Longitudinal changes in body weight of breastfeeding mothers in the first year after delivery and its relationship with human milk composition: a combined longitudinal and cross-sectional cohort study
Huijun Run,Yjie Zhng,Qingy Tng,Xun Zho,Xuelin Zho,Yi Xing,Wei Geng,Yi Feng,Wei Ci,c,*
a Department of Clinical Nutrition,Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine,Shanghai 200092,China
b Shanghai Key Laboratory of Pediatric Gastroenterology and Nutrition,Shanghai Institute of Pediatric Research,Shanghai 200092,China
c Department of Pediatric Surgery,Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine,Shanghai 200092,China
Keywords:Human milk Milk composition Body weight Body mass index (BMI)Weight gain Postpartum weight retention
ABSTRACT Objective:Postpartum weight retention (PPWR) is a common problem among women after childbirth.The main objectives of this study are to understand the changes in body weight of breastfeeding mothers during long-term follow-up and preliminarily explore the relationship between maternal body weight and human milk composition,including macronutrients,leptin,and adiponectin.Methods:The study included a longitudinal cohort (122 mothers),and a cross-sectional cohort (37 mothers).The human milk,maternal weight,and dietary surveys were collected in the longitudinal cohort at different follow-up time points (1−14 days postpartum,2−4 months postpartum,5−7 months postpartum,and 12−17 months postpartum).The maternal body weight was analyzed using the responses in the survey questionnaires.A milk analyzer based on the mid-infrared spectroscopy (MIRS) was used to determine milk composition,and nutrition analysis software evaluated dietary intakes.In the cross-sectional cohort,participating mothers were asked to provide blood and human milk samples and pertinent information related to maternal body composition.Maternal body composition was measured by bioelectrical impedance analysis (BIA),while ELISA analyzed leptin and adiponectin in milk and serum.Results:At 5 −7 months postpartum,the PPWR of breastfeeding mothers was (2.46 ± 3.59) kg.At 12−1 7 months postpartum,the PPWR was (0.98 ± 4.06) kg.PPWR was found to be negatively correlated with milk fat content within 14 days postpartum and positively correlated at 2−4 months postpartum.In addition,the maternal weight and body muscle mass were positively correlated with leptin and adiponectin in milk.Plasma leptin was positively correlated with the mother’s body weight,body mass index (BMI),FAT percentage,and body fat mass,while plasma adiponectin did not correlate with any parameter.The results also indicate that the PPWR did not correlate with leptin and adiponectin in plasma or milk.Conclusions:Breastfeeding mothers may retain considerable weight gain one year after delivery.Human milk composition may be related to changes in maternal body weight.Leptin and adiponectin in breast milk and leptin in plasma are associated with the maternal body composition.This study supports the notion that maternal nutritional status may affect offspring health through lactation,and future research should focus on exploring weight management of postpartum mothers.
1. Introduction
Obesity is widely considered a global epidemic disease[1,2].Studies have found that women are more likely to be prone to obesity than their male counterparts,profoundly impacting reproductive health,especially during pregnancy[3,4].Women suffering from obesity may also face increased risks of gestational diabetes,preeclampsia,operative delivery,fetal macrosomia,and neonatal morbidity[2,3,5,6].In addition,maternal obesity is a risk factor for childhood obesity[7,8].A common problem among women after childbirth is postpartum weight retention (PPWR),which refers to the value of postpartum weight minus the pre-pregnancy weight[9].Excessive weight gain can lead to short-and long-term adverse health effects for mothers,babies,and even future pregnancies.It could also exacerbate the long-term risk of obesity and increase the likelihood of diabetes and cardiometabolic diseases after childbirth[10-12].
Fortunately,breastfeeding is an effective way for mothers to lose excess weight[11].Several studies have confirmed the positive relationship between breastfeeding and postpartum weight loss[11,13-16],whereby the longer the breastfeeding duration,the more weight mother loses[16,17].However,some studies have concluded a U-shaped curve between maternal postpartum weight and breastfeeding duration,although the reasons remain unclear[15].In breastfeeding research,much attention has been paid to the human milk composition in the last few decades.Human milk varies in composition due to differences in maternal diet and human nutritional status,including muscle content,fat content,and nutrient reserves[18-24].
