity and abolished the neuroprotective effects of TFA,further suggesting the essential role of SLC7A11/GPX-4 signaling axis in TFA mediated neuroprotection.
It has also been demonstrated that iron chelators may exert neuroprotection through inhibiting ferroptosis in PD.Since the use of synthetic iron chelators may cause side effects such as hypoxia[31],natural iron chelators with high safety profiles are preferred.A.membranaceusis generally regarded as a safe food supplement with minimal side effects.It is used as a functional food according to the China National Medical Products Administration (NMPA).The effective dose of TFA used in our study is 100 mg/kg,which is equal to 8 mg/kg in humans based on body surface area method[32].This was estimated to be a daily consumption of 560 mg TFA for a 70 kg adult,to achieve neuroprotective effects.The recommended daily dose forA.membranaceusin China is 9-30 grams and total flavonoids amount in the herb is around 11 mg/g[33,34].This equates to a maximum of 330 mg of TFA that can be consumed through daily consumption ofA.membranaceus.However,our results still indicate the dietary consumption of Astragalus flavonoids will benefit patients with PD.Bioavailability and permeation of blood-brain barrier are the challenges for the development of therapeutic agents in the neurodegenerative disorders.Flavonoids and their metabolites can cross the blood-brain barrier during chronic or acute administration and exert various effects on the central nervous system[35,36].The major flavonoids isolated fromA.membranaceusare isoflavones,such as formononetin,ononin and calycosin[37].Research has demonstrated that isoflavones have higher absorption rate compared to other flavonoids.One study shows the bioavailability of formononetin and ononin are 21.8% and 7.3%,respectively[38].Another study found that calycosin and formononetin can improve brain function by modulating the brain-gut axis[39].Our present study demonstrated that TFA attenuated MPTP induced motor deficits and dopaminergic neurodegeneration,indicating its ability to cross blood-brain barrier.However,future studies are needed to evaluate the bioavailability of TFA,as well as its ability to enter the central nervous system.
In conclusion,we demonstrated that TFA was neuroprotective against MPTP and MPP+induced neurodegeneration through the inhibition of ferroptosisin vivoandin vitromodels of PD.Mechanistically,it exerts these effects by increasing antioxidant capacity,and inhibiting ferroptosis through the regulation of SLC7A11/GPX-4 axis.Our results seem to suggest potential benefits in supplementingA.membranaceusto foods,or using flavonoids extracted fromA.membranaceusas a dietary supplement to benefit PD patients.These results might also point to the modulation of ferroptosis as a potential therapeutic target for PD treatment.
Conflict of interest
The authors declare they have no competing of interests.
- 食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics

