Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
Ynxing Bi,Jibo Ni,Xiofeng Xue,Zidn Zhou,Wenli Tin,Vlérie Orst,Sh Yn,Wenjun Peng,*,Xioming Fng,*
a State Key Laboratory of Resource Insects,Institute of Apicultural Research,Chinese Academy of Agricultural Sciences,Beijing 100093,China
b College of Engineering,China Agricultural University,Beijing 100083,China
c Bioresource Engineering Dept.,McGill University,Ste-Anne-de-Bellevue QC H9X 3V9,Canada
Keywords:Drying Bee pollen Free amino acids α-Dicarbonyl compounds Volatile compounds
ABSTRACT The significant demand for high quality food has motivated us to adopt appropriate processing methods to improve the food nutritional quality and flavors.In this study,the effects of five drying methods,namely,pulsed vacuum drying (PVD),freeze drying (FD),infrared drying (IRD),hot-air drying (HAD) and sun drying(SD) on free amino acids (FAAs),α-dicarbonyl compounds (α-DCs) and volatile compounds (VOCs) in rape bee pollen (RBP) were determined.The results showed that FD significantly released the essential amino acids(EAAs) compared with fresh samples while SD caused the highest loss.Glucosone was the dominant α-DCs in RBP and the highest loss was observed after PVD.Aldehydes were the dominant volatiles of RBP and SD samples contained more new volatile substances (especially aldehydes) than the other four drying methods.Comprehensively,FD and PVD would be potential methods to effectively reduce the quality deterioration of RBP in the drying process.
1. Introduction
Currently,the plant-based healthy foods have spurred a huge interest from consumers.The foods can not only provide rich nutrients but effectively prevent malnutrition,heart disease and cancer[1].Bee pollen,which is on average of 54.22% carbohydrates,21.30%proteins,5.31% lipids and other beneficial ingredients,including amino acids,polysaccharides and flavonoids,is considered as “the world’s best food product”[2,3].However,due to the high moisture cotent of 21.00%–30.00% (wet basis) and inherent enzymes,fresh bee pollen is extremely susceptible to microbial contamination and spoilage after its collection,thus can reduce its commercial value and endanger consumers’ health[4].
Drying is an essential operation for long-term storage of bee pollen and other agricultural products by inhibit microbial growth and biochemical reactions,playing a crucial role in improving food security[5].However,drying is a complex process that will lead to changes of the key flavor compounds and damage the nutritional and sensory value of the final product.Bee pollen contains an average of 21.30% protein including more than 20 free protein amino acids and a large number of non-protein amino acids[3]. Drying of bee pollen is considered harmful to its proteins due to the thermal stress triggered by increased temperatures,that result in conformational instability. Meanwhile,protein can lose its hydration shell during drying process that cause protein denaturation,degradation,and further lead to loss of its functionality.Besides,amino acid oxidative modifications can enhance protein oxidation which leads to an increase of carbonyl groups within the protein side chains and the formation of carbonyl compounds during drying.These active carbonyl compounds participate in the Maillard reaction to produceα-dicarbonyl compounds (α-DCs)[6].α-DCs are highly reactive critical intermediates in the Maillard reaction as well as the precursor of advanced glycation end-products (AGEs),which are linked to chronic diseases like diabetes and cancer[7].Previous study found that nineα-DCs levels varied by processing and storage conditions,among which 3-deoxyglucosone may be used as a reliable marker to distinguish fresh acacia honey and artificially heated acacia honey[8].It has been reported that the formation ofα-DCs during the dehydration process or roasting of dried fruits and edible seeds,indicating that the presence ofα-DCs can convert into more toxic compounds when their structure changes in the food product[9].To the best of our knowledge,research findings on the influence of processing on the amino acid composition of bee pollen and the correlation analysis with carbonyl compounds are still not available.
Flavor,as an important indicator to evaluate the sensory properties and freshness of bee pollen,is closely related to the acceptance from consumers.A hundred and six kinds of volatile organic components(VOCs) have been identified as leading to the aroma and flavor of different bee pollen sources[10].A few VOCs,including styrene,limonene,nonanal,and hexanal,are considered to be the key components that provide the characteristic aroma of green and yellow bee pollen.1-tridecene is considered to be the main volatile component of brown bee pollen,accounting for 43.30% of the total mass of volatile components and a key element of quality assessment[11].In addition,during postharvest processing and mishandling that may lead to spoilage,changes in the sensory aromatic profile of foods usually involve the volatilization of carbonyl compounds.During the thermal processing,theα-DCs generated by Maillard reaction can act as important precursors to participate into the changes of major volatile flavor chemicals[12].Therefore,studies at the molecular level to explore the effect of dehydration processes on the flavor attributes of bee pollen are still needed.The freshness evaluation of bee pollen should be based on flavor characteristics,carbonyl compounds and their key reactant-amino acids.
Drying technologies such as freeze drying (FD),infrared drying(IRD),and hot air drying (HAD) have been widely used in industrialscale food processing[13].A suitable drying method is important for bee pollen to maintain high quality.Sun drying (SD) and HAD are the traditional methods of bee pollen due to the simple operation and low cost[4].However,some studies have investigated that the chemical characteristics of fresh bee pollen is influenced by the treatment process,especially in heat process[14].In addition,FD is considered as one of the best drying methods for the production of high-quality food,but it is quite expensive[15].Therefore,several advanced drying techniques including pulsed vacuum drying (PVD),infrared drying,and have been explored to food drying,especially in functional food.PVD is a relatively novel drying technology developed in recent years,which involves changing the drying chamber environment into an alternating state of vacuum-atmospheric to promote quick moisture transfer during the drying process.PVD technology can break the equilibrium state of partial pressure of water vapor inside and outside and generate porous structures in the materials,thus increasing the drying rate.It has been employed in the dehydration of grape,lemon,rhizoma dioscoreae,wolfberry.Vacuum drying of banana puree results in better retention of volatile compounds than hot air drying[16].Hence,the final products obtained from these methods may differ in nutritional properties and sensory quality,which need to be further studied.
Therefore,this study aimed to investigate the effect of the five different drying methods on theα-DCs,amino acids and volatile compounds and their changes as a function of the drying process and drying parameters used.Qualitative and quantitative analyses ofα-DCs,amino acids and volatile compounds formed in the drying process of bee pollen was studied and presented here for the first time.Meanwhile,the transformation and synthesis pathways ofα-DCs were analyzed preliminarily.The findings of this research provide a theoretical basis for selecting drying methods to maximize bee pollen product quality and provide a reliable theoretical support for the development and processing of bee products.
2. Materials and methods
2.1 Materials
Fresh rape bee pollen (RBP) was purchased from a bee keeper in Xining City,Qinghai province,China.The initial moisture content was determined via vacuum drying at 70 °C for 24 h and the result was (19.17 ± 0.16)% on wet basis.The fresh pollen was packed in polypropylene bags and stored in a refrigerator at-18 °C before further use and analysis.The experimented samples with consistent size were carefully selected to ensure the uniformity of the physicochemical properties.
2.2 Drying methods
The samples were subjected to the following five drying methods:PVD,FD,IRD,HAD and SD.The drying was conducted until the samples reached the final moisture content of approximately 5% (wet basis),which is recommended for long-term storage of bee pollen[17].To avoid the formation of Maillard compounds,and according to preliminary trials,the drying temperature of PVD,IRD,and HAD processing was set at 45 °C and the experiments were carried out in laboratory-scale dryers installed in the College of Engineering of China Agricultural University,Beijing,China.In the PVD method,the durations of vacuum and atmospheric pressure periods were set for 12 and 3 min,respectively.The samples for FD were previously frozen at -40 °C for 16 h and then dried in a freeze dryer at 25 °C and 50 Pa.The IRD samples were performed in a YXD-F9 infrared drying oven (Shanghai Jihang Machinery CO.,LTD,China) at 1000 W.HAD samples were conducted at the temperature of 45 °C with the air velocity set at 3 m/s.In the SD method,the fresh RBP samples were placed on the surface of drying trays at room time (25 °C).All of the drying experiments were conducted in triplicate.
2.3 Identification and quantification of α-DCs in RBP
The identification and quantification ofα-DCs in RBP were based on a published method with slight modifications[18].Fresh and dried samples were ground into powder with an analytical grinder (IKA®A11 basic) for one minute and then stored at 4 °C until analysis.A sample of 1.0 g of ground bee pollen was added into centrifuge tube and extracted with 5 mL of 10% aqueous methanol and then centrifuged at 10 000 ×gat 30 °C for 5 min.Supernatant was mixed with 0.8%o-phenylenediamine (OPD),the tube was shaken without heating until complete dissolution and then derivatized for 12 h in an orbital shaker at 30 °C.The reaction solution was purified using C18solid-phase extraction column (6 mL,500 mg,Waters,Milford,Masssachusetts USA).The eluent was dried with nitrogen and redissolved with methanol.The solutions were filtered with a 0.22 μm nylon membrane filter for further analysis.
α-DCs were determined by using an Agilent 6560 Ion Mobility Q-TOF LC/MS.Agilent Poroshell 120 SB-C18(4.6 mm × 50 mm,2.7 μm) column was selected and the column temperature was set at 30 °C.Samples were eluted using a step-wise gradient of 0.1%formic acid aqueous solution (A) and methanol (B) as follows:0–1.5 min,95% A;1.5–10 min,45% A;10–20 min,5% A;20–23 min,5% A and 23–24 min,95% A at a flow rate of 0.3 mL/min.The MS was operated in electron spray ionization (ESI)-positive ion mode with a scan mass-to-charge ratio (m/z) from 65 to 950,and nitrogen was the nebulizing gas.The MS parameters were as follows: gas temperature of 260 °C;drying gas flow of 8 L/min;and nebulizer pressure set at 40 psi.Sheath gas temperature was 360 °C and its flow rate was 12 mL/min.A 0.5 μL sample was injected for each run.
2.4 Determination of volatile compounds
The volatile compounds of the samples were analyzed by FlavourSpec®flavor analyzer (Hanon,Jinan,China).Take 2.0 g of RBP sample into a 20 mL headspace vial.Each sample was incubated for 15 min at 5 000 ×gat 60 °C.The temperature of the autosampler was set at 85 °C and the injected volume was 500 μL.Gas chromatography (GC) separation was carried out with a FS-SE-54-CB-1 column (15 m × 0.53 mm ID) at 60 °C.Ultra-high purity nitrogen (150 mL/min,constant flow mode) was used as the carrier gas.The GC Analytical Conditions are as follows: 2 mL/min for 2 min,10 mL/min for 8 min,100 mL/min for 10 min,150 mL/min for 10 min.The drift gas flow was set to 150 mL/min.