Dietary factors have also significantly impacted human milk composition[22,25].In addition to nutrients,human milk also contains many hormones.Adiponectin and leptin are the two most commonly studied adipocyte-derived hormones and have been reported to be present in human milk[25,26].Studies have shown that leptin and adiponectin levels in human milk are associated with both the short-term and long-term growth of infants,which indicates that maternal weight may affect the development of infants through breastfeeding[27-29].Additionally,several studies have examined the effects of dietary intake and patterns on maternal weight[30,31].However,the relationship between maternal weight and human milk composition remains unclear[18-20,23,25,32].To address this knowledge gap,we conducted a longitudinal observational study to explore the weight status of Chinese mothers and preliminarily investigate the relationship between maternal weight and human milk composition.We also analyzed adiponectin and leptin levels in maternal blood and milk to understand their relationship with maternal body composition through a cross-sectional cohort.The results of this study help to provide novel insight into the relationship between maternal physiology and human milk composition in Chinese mothers and provide a theoretical basis for promoting the optimal nutrition of lactating women.
2. Materials and methods
2.1 Objects of study
The study includes a longitudinal cohort and a cross-sectional cohort.The researchers recruited the participants of the breastfeeding study cohorts of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai,China) from December 2019 to December 2020.This study was approved by the Ethics Committee of the Hospital with approval number XHEC-C-2020-081,and written informed consents were obtained from all participants.The flow chart is shown in Fig.1.Additional information on the participant population is available in a previous study[33,34].
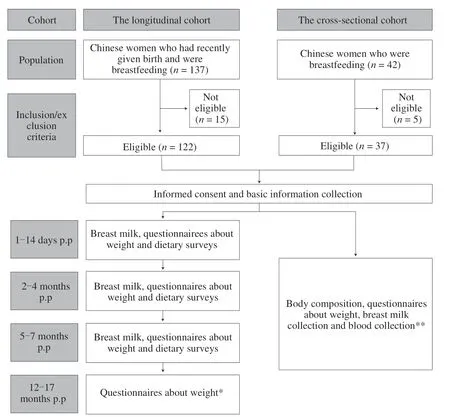
Fig.1 Study design flow chart.* At this follow-up point(12−17 months postpartum),almost all mothers stopped breastfeeding (only 4 of 122 were still breastfeeding),so only maternal weight information was collected.** The collection of relevant information,samples,and anthropometric measurements were carried out during the mature milk period of the mothers (2−12 months postpartum).p.p: postpartum.
2.1.1 Longitudinal cohort
All participants from the longitudinal cohort were Chinese women who had given birth and had been breastfeeding.Before the recruitment confirmation,we conducted a preliminary experiment with 20 mothers to test the experimental design and make any necessary improvements.Based on the preliminary results,we calculated the needed sample size for the study.Using an attrition rate of 20%,the computed sample size required for the study was 120.After appropriate expansion,we finally recruited 122 mothers for the longitudinal study.Follow-up tests were conducted 12−17 months after delivery to monitor the maternal postpartum weight.The inclusion and exclusion criteria for the longitudinal cohort are as follows:
Inclusion criteria: Chinese mothers who had been breastfeeding within 14 days postpartum and lived in Shanghai or neighboring provinces and cities for more than six months.
Exclusion criteria: Lack of human milk led to a cessation of breastfeeding at the time of recruitment;inability or unwillingness to provide milk at the time of recruitment;unable to communicate due to language barriers or mental problems;severe medical condition requiring medication;failure to collect or store human milk as required[33,34].
2.1.2 Follow-up visits in the longitudinal cohort
The follow-up time of the longitudinal cohort was 1−14 days postpartum,2−4 months postpartum (60−120 days),5−7 months postpartum (150−210 days),and 12−17 months postpartum(365−547 days).Each mother collected milk at every followup period (1−14 days postpartum,2−4 months postpartum,and 5−7 months postpartum).At the final follow-up point,almost all mothers had stopped breastfeeding (only 4 out of 122 people were still breastfeeding),so only the maternal weight was followed up.The average follow-up time was (10.17 ± 3.05),(80.66 ± 21.18),(164.75 ± 14.92),and (429.43 ± 37.98) days,respectively.
2.1.3 Cross-sectional cohort
To supplement the analysis of the longitudinal cohort to explore the parameters of maternal body composition and analyze the relationship between leptin and adiponectin in human milk and blood,we conducted a cross-sectional study in which mothers recruited needed an additional fasting blood collection and human composition analysis in addition to breast milk.A total of 37 lactating mothers were recruited as the cross-sectional study population.The inclusion criteria were as follows: (1) breastfeeding mothers,(2) able to provide for blood and milk collection and body composition analysis,and (3)during the mature milk period (2−12 months postpartum).
2.2 Milk sample collection
The mothers in the longitudinal cohort provided milk at each designated collection time according to the requirements,while the mothers in the cross-sectional cohort completed one milk sample collection during the mature milk period (2−12 months postpartum).The volunteers were first given a detailed explanation face-to-face or via WeChat regarding the unified sample collection procedure.Then,mothers from both cohorts performed the same breast milk collection method and mixed samples according to uniform requirements.The participating mothers were asked to collect the samples using an efficient electric breast pump at home.The milk samples were collected before the babies were fed (8:00 AM to 9:00 AM).The mothers needed to fast for more than 8 h before providing the samples.After emptying the milk from one breast,the mother would have to carefully shake the container into which the milk was pumped for at least 10 s,and splashing or foaming should be avoided.Then the mother takes 5 mL of the mixture and puts it into the unified breast milk collection bag.The sample was frozen in the household refrigerator at −20 °C for temporary storage.Mothers were provided a cold box and ice bags to store the milk samples to ensure that they were kept at low temperatures.We then arranged for the transfer of milk through the cold chain express.After the milk was transferred to Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai,China),the researchers evaluated the characteristics of the collected milk.A contingency plan was put in place to re-collect milk samples in cases of deterioration,impurities,and rupture of the milk container.The milk samples in the crosssectional cohort were homogenized and repackaged for macronutrient and ELISA determination later.The collected milk was stored in a lab freezer at −80 °C until further analysis,and this process was performed for each milk sample collection.For more details on the collection process,transportation,and human milk storage,refer to the previous studies[25,33-35].
2.3 Individual questionnaire
After the subjects for the longitudinal and cross-sectional cohorts were formally recruited,the mothers completed the personal questionnaire,gathering information including the mother’s birth date and height,baby’s date of birth,pre-pregnancy weight,current weight,and infant feeding methods,and contact details.
2.4 Dietary survey
The dietary survey was conducted at each follow-up visit with the participants in the longitudinal cohort,adopting the 24-h dietary survey method.Each participant was given a questionnaire,which included a 24-h diet diary and a unified food scale for quantification.The participants were requested to record their food intake,other than pure water,for at least 24 h before collecting the milk samples (from 8:00 AM to 8:00 AM the following day).After recovering the dietary survey records,we assessed the quality of each survey response.An American registered dietitian with 6 years of work experience then carried out additional dietary evaluation using the Nutrition Star diet analysis software (ZHENDING Co.,Nutrition Star,V5.20,Shanghai,China).
2.5 Analysis of human milk
2.5.1 Determination of macronutrients in human milk
In this study,we used a milk analyzer (BETTERREN Co.,HMIR-05,Shanghai,China) based on the mid-infrared spectroscopy (MIRS)method to determine lactose (g/dL),protein (g/dL),and fat (g/dL),as well as energy (kJ/dL) in the human milk[34,36-38].The instrument used was patented by the State Intellectual Property Office of China (CN 108760671 A) in 2018.The manufacturer’s unique quality control material was used for calibration before the daily sample testing.A milk sample of 0.5 mL was used for one test of macronutrients,and the analysis was performed per the instrument operating instructions[34].
2.5.2 Determination of leptin and adiponectin in milk
Thirty-two milk samples from the cross-sectional cohort were measured for adiponectin and leptin.The pre-treatment and detection process of milk adiponectin and leptin were referred to the manufacturer’s instructions (Elabscience Biotechnology Co.,Ltd.,Wuhan,China).Milk leptin was detected using whole milk stock solution (100 μL),while whole milk stock solution (5 μL) was diluted 20 times for adiponectin determination.The technician determined wells for diluted standard,blank,and sample and added 100 μL of each into the appropriate wells.The plate was then covered with the sealer provided in the kit and was incubated for 90 min at 37 °C.The technician decanted the liquid from each well and immediately added 100 μL of Biotinylated Detection Ab working solution to each well.The plate was then covered with a new sealer and incubated for 1 h at 37 °C.Then,the technician decanted the solution from each well,adding 350 μL of wash buffer to each well.After soaking for 1 min,the solution was aspirated (or decanted) from each well and dried using clean absorbent paper;the wash procedure was repeated three times.
Each well was then added with a 100 μL of HRP Conjugate working solution,covered with a new sealer,and incubated for 30 min at 37 °C.The technician then decanted the solution from each well and repeated the wash process 5 times.Afterwhich,90 μL of substrate reagent was added to each well.The plate was again covered with a new sealer and incubated for about 15 min at 37 °C.Finally,50 μL of stop solution was added to each well.Each well’s optical density(OD value) was measured with a microplate reader set to 450 nm.Pre-experiments were first carried out for each determination,and the results of each sample were tested by enzyme-linked immunosorbent assay in duplicated wells.
2.6 Collection and analysis of blood samples
In the cross-sectional cohort,32 mothers who fasted for more than 8 h provided blood samples from 8:00 AM to 10:00 AM in the morning,while plasma was obtained after centrifugation.ELISA detected adiponectin and leptin following the manufacturer’s instructions (Elabscience Biotechnology Co.,Ltd.,Wuhan,China).The serum (5 μL) was directly diluted 20 times for leptin determination and 5 000 times (multiple dilutions) for adiponectin determination.Other detection processes were similar to those previously discussed.Pre-experiments were first conducted for each determination,and ELISA tested the results of each sample in duplicated wells.These two indicators had been extracted from the original data from previous research and were analyzed twice[33].
2.7 Maternal body weight and body composition
In both the longitudinal and cross-sectional cohorts,the mother’s pre-pregnancy weight and height were self-reported at the postpartum follow-up using questionnaires.The questionnaires,dietary surveys,and milk samples were returned via the cold chain express to the research group.A highly qualified nutritionist preliminarily assessed the quality and completeness of the responses.If important information was missing or some data was in doubt,the subject was contacted immediately through WeChat or telephone for verification.Body mass index (BMI) was also obtained following this step,and it is calculated using the following formula: BMI=Body weight (kg)/ Height (m2).The change in BMI (ΔBMI) was calculated by subtracting the BMI pre-pregnancy from the followup BMI.Similarly,the difference in body weight (Δbody weight)was computed by the follow-up weight minus the weight before pregnancy.
In the cross-sectional cohort,maternal body composition was measured by bioelectrical impedance analysis (BIA) using Inbody 720 (Biospace Co.,Ltd.,Seoul,Korea).The measured parameters included body fat percentage (%),body fat mass (kg),and body muscle mass (kg)[33,34].
2.8 Statistical analysis
Statistical analysis was performed using SPSS Statistics 25.0 statistical software (IBM Co.,Armonk,NY,USA).Continuous variables were presented as mean ± standard deviation (SD).One-way analysis of variance (ANOVA) was used to compare the difference between groups,while the least square distance (LSD) method was used to compare data between within groups.Bonferroni correction was used to adjust the results of multiple comparisons.The formula of Bonferroni correction isp(1/n),wherepis the actual threshold (0.05),andnis the total number of tests.The missing data were supplemented by maximum likelihood estimation (ML) as recommended in existing literature[39].Pearson’s correlation analysis assessed the correlation between weight/BMI and other indicators,while partial correlation analysis was used after controlling the variables.Multiple linear regression was employed further to analyze the relationship between maternal weight and milk composition,and collinearity diagnostics were carried out using the variance inflation factor (VIF)[40].P<0.05 indicated statistical significance.R-language and Prism 9.0 were used to draw figures.
3. Results
3.1 Characteristics of the subjects
In the longitudinal cohort,122 mothers were included in the study.The average age and height were (31.57 ± 4.10) years old and (161.54 ± 5.65) cm.The average follow-up time was(10.17 ± 3.05),(80.66 ± 21.18),(164.75 ± 14.92),and (429.43 ± 37.98) days,respectively.In total,578 weight measurements were obtained at 5 intervals (including the pre-pregnancy weight).Of the 341 dietary questionnaires distributed,93 were accepted after screening and used in the evaluation.The detailed characteristics involved are summarized in Table 1.
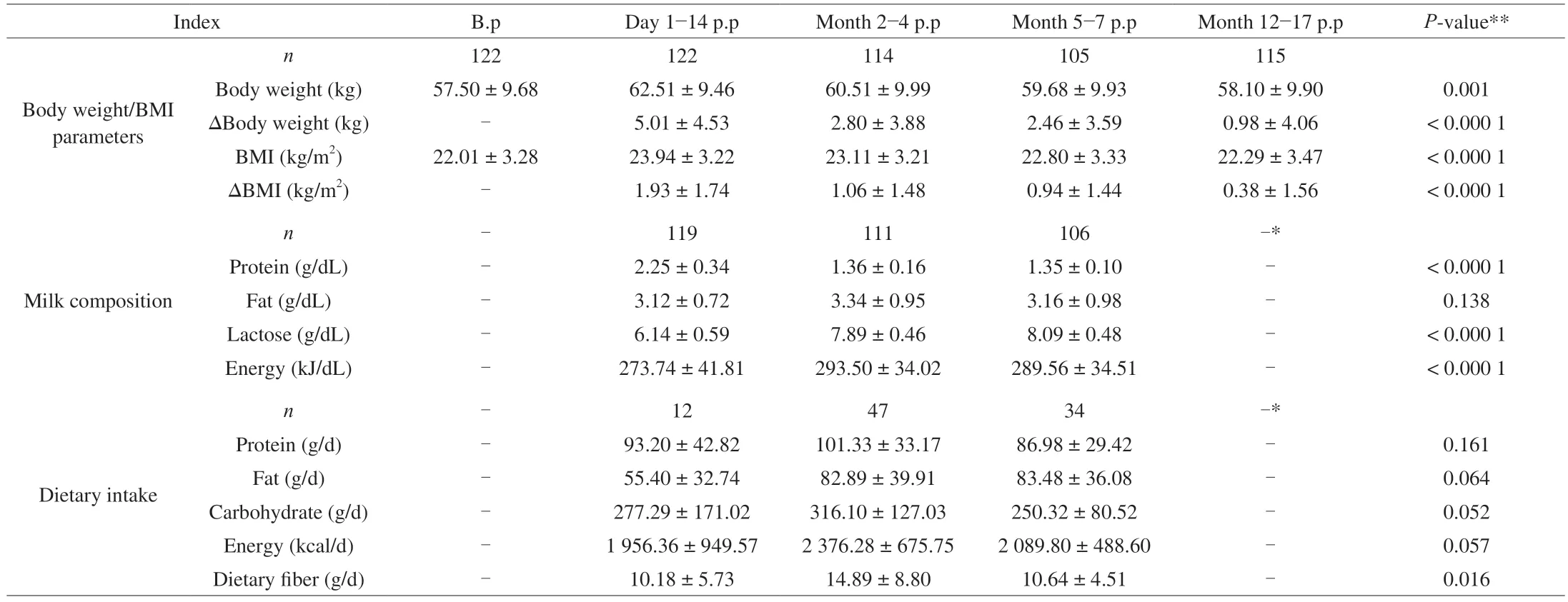
Table 1 Characteristics of the subjects in the longitudinal cohort.
In the cross-sectional study composed of 37 mothers ((31.33 ± 3.29)years old,(179.32 ± 106.30) days postpartum),33 were included in the body composition analysis,32 of whom provided milk and blood.The pre-pregnancy weight was (55.30 ± 7.60) kg,the current weight was(58.02 ± 8.56) kg,the PPWR was (5.71 ± 13.36) kg,FAT percentage was(30.70 ± 6.31)%,body fat mass was (17.94 ± 5.66) kg,body muscle mass was (21.22 ± 2.77) kg,and BMI was (22.15 ± 2.80) kg/m2.
3.2 Maternal body weight,BMI,ΔBody weight and ΔBMI
As shown in Fig.2,we found significant differences in the body weight/BMI pre-pregnancy and the bodyweight/BMI at different stages of postpartum (P<0.05).After Bonferroni correction,there was still a significant difference between groups (except body weight between 2−4 months postpartum and 5−7 months postpartum,BMI between 2−4 months postpartum and 5−7 months postpartum,BMI between 5−7 months postpartum and 12−17 months postpartum)at the level of adjustedP=0.005.As shown in Fig.3,there were noticeable changes in pre-pregnancy and postpartum body weight and BMI,and the difference between groups was statistically significant(P<0.05).After Bonferroni correction,there was still a significant difference between groups at the adjustedP=0.008 3.

Fig.2 Maternal weight (A) and BMI (B) before pregnancy and postpartum in the longitudinal cohort.B.p: before pregnancy;p.p: postpartum;BMI: body mass index.