2.5 Determination of free amino acids
Samples of 2.0 g were precisely weighed into a centrifuge tube and 10 mL extra-pure water was added.After 15 min sonication,the samples were centrifuged at 10 000 ×gfor 10 min.Then,the volume of 1 mL supernatant was mixed with 1 mL 8% sulfosalicylic acid extraction solution to remove the protein from the RBP and then centrifuged at 10 000 ×gfor 15 min.The volume of 1 mL supernatant was dried with nitrogen and redissolved with 2 mL 0.02 mol/L hydrochloric acid.The solutions were filtered using 0.22 μm nylon membrane filter for further analysis.
The samples were analyzed by the amino acid analyzer (HITACHI L-8900,Japan) attached HITACHI HPLC Packed Column with Ion-exchanging Resin No.2622 PF (4.6 mm × 60 mm) and UV detector(VIS1: 570 nm,VIS2: 440 nm).Wako L-8500 buffer solution PF-1,2,3,4 and RG were used in this study.20 μL of sample was automatically injected and determination was performed using ninhydrin reagent set (Wako Chemical Inc,Japan).
2.6 Statistical analysis
All analyses were performed in triplicate,and the results were expressed as the mean ± standard deviation.In each parameter,the differences between samples were determined by one-way analysis of variance (ANOVA) and Waller-Duncan’s multiple range test at 5% probability level using SPSS 26.0 (SPSS Inc.,Chicago,IL,USA)and Origin Pro 2021 (OriginLab Inc.,Northampton,MA,USA).GC-IMS technology was equipped with analysis software including LAV (Laboratory Analytical Viewer),three plug-ins (Reporter,Gallery Plot and Dynamic PCA) and GC × IMS Library Search,which allows samples to be analyzed from different perspectives.Qualitative analysis and identification of compounds depended on the IMS database and built-in database.
3. Results and discussion
3.1 Free amino acid profile
The composition and profile of free amino acids are commonly used as indicators for assessing the nutritional value of bee pollen.As shown in Table 1,a total of 33 free amino acids were detected in RBP samples.The total AA contents of the fresh and dried BP were significantly different,varying from 2.39 to 3.09 mg/kg.The highest concentration was found in HAD samples.This phenomenon can be attributed to the Maillard reaction between the amino acids and reducing sugars in bee pollen.Heating facilitated the generation of free AA from protein.Most of the amino acids are located in the outer membrane-exine of pollen.As a result of high content of protein,the bee pollen may suffer proteolysis,lipolysis or enzymatic oxidation when exposed to oxygen,heat,or action of enzymes.Proteins are hydrolysed by proteases,rendering large peptides,which are further hydrolysed to small peptides and free amino acids[19].Hence,when the amount of amino acids produced by the protein was greater than those consumed in the Maillard reaction,the final total AA concentration would be higher than that of the fresh samples.The similar results were also found in the study on the effects of different drying methods on the total AA content ofPleurotus eryngii[20].

Table 1 Effects of different drying methods on bee pollen amino acids.(mg/kg).
γ-Aminobutyric acid (GABA),found in highest amounts in all RBP samples,is a common non-protein amino acid that can relieve depression and lower blood pressure while boosting metabolism.It is synthesized from glutamic acid by catalytic reaction with glutamate decarboxylase.Compared to fresh sample,SD and PVD samples showed significant decrease (P<0.05).The increase is justified by the fact that GABA can quickly accumulate under environmental stress conditions such as hypoxia,drought,and cold temperature to adjust the organism’s own environmental adaptability[21].It has also been observed that the increase of exogenous GABA can improve the tolerance of tomato seedling under low temperature stress[22].
Over and above the 26 non-essential amino acids,seven essential amino acids,including isoleucine,leucin,methionine,phenylalanine,threonine,valine and histidine,were detected in fresh RBP.The essential amino acids accounted for 12.59% of the total amino acids content.Interestingly,among all drying methods,the content of essential amino acids in PVD and FD samples were the highest,while the lowest essential amino acids content was observed in SD samples.This may be due to the dehydration stress that led to the release ofamino acids during protein solubilization,which,also promoted the Maillard reaction and the formation of carbonyl compounds(under high temperature conditions).Compared with other amino acids,the essential amino acids are the most important key reactants that promote the Maillard reaction.Therefore,it is reasonable that the content of essential amino acids in HAD and IRD samples is relatively low.The loss of free amino acids increased may also be related to Maillard reaction.Meanwhile,SD samples preserved the lowest amount of total amino acids,where the SD method reduced the amino acid content,which is not favorable.This result can be explained by the long heating time which favor the decomposition of heat sensitive AAs[23].
Most of the amino acids are located in the outer membrane-exine of pollen.Due to high content of protein and lipid,the bee pollen may suffer proteolysis,lipolysis or enzymatic oxidation when it is exposed to oxygen,heat,or action of enzymes,and this will enhance Maillard reactions and Strecker degradation,contributing to form special flavor compounds.Proteins are hydrolysed by proteases,rendering large peptides,which are further hydrolysed to small peptides and free amino acids (FAA).Meanwhile,FAA are precursors of many volatile compounds[24].Since proteins are the major constituents of bee pollen,proteolysis and the production ofα-DCs will adversely affect the quality of bee pollen.
3.2 α-Dicarbonyl compounds
α-DCs are linked to chronic conditions like diabetes,alzheimer disease,and cancer[7].From this point of view,it is very important to monitor the additional production ofα-DCs during food processing.The samples were directly infused into the Q-TOF using iterative MS/MS acquisition mode.Theα-DCs were unambiguously identified according to their mass and production ions in agreement with published structural data[25].In this study,nineα-DCs were identified and quantified,including glucosone (GS),3-deoxyglucosone(3-DG),methylglyoxal (MGO),1-deoxypentosulose (1-DP),3,4-dideoxyglucosone-3-ene (3,4-DGE),1,4-dideoxyglucosone(1,4-DDG),2,3-butanedione (2,3-BD),1,4-dideoxypentosulose(1,4-DDP) and 2,3-pentosone quinoxaline (2,3-PS).The MRM information on the 9α-DCs was presented in Table 2.The standard curve for quantitative analysis had a linear relationship in the range of 5–1 000 ng/mL,with all of the correlation coefficients (R2) greater than 0.998.LOD was 0.002–0.075 mg/kg,and LOQ was 0.005–0.220 mg/kg.The average recovery test results ranged from 80% to 103%.The results ofα-DCs for the treated samples are shown in Fig.1.Among the 9 compounds,GS is the dominantα-dicarbonyl compound (α-DC) found in RBP samples.The IRD samples had the highest GS content,which was 1013 mg/kg,while the content of PVD and FD samples had no significant difference compared to fresh samples,which were 278 mg/kg and 300 mg/kg,respectively.The moisture of RBP moved very slowly thus extending exposure to air in SD process,which caused the highest content of 3-DG in SD samples,further accelerating the accumulation of 3-DG at lowawlevels[7].In summary,although there was a general decrease of GS content,the five drying methods increased the content of 3-DG,to varying degrees.GS is a degradation product that facilitates the formation of AGEs.It is formed from the oxidation of sugars catalyzed by transition metal ions and/or oxidation of Amadori products by hydrolysis and will undergo C1−C2 bond cleavage to yield D-ribulose at 37 °C[26,27].It was reported that the decrease in honey GS content was always accompanied by an increase in 3-DG content during storage (40 °C for 12 months),which is consistent with the trends we obtained[28].The formation of 3-DG may be due to the existing monosaccharides through 1,2-enolization with the removal of water during the Maillard reaction or caramelization[29].

Fig.1 Effects of different drying methods on the concentration of α-DCs in RBP.(a):GS;(b):3-DG;(c):MGO;(d):1-DP;(e):2,3-BD;(f):1,4-DDG;(g):3,4-DGE;(h):2,3-PS;(i):1,4-DDP.* means values are significantly different, P <0.05.** means values are extremely significantly different,P <0.01.

Fig.2 GC-IMS of RBP in different treatment groups (Top View).
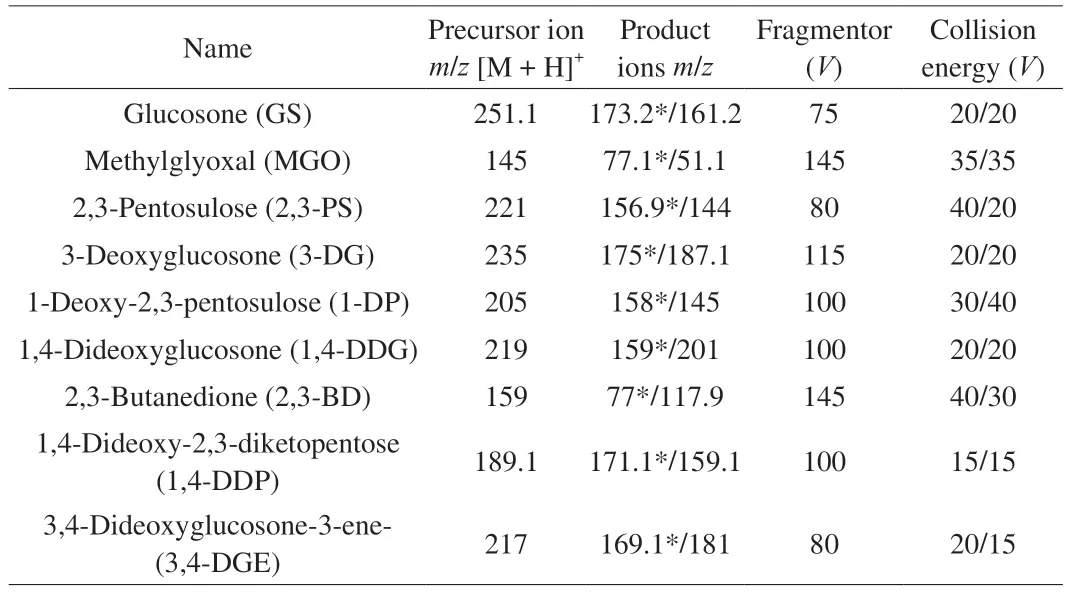
Table 2 The MRM information on the 9 α-DCs.
As a decomposition product ofα-DCs,the content of MGO was lower than that of 3-DG and GS.It is a typicalα-DC as well as an important precursor of AGEs,which is associated with a number of chronic diseases[8].The formation of the low molecular weightα-DC occurred mainly in the advanced stage of the Maillard reaction.The variation of MGO presented a similar trend to 3-DG in different drying methods.The degradation of 3-DG to MGO is well known to accelerate under alkaline conditions,thus its accumulation was lower than 3-DG.Except GS,1-DP and 2,3-PS,the content of the remainingα-DCs in SD samples was generally higher than that of the other four methods.In the thermal processing of foods,sugars are prone to a series of reactions like isomerization,dehydration,and oxidation in acidic conditions,contributing to the formation ofα-DCs including 3-DG,GS,and 3,4-DGE.Following the formation of intactα-DCs,retroaldol reactions,fragmentation,and water elimination reactions lead to the generation of shorter chainα-DCs such as MGO and 3-DP[30].To the best of our knowledge,this is the first quantitative data onα-DCs in bee pollen.
3.3 Volatile compounds
3.3.1 Comparative analysis of GC-IMS
Most of the volatile compounds are formed from the drying process and the degradation of lipids and proteins[31].Considering that bee pollen is usually processed before being consumed,it is necessary to analyze the VOCs generated during this period.GC-IMS has been used to analyze the volatile compounds in the samples treated by different drying methods.
The top-view plots (Figs.2 and 3) of GC-IMS were obtained.The whole spectrum represented the total flavor compounds of the RBP treated in different ways.The background of the whole image is blue,the red vertical line at Abscissa 1.0 is reactant ion peak (RIP),and the two on the right side of RIP are ethanol peak.Each point on the RIP’s right side represented the VOCs detected from the sample.Most of the signals appeared in the retention time of 100–800 s and the drift time of 1.0–1.5 s.The color represented the concentration of the substance,white indicated lower concentration,red indicated higher concentration.The darker the color was,the greater the concentration was.
Fig.3 compared the difference in the contents of each volatile compounds of samples dried by different ways.The blue area indicates that the concentration of the substance is lower than that of fresh sample in Fig.3.If the VOCs were the same,the background of the topographic map deducted from other samples was white,and the darker the blue was,the lower the concentration was;the red area indicates that the concentration of the substance is higher than that of the control sample,and the darker the red was,the higher the concentration was[32].The VOCs of RBP were different due to the different treatment time and biochemical reaction intensity of different drying methods.
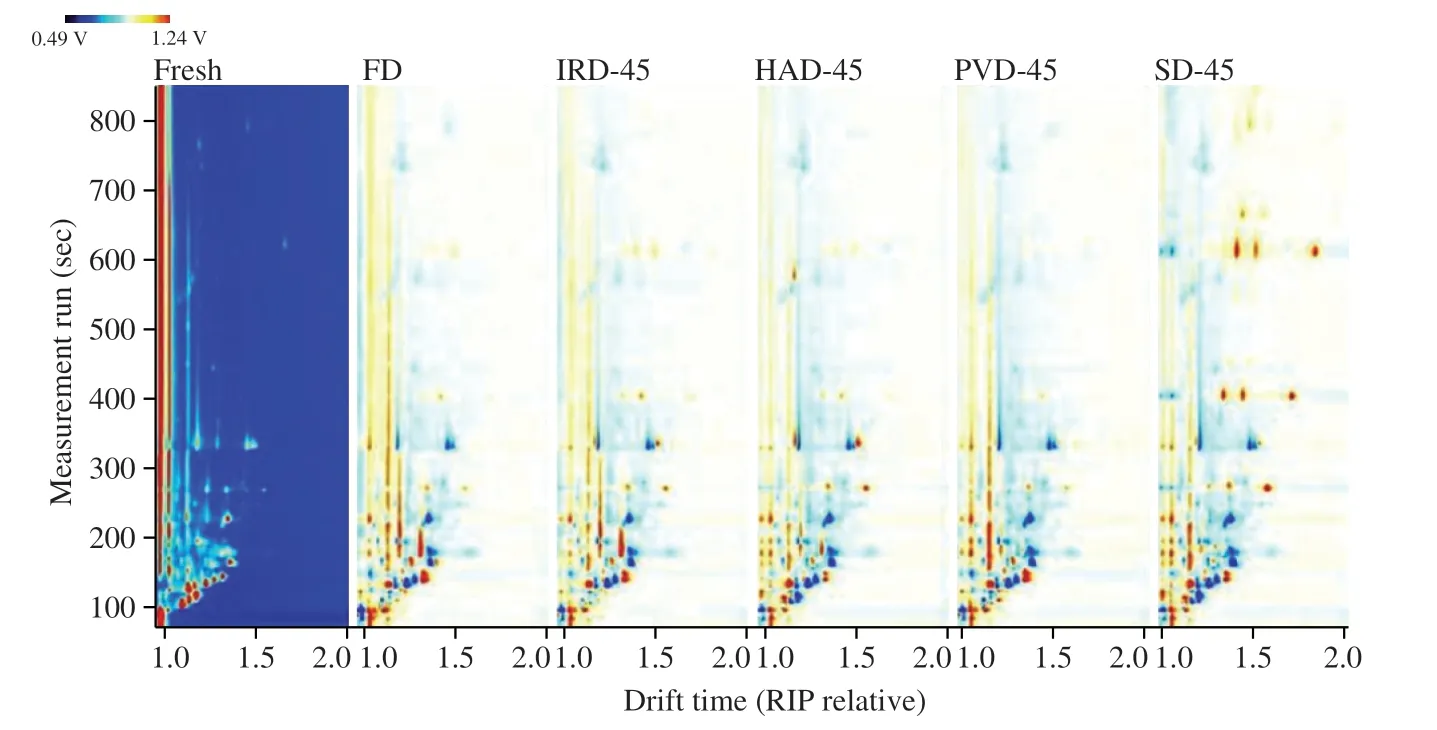
Fig.3 GC-IMS of RBP in different treatment groups (difference map).
3.3.2 Qualitative analysis of VOCs in different drying methods of RBP
As shown in Table 3,a total of 29 volatile flavor compounds were identified,including 13 aldehydes and ketones,7 esters,3 acids,2 terpenes,3 alcohols and thiophene.The aldehydes comprised the dominant components of RBP aroma among the 7 classifications.Aldehydes was good descriptors of dried odor as it was related to the proteolysis phenomena and amino acid degradation[33].Ester compounds were derived from condensing alcohol and a coenzyme-A-activated acid[34].Alcohols were formed from lipid and amino acid oxidation and were responsible for the fatty aroma[35].Ketonic compounds were the products of fatty acid or alkane degradation,Maillard reaction and microbial esterification.It can produce the flavor of butter,blue cheese and pungency at low concentrations[33].The following preliminary conclusions can be drawn from combining Table 3 with Fig.2: after drying,some of the original volatile substances of RBP were lost,while new volatile substances were released.The volatiles of FD,IRD,HAD and PVD samples were similar in their composition,while the SD samples contained more new volatile substances.It was obvious that the ethanol content of RBP increased after drying on account of high proton affinity of ethanol,promoting the formation of dimers or trimers during the migration process[36].The branched acid such as 2-methyl-propanoic acid,was most likely derived from the oxidation of its Strecker aldehyde 2-methyl-propanal[33].
Table 3 The information on detected VOCs of RBP treated by different methods by GC-IMS.
3.3.3 Comparative analysis of volatile compounds fingerprint
To further compare the differences in the volatile compounds of RBP from different drying methods,the sample information is shown in the perceptible visual plots.Each row represents a treatment group,and the higher the volatile compound content is,the redder and brighter the color is.Unidentified substances are presented by numbers.Three areas A,B,and C are marked in Fig.4,which means that different drying methods have different effects in different areas.
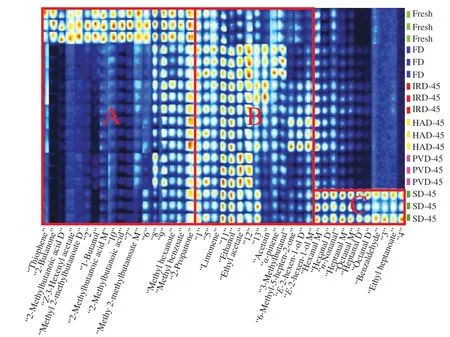
Fig.4 Fingerprint of VOCs in RBP of different drying methods.
It can be seen from region A in Fig.4 that the fresh sample was mainly composed of 3 acids (2-methylbutanoic acid D,2-methylbutanoic acid M and 2-methyl-propanoic acid),five esters(methyl 2-methylbutanoate M,methyl 2-methylbutanoate D,Z-3-hexenyl acetate,methyl hexanoate,methyl benzoate),one alcohol(1-butano),two ketones (2-butanone,2-propanone) and thiophene.These compounds were typical volatile compounds in fresh samples.In addition,the content of these compounds was greatly reduced or even disappeared after drying.Among them,the acid represented by 2-methylbutyric acid (rancidity,sour taste) produced an unpleasant acidic pungent odor.Its concentration was significantly reduced after drying.Therefore,proper heat treatment helped to eliminate the peculiar smell in RBP.Generally,the reduction of acid substances can be attributed to: (1) The esterification reaction of acid substances and alcohol substances which produce ester substances.(2) The degradation and/or oxidation reaction of lipids into acid substances[37].However,the only ester compound produced after drying bee pollen was ethyl acetate,and its content was higher than that of fresh samples.Therefore,the disappearance of fresh bee pollen acids was not only caused by the high fat content of pollen,but also had a lot to do with its participation in the esterification reaction to form ethyl acetate.It was reported that the content of volatile acetic acid in dried sausages was reduced by more than half compared to fresh ones and volatile isooctanoic acid even disappeared,which was consistent with the results of our study[37].Although drying was not conducive to the formation of acid,it was reported that the drying temperature had a significant effect on alcohols and ester compounds[38].We found that the contents of methyl hexanoate,methyl benzoate,andZ-3-hexenyl acetate were reduced after drying,and there was no significant difference between different drying methods.At the same time,drying also produced some negative effects,such as the decrease in butanone and 2-acetone content in RBP.It was reported that ketone compounds with creamy and fruity aroma can enhance the flavor of food[38].
A total of three alcohols (ethanol,E-2-hexen-1-ol D,E-2-hexen-1-ol M),2 terpenes (limonene,alpha-pinene),1 aldehyde(3-methylbutanal),1 ketone (acetoin) and other volatile compounds,including 6-methyl-5-hepten-2-one and ethyl acetate were detected besides the unknown five components in region B.It can be seen from Fig.4 that the ethanol content increased after each drying treatment and the intensity of 6-methyl-5-hepten-2-one andE-2-hexen-1-ol from HAD samples were much higher than other samples while acetoin,alpha-pinene,3-methylbutanal were detected in large quantities in the FD samples.Alcohols are produced by reduction of their corresponding aldehydes,and secondary alcohols (such asE-2-hexen-1-ol M andE-2-hexen-1-ol D) arise from certain organisms viaβ-oxidation of free fatty acids and hydrolysis to the correspondingβ-keto acid[39].Furthermore,ketones can be generated by the oxidation of secondary alcohols,and this was the reason for lower levels of ketones in FD and PVD than other aerobic drying methods.One more reason mentioned was the heat stress.High temperature reduced the solubility of oxygen and inhibited the aerobic respiration of plants simultaneously.The long period of anaerobic respiration accumulated ethanol.Acetoin was the only ketone detected.It was reported that acetoin could be produced by alcohol fermentation from pyruvate and butanodiol[40].Aldehydes are the volatiles derived from lipid with strong smell of fat,fruit and spices[41].In this study,the content of 3-methylbutanal in FD samples was increased while it was decreased in other drying methods,especially in SD samples.There were two main causes of this phenomenon,one was the degradation of microorganism and the other was the degradation of amino acids via Strecker degradation.The latter was more possible in this study since the Strecker degradation requires heat and was promoted by relatively low water content.It has been reported that aldehydes can be converted from flavor precursors during drying,while flavor development continues with the removal of volatile acids and the elimination of moisture[42].Previous study also found that heating treatment can promote the formation of aldehyde and ketone compounds in milk powders[43].Moreover,it was reported that high concentrations of aldehydes and ketones contribute to the quality of cocoa,producing a fruity and floral aroma[38].
Ethyl acetate is a product of esterification from acetic acid and ethanol,which are responsible for fruity odor[44].Obviously,the content of ethyl acetate increased to varying degrees after drying treatment,thus,a proper drying treatment contributes to mask rancid and vegetable cooked odors in samples.The result showed a higher volume in FD and PVD than that in IRD,HAD and SD.The amount of ethyl acetate may be derived from the interaction of alcohol and free fatty acids produced by lipid oxidation in the sample,which may well explain the presence of esters according to the content of alcohols and acids in the treatment group.Meanwhile,esterification of acids formed by oxidation of aldehydes is also an important source[45].The specific mechanism needs to be further studied.In addition,a previous study has shown that heating was not conducive to the formation of ester compounds,which may even promote the volatilization of esters[31].It has been that observed that the area of ethyl acetate was increased after the SD,which was similar to the change in acetic acid production during the drying process[42].Previous research has also found that the volume of acetic acid increased to maximum at 8 days of air-drying[39].This may be due to the carbohydrate fermentation from microorganism.Additionally,the author indicated that esterification between ethanol and carboxylic acid also occurs at 45–50 °C,which was consistent with the results we obtained about the content of ethanol and ethyl acetate.Both alpha-pinene and limonene belong to the terpenes,which have characteristic sensory properties and putative antibacterial activity[15].In this study,compared to fresh samples,the volume of alpha-pinene increased after FD,IRD and HAD,whereas it decreased by SD and PVD.It was studied that the influence of different drying methods on terpenoid compounds in commercial strawberry jams,and found that total terpene percentage increased after FD and oven-drying (60 °C) but oven-drying(45 °C) and shade air-drying decreased this percentage[15].This is in agreement with our result,where the difference of the temperatures of HAD (45 °C) on terpene content may be due to the difference of materials.
Region C consists of aldehyde compounds other than ethyl heptanoate.Aldehydes usually have low threshold values and exert a significant influence in the development of food flavor.Previous study testified the increase of aldehydes,alcohols,and some other volatiles during dehydration inF.velutipes[46].In this research,a large number of aldehydes such as hexanal,nonal,octanal and heptanal were detected in SD samples and the concentrations of these compounds showed a difference in other drying methods.Nonanal and hexanal are products of oleic and linoleic acids formed by the fatty oxygenase pathway and the oxidative degradation of N-6 and N-9 polyunsaturated fatty acids,respectively[32].Octanal was the most abundant aldehydes after drying,generated by the autoxidation of oleic acid,which was in agreement with a previous study[39].Meanwhile,the author also indicated that corresponding acids and esters were formed due to the oxidization of octanal in the later stage of air-drying.Heptanal imparts fatty and rancid odors,which can be considered as a good indicator of the oxidation level.Benzaldehyde is the only aldehyde detected in the SD.It can arise from methionine and phenylalanine through Strecker degradation reaction,possessing a characteristic almond and cherry smell[32].These aldehydes are characterized by a pleasant flavor at low concentrations while most of their odors are unacceptable at high concentrations.In this study,these aldehydes contributed a grass,roasted and fat odor,which is directly related to the overall flavor of bee pollen after drying.In conclusion,the prolonged and continuous SD lead to the increase of lipoxygenases,resulting in the acceleration of the Strecker degradation of reducing sugars and amino acids[47].Additionally,autoxidation exerts a dynamic effect on catalyzing unsaturated fatty acids into C6 and C9 volatiles (aldehydes),affecting the development of distinct smells[48].It was studied that the increase of total aldehyde content in sesame samples after sun-drying was related to the Maillard reaction[41].
3.4 Principal component analysis (PCA) of different drying methods
The variables for each sample were grouped based on the results of the content of AAs,α-DCs and VOAs.Fresh samples were compared to all 15 samples from five drying treatment groups.Samples were analyzed in triplicate for each treatment group for a total of 18 samples.The PCA plot (Fig.5a) showed overall differentiation of the drying characteristics of RBP samples with different treatment groups.