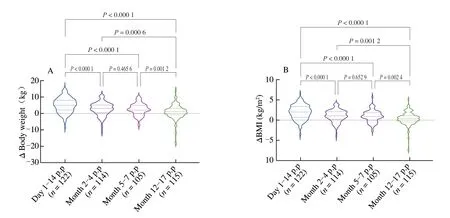
Fig.3 Changes in maternal weight (A) and BMI (B) after delivery compared with the prenatal status in the longitudinal cohort.p.p: postpartum;BMI: body mass index.Δ: refers to postpartum weight/BMI minus pre-pregnancy weight/BMI.
We categorized the participants based on their BMI: underweight(BMI <18.5 kg/m2),normal weight (18.5 kg/m2≤ BMI <24 kg/m2),overweight (24 kg/m2≤ BMI <28 kg/m2),and obesity (BMI ≥28 kg/m2).As shown in Fig.4,at 5−7 months postpartum,theproportion of mothers with normal weight status decreased by 6.55% compared to the pre-pregnancy value,while the proportion of mothers with overweight status increased by 9.59%.At 12−17 months postpartum,the rate of mothers with overweight status (23.48%) was still higher than pre-pregnancy (18.03%),while the rate of mothers with normal weight status (60.87%) decreased by more than 10%compared to the pre-pregnancy rate (71.31%).

Fig.4 Percentage of mothers’ body shape before pregnancy and postpartum in the longitudinal cohort.(A) B.p;(B) day 1−14 p.p;(C) month 2−4 p.p;(D) month 5−7 p.p;(E) month 12−17 p.p.B.p: before pregnancy;p.p: postpartum;malnutrition: refers to BMI <18.5 kg/m2;normal weight: refers to 18.5 kg/m2 ≤ BMI <24.0 kg/m2;overweight: refers to 24.0 kg/m2 ≤ BMI <28 kg/m2;obesity: refers to BMI ≥ 28 kg/m2.
3.3 Human milk composition
We had found that the milk protein showed a significant downward trend,while the milk lactose and milk energy increased significantly (P<0.05) (Fig.5).After Bonferroni correction,at the level of adjustedP=0.017,there were still significant differences in milk protein,lactose,and energy.

Fig.5 Content and change trend of human milk composition in the longitudinal cohort.p.p: postpartum.
3.4 Dietary intake of lactating mothers
Ninety-three effective dietary questionnaires were collected.There was no significant difference in the energy,protein,fat,and carbohydrate intakes at different lactation periods (P>0.05),but there was a significant difference in the dietary fiber intake at other times(P<0.05).The exact values can be found in Table 1.
3.5 Relationship between ΔBody weight,ΔBMI and human milk composition
In different postpartum periods,ΔBody weight and ΔBMI were related to human milk composition,as shown in Fig.6.There was a negative correlation between milk fat and ΔBody weight and ΔBMI in 1−14 days postpartum,but the relationship disappeared after adjusting the dietary energy intake.In 2−4 months postpartum,milk energy and milk fat positively correlated with ΔBody weight and ΔBMI.This positive correlation still existed after adjusting the dietary energy intake.At 5−7 months postpartum,no milk composition correlated with ΔBody weight and ΔBMI.
After collinearity diagnostics,we incorporated candidate indexes into the multiple linear regression model to explore the relationship between maternal weight and human milk composition.Using the stepwise regression method,we introduced milk fat (day 1−14 postpartum and month 2−4 postpartum) into the model.At this time,two conditions were met: (1) the overall model was significant(P<0.05),and (2) the independent variable coefficient was significant (P<0.05).During 5−7 months postpartum,no parameterswere successfully introduced into the model.The specific statistics are listed in Table 2.

Table 2 Statistics of multiple linear regression.
3.6 Relationship between leptin,adiponectin,maternal weight,and maternal body composition
In maternal plasma,the adiponectin content was (11 993 042 ±5 101 614) pg/mL,while the leptin content was (7 431.78 ± 6 592.85) pg/mL.In milk,the adiponectin content was (9 859.41 ± 6 512.46) pg/mL,and the leptin content was (174.71 ± 397.67) pg/mL.After correlation analysis,leptin and adiponectin in milk were positively correlated with plasma leptin.Current maternal weight and body muscle mass were positively correlated with leptin and adiponectin in human milk.Plasma leptin was also positively correlated with the mother’s current body weight,BMI,FAT percentage,and body fat mass,while plasma adiponectin did not significantly correlate with other parameters.In addition,PPWR did not correlate with leptin and adiponectin in plasma or milk.The complex relationship between parameters is presented in Fig.7.

Fig.7 Relationship between maternal body weight,body composition,adiponectin,and leptin in plasma and milk in the cross-sectional cohort.Correlation coefficients between the variables.Only significant correlations were shown (P <0.05) either in blue (positive) or in red (negative).The color intensity and size of the ellipse were proportional to the correlation coefficients.* Only significant correlations were shown (P <0.05).ADP:adiponectin;LEP: leptin;B.p: before pregnancy;BMI: body mass index;FAT:percentage of body fat;Current weight: refers to the bodyweight at the time of body composition analysis after delivery.
4. Discussion
In the present study,we described the long-term trend of weight changes in Chinese breastfeeding mothers and analyzed the dynamic relationship between changes in weight and human milk composition in a longitudinal cohort.We also explored the relationship between maternal weight and adipocyte-derived hormones in blood and milk using a cross-sectional cohort.The results show that maternal weight change is correlated with milk composition and that adipocyte-derived hormones in milk and blood are related to the mother’s body composition.
Many mothers went from normal to overweight status after experiencing pregnancy.The results suggest that maternal weight gradually decreases as lactation progresses.Lactation is a process with high energy demand;it may help mobilize fat deposits accumulated during pregnancy and subsequently cause weight loss[15].Another important reason for the rapid bodyweight reduction 6 months postpartum is the diet of lactating mothers.In our study,except for the 2−4 months postpartum,the dietary energy intake among the study participants was less than the recommended value by the China Nutrition Society for lactating mothers of 2 300 kcal/d.These results are similar to the findings of another study on Chinese lactating mothers[30].In addition,a previous study found that ovarian hormone itself affected women’s fat metabolism.Estrogen increases dramatically during pregnancy but quickly drops below pre-pregnancy levels at birth and remains suppressed during the postpartum period,so changes in hormone levels may partly affect postpartum weight[41,42].
Our study also found that during the period of colostrum and transition milk (1−14 days postpartum),more PPWR was related to the less fat content in human milk,which has not been previously reported.Studies have found that postpartum milk secretion is delayed among women with obesity[32,43].Moreover,at 6 weeks postpartum,the rate of initiation of breastfeeding and breastfeeding maintenance among women with obesity are also lower than healthy weight women[43].Delayed lactogenesis in mothers with obesity may be related to the difference in human milk composition.Given the significant variability in fat concertation of breast milk,further research is needed to confirm and explain the results.
In the stage of mature milk (2−4 months postpartum),higher fat content in milk is related to more PPWR.Several studies have found that the nutritional composition of human milk may be related to maternal dietary intake[20,21,23].In this study,at 2−4 months postpartum,maternal nutritional energy intake was more remarkable compared to other lactation periods.Possible reasons are that in the early postpartum period (from delivery to 1 month postpartum)the mother’s body does not fully recover from both physical and physiological changes of during pregnancy and delivery and that her diet potentially may be limited due to the Chinese postpartum custom of “sitting the month” or “postpartum confinement”[44,45].Then,within 1−6 months postpartum,the mother secretes the most milk,making her use a lot of energy[46].Increased dietary energy may also lead to changes in milk composition (changes in milk fat) and promote PPWR[30,31].After adjusting for dietary factors,the relationship between body weight change and milk fat still exists,indicating that the relationship between milk fat and maternal weight change may be independent of dietary factors.A previous systematic review found that maternal BMI is positively correlated with fat in human milk[18].However,the previous study did not analyze the relationship between BMI change and human milk composition;this study addresses such concern.Further research is still needed to confirm and explain the above results considering the various factors affecting the fat content in human milk.