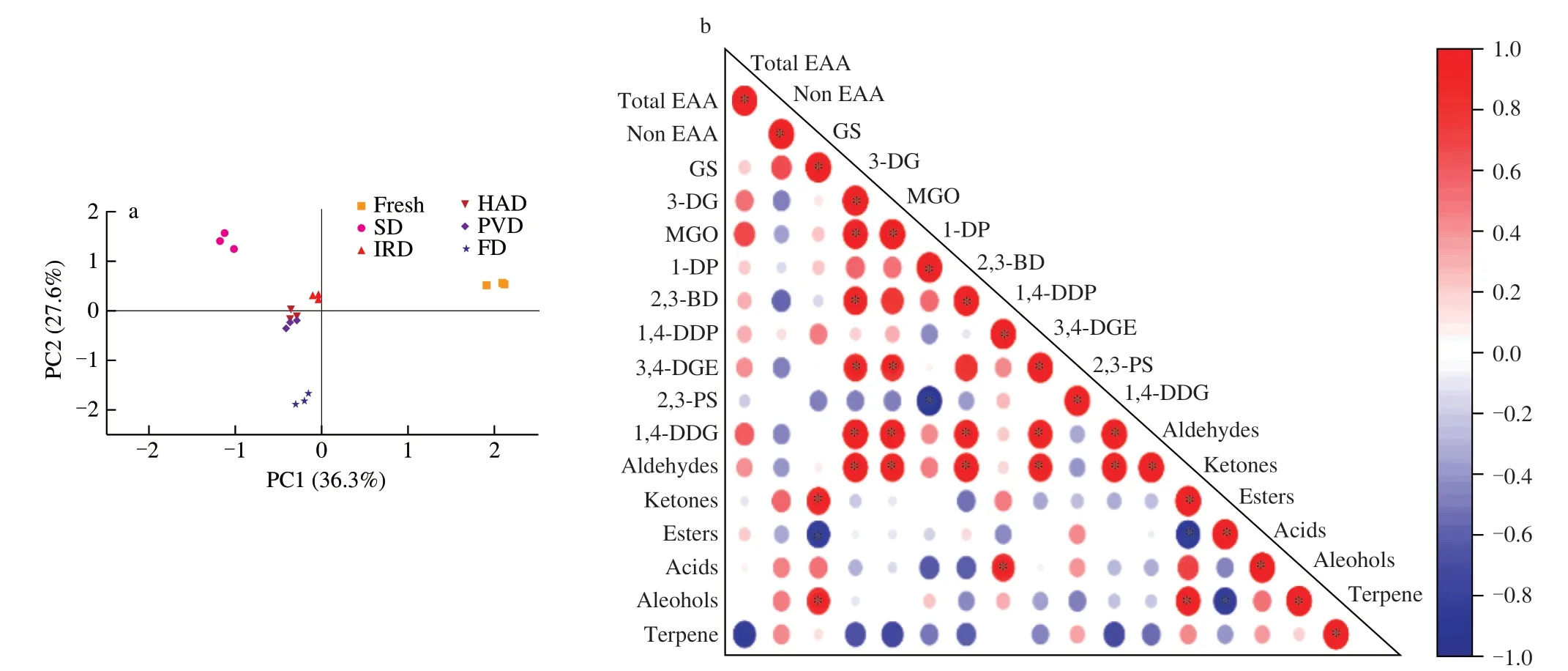
Fig.5 (a) is PCA of RBP with different drying methods.(b) is Pearson’s correlation analysis among AAs,α-DCs and VOCs of RBP by different drying methods.
Significant differences between the fresh and dried samples were observed.The SD and FD samples were obviously distinguished from the other three drying methods,indicating that the different treatment groups varied considerably in chemical composition.According to the results shown in Fig.5(a),the cumulative contribution rate was 63.90%,and the first and second PCs explained 36.30% and 27.60% of the variance,respectively.SD was located on the positive part of PC2 while FD was positioned on the negative part of the same dimension.IRD was located in the center of PC1 and positive PC2.HAD and PVD clustered together on the negative PC1 and the center of PC2 on the PCA plot.This showed that the low temperature without oxygen environment of FD was rather distinct from the other three thermal treatments.Compared with HAD and IRD,the vacuum environment during the PVD process inhibited oxidative reactions and reduced the degradation of the heat-sensitive nutrients,thus improving the drying quality of materials.SD experienced the longest drying period that aggravated the lipid auto-oxidation and thermal oxidation or degradation of unsaturated fatty acids,producing more carbonyl compounds and a large number of aldehydes,thus had a serious impact on the nutrients in RBP.The results of PCA showed that different drying methods exerted important influences on the components of AAs,α-DCs and VOAs of RBP that make differences in biological activity.
3.5 Pearson’s correlation analysis of RBP by different drying methods
Pearson’s correlation analysis between AAs,α-DCs and VOCs of RBP in different drying conditions is shown in Fig.5(b).Among the VOCs,aldehydes have a significant effect ofα-DCs on the RBP samples and positively correlated to 3-DG,MGO,2,3-BD,3,4-DGE and 1,4-DDG (P<0.05).Both ketones and alcohols showed significant positive correlations with GS (P<0.05) while esters showed significant negative correlations (P<0.05).Furanone derivatives,hydroxylketones and dicarbonyl compounds can be produced in the glucosamine stage of the Maillard reaction[49].In consequence,these intermediate dicarbonyl products might form the carbonyl center of the aldehydes and further combine with a hydrogen and to an R group to form alkyl aldehydes,from which pentanal,hexanal or nonanal can be formed.It has also been reported that 2,3-BD or MGO can provide theα-DC reactant for the stage of Strecker degradation,which accounts for the generation of Strecker aldehydes and aminoketones[50].
4. Conclusion
The drying methods exert significant effects on the composition and content of AAs,α-DCs and VOCs of RBP.The loss of total AAs in SD samples was the highest while FD effectively improved the content of EAAs.FD was the best method of drying in terms of totalα-DCs content,which was only slightly generated with respect to the control (fresh bee pollen).Most abundant VOCs in RBP were aldehydes and the VOCs number of SD samples were more than that of other drying methods.From our results,FD and PVD would be potential methods to effectively reduce the quality deterioration of RBP in the drying process.This study provided a new evaluation of bee pollen and a theoretical reference for maximizing the product quality during the drying process.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos.31871861 and 31501548),The Apicultural Industry Technology System (NCYTI-43-KXJ17),and The Science and Technology Innovation Project of Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2015-IAR).The authors also would like to express their gratitude to the anonymous referees for their valuable comments and suggestions.
- 食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics
- A texture-modified dessert with high nutritional value designed for people with dysphagia: effect of refrigeration and frozen storage