In the stage of mature milk at 5−7 months postpartum,the results indicate that no milk composition is related to PPWR.The possible explanation for this result is that in all postpartum periods,many factors (such as lactation,diet,lifestyle,and physical activity) can significantly affect the change in maternal weight.Taking lactation as an example,compared with that before 5 months postpartum,difference in the mother’s weight caused by lactation may be less influenced during the 5−7 months postpartum period.In addition,considering the significant variability of milk fat,further correlation research needs to further judge the current results by measuring the milk fat production of the mother for 24 h rather than the fat concentration in a single milk sample.Adiponectin stimulates fatty acid oxidation in skeletal muscle and inhibits glucose production in the liver,improving whole-body energy homeostasis[26].Leptin is involved in appetite control and weight regulation[47].Leptin and adiponectin in human milk have been implicated as potential regulators in early-life metabolic programming.Our study found that leptin and adiponectin in milk are associated with plasma leptin,while maternal weight and body muscle mass are correlated with leptin and adiponectin in milk.Our results support previous studies that found that maternal body composition is related to blood circulation and milk hormones[25,26,48]and that human milk hormones may continuously affect infants[27-29].Hence,the findings of other studies justify the many ways the mother’s nutritional status affects her offspring.
In a previous study investigating leptin levels in breast milk 6 weeks postpartum,the leptin level in milk was negatively correlated with children’s long-term BMI[28].In another study,adiponectin levels in human milk had a significant negative correlation with anthropometric values among term infants;but in preterm infants,adiponectin levels were positively correlated with infant BMI[27].Based on these results,mothers need to maintain good nutritional status before pregnancy and after delivery.However,further studies are required to analyze the growth mechanism in offspring regulated by milk hormones.
The World Health Organization recommends that infants be exclusively breastfed for up to six months and that breastfeeding continues to be an essential part of the diet until at least 2 years of age[49,50].However,most of the participants in our study did not continue breastfeeding past 7−8 months postpartum.Several studies have shown that breastfeeding can reduce maternal risks of breast cancer,endometrial cancer,ovarian cancer,type 2 diabetes mellitus,and heart disease and aid in birth spacing[14,51-59].Similar to the conclusions in previous studies,our results indicate that extending breastfeeding duration is conducive to reducing postpartum weight gain and can bring many benefits to mothers[15,60].
This study preliminarily explored weight changes among Chinese mothers and assessed the relationship between human milk composition and weight change using a one-year follow-up monitoring the maternal weight,milk,and hormones in milk and blood.The study found that that maternal adipocyte-derived hormones in milk and plasma are significantly correlated with maternal weight and body composition.
This study has some shortcomings.Other influencing factors of PPWR were not studied in this article.In the methodology of breast milk collection,we have not fully considered the considerable variability of breast milk components.Besides,the milk collection is carried out by the mother herself,which may increase the variability of human milk composition,especially milk fat.The dietary survey method was used to account for milk composition changes that could have been caused by maternal diet.However,we did not explore the long-term impact of dietary patterns on milk composition.Combining the food frequency questionnaire (FFQ) with the 24-h dietary survey method can be considered in future research.Another disadvantage is that self-reporting of maternal pre-pregnancy weight (and BMI) may impact the results,as women tend to under-reported pre-pregnancy weight and over-reported gestational weight gain[61].Besides,this is a single-center study,so the results reflect the situation of a specific population and may not be extendable to other countries and regions.In addition,although the follow-up time was long,few mothers were breastfeeding for more than a year,and due to the study design,mothers were not required to report the weaning time accurately.
5. Conclusions
The present study found that breastfeeding mothers may retain considerable weight gain one year after delivery.Changes in maternal body weight may be related to milk fat concentration but have no correlation with adiponectin and leptin in breast milk.Leptin and adiponectin in breast milk and leptin in plasma are associated with the maternal body composition.This study supports the notion that human milk composition may be affected by maternal body composition.Future research should focus on exploring breastfeeding following the recommendations of the World Health Organization and the weight management of postpartum mothers.
Statement of Ethics
This study was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine with the approval number XHEC-C-2020-081 and with appropriate participants’ informed consent in compliance with the Helsinki Declaration.Written informed consents were obtained from the participants.
Declaration of Competing Interest
The authors have no known competing financial interests relevant to this article.
Acknowledgments
We thank all participants in this study.We thank Mr.Zhongliang Jin for his help in drawing.We also thank all colleagues in the Department of Obstetrics and Gynecology of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine,Ms.Jing Yang of the Brand Development Department,and Ms.Heng Zou of the Outpatient Department for their support in the recruitment process.This study was supported by grants from the Shanghai Key Laboratory of Pediatric Gastroenterology and Nutrition (17dz2272000),Foundation of Shanghai Municipal Health Commission (Key weak discipline construction project 2019ZB0101),and the Scientific research fund of China Nutrition Society (CNSHPNK2021-16).
- 食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics

