Effects of potassium lactate on sensory attributes,bacterial community succession and biogenic amines formation in Rugao ham
Renyong Lio,Ying Wng,Qing Xi,Chngyu Zhou,*,Fng Geng,Dodong Pn,Jinxun Co*
a State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products,Key Laboratory of Animal Protein Food Processing Technology of Zhejiang Province,Ningbo University,Ningbo 315211,China
b Meat Processing Key Laboratory of Sichuan Province,School of Food and Biological Engineering,Chengdu University,Chengdu 610106,China
c School of Food and Health,Beijing Technology and Business University,Beijing 100048,China
Keywords:Rugao ham Potassium lactate Biogenic amines Microbial community Metabolic pathways
ABSTRACT To deepen the understanding in the effect of potassium lactate on the sensory quality and safety of Rugao ham,sensory attributes,physicochemical parameters,total volatile basic nitrogen (TVBN),microorganism community and biogenic amines of Rugao ham manufactured with different potassium lactate levels (0%,0.5%,1%,2%) were investigated;the relationship between microbial community and the formation of TVBN and biogenic amines was further evaluated.With the increase of potassium lactate from 0% to 2%,the increased sensory scores and the decreased total aerobic bacterial count and TVBN were observed; the abundance of Staphylococcus increased,while the content of Halomonas decreased. LDA effect size (LEfSe)and correlations analysis showed that Staphylococcus equorum and Lactobacillus fermentum could be the key species to improve sensory scores and decrease biogenic amines and TVBN.Metabolic pathway analysis further showed that amino acids metabolism and nitrogen metabolism were mainly involved in decreasing TVBN and biogenic amines in the treatment of 2% potassium lactate.
1. Introduction
Chinese Rugao ham is one of the most famous dry-cured meat products and is appreciated by consumers because of their special flavor and texture characteristics in China[1].The salting is the key process in the production of Rugao ham,which requires sodium chloride as the crucial ingredient owing to its preservative properties,and it has an important effect on sensory quality and safety of the products,such as texture,flavor,microbial stability and sensory properties[2].However,Chinese Rugao ham is usually salted with a large amount of salt (10%−12% NaCl) during the traditional processing procedure,which may lead to high level of salt content in the final products.The consumption of dry-cured ham with high salt is related to the raise of blood pressure,further increasing the risk of cardiovascular diseases[2,3].Therefore,it is particularly important to reduce the amount of salt to improve its impact on consumer health during the modern processing procedure.
The main strategies of salt reduction were to directly reduce the NaCl content at salting stage or partly replace of NaCl with other ingredients[4]. However,directly reducing the salt content added in the salting process of dry-cured ham may cause excess proteolysis,lipid hydrolysis and lipid oxidation due to high activity of cathepsins B,L and H,aminopeptidase and lipolytic enzymes occurring[2,5,6],which could result in the defective texture and unpleasant flavor of products.Salt reduction will also lead to the rapid growth of microorganisms,increasing the activity of microbial decarboxylase,which could promote the decarboxylation of specific amino acids for the generation of biogenic amines connected with the occurrence of adverse cardiovascular and nervous system reactions[7].Furthermore,these may result in spoilage or unsafety for the products[2].Accordingly,salt reduction is usually achieved by using other alternatives,such as KCl,which is a widely used substitute for salt reduction due to the similar properties to NaCl,but it may also be limited by negative sensory attributes,which could show intense bitter and astringent taste[8].
Potassium lactate has been used as a food additive in meat products,because it can not only inhibit spoilage bacteria and pathogenic bacteria,but also maintain color stability,and increase cook yields and flavor[9-11].Quilo et al.[12]used potassium lactate on ground beef patties and found it could improve sensory,shear and cooking characteristics.Astruc et al.[13]found that the using of potassium lactate in beef sausage increased the salty taste.Muchaamba et al.[14]reported that potassium lactate could inhibit the growth ofListeriamonocytogenesin Salami without affecting the product quality,and it also contributed to reducing microbiota in recombinant ham[4].Therefore,it could be a potential substitute for sodium chloride in the process of dry-cured meat products[15].Fulladosa et al.[16]found that potassium lactate could improve color,tenderness and texture of restructured ham,and Costa-Corredor et al.[2]also confirmed that potassium lactate decreased water activity (aw),proteolysis and softness of salt-reduced restructured ham.Although potassium lactate could improve the quality and safety of meat products,there is no research to evaluate the effect of potassium lactate on quality and safety of Rugao ham.
Most of researchers studied the effect of potassium lactate on microorganisms in dry-cured meat products by cultured enumeration[4,17],while the variation of microbial community structure in dry-cured meat products has not been investigated in detail when potassium lactate was added as curing agent.Recently,high-throughput sequencing (HTS) technology can deeply and reliably characterize microbial community,which relies on the amplification of specific regions in the bacterial 16S rDNA gene[18].This technology has been successfully used to monitor the changes of microbial community structure and species abundance during the processing or storage periods of dry-cured ham[19,20].
Therefore,this work was to evaluate the sensory quality and safety characteristics of Rugao ham manufactured with different levels of potassium lactate through sensory evaluation,physicochemical determination,microbial counts,biogenic amines levels,and microbial community composition analysis by HTS of bacterial 16S rDNA gene,and the relationship between microbial community and the formation of TVBN and biogenic amines was further discussed.These findings could be helpful to understand the effect of potassium lactate on sensory quality and safety of Rugao ham in terms of microbial community and biogenic amines,and provide valuable information to study on the strategy in the salt reduction of dry-cured ham in the future.
2. Materials and methods
2.1 Processing and sampling of Rugao ham
The Rugao hams were manufactured by Food Company of Jiangsu Province in China.A total of 200 fresh hind legs of about 8 kg from native pigs randomly and equably divided into four groups.The manufacture procedure of Rugao ham were performed according to methods of Wei et al.[1].Four group of fresh hind legs were salted for 40 days using 6% NaCl,0.015% NaNO2and potassium lactate(0%,0.5%,1%,2%,respectively) at 0−8 °C and 60%−80% relative humidity.After salting,the hams were washed and sun-dried (10 °C and 60%−70% relative humidity) for 15 days,then the hams were transferred to the dry-ripening room for ripening over a period of seven months under natural conditions;the hams were post-ripened at room temperature for two months after ripening.At the end of the process,6 hams were randomly taken from each group;after removing the oxide layer on the surface of ham and bones,thebiceps femorisandsemimembranosusfrom each ham were quickly separated by sterile knife and sampled.Then the samples were vacuum packed and stored at −20 °C until analyzed.
2.2 Sensory analysis
According to the descriptions of Zhou et al.[21]and Liao et al.[22],thebiceps femorisandsemimembranosussamples were evaluated by 10 expert sensory assessors (5 females and 5 males,mean age 23) with rich experience in assessing dry-cured ham.Before the test,panelists received a total of three sessions for training in sensory characteristics of dry-cured ham to reach a consensus on the use of attributes and scales.The samples were cut into pieces (1 mm) and presented to the assessors in the laboratory (25 °C) for no more than 15 min before sensory analysis.The followed indicators were evaluated on a scale ranging from 0 to 10: color (0 means dark gray and dull,10 means bright red and shiny),texture (0 means loose and soft,10 means dense and elastic),odor (0 means unpleasant odor,10 means rich and fresh aroma),taste (0 means too salty or unpleasant taste,10 means moderate saltiness and delicious taste) and overall acceptance (0 means unacceptable,10 means very favorite).Individual ratings for each indicator did not vary more than ± 0.5 from the average panel score.The values were expressed as the average of 6 ham samples of each group.
2.3 Physicochemical determination
The samples (5 g) were homogenized in 45 mL of distilled water at 12 000 r/min for 30 s in the XHF-D high-speed dispensator (Scientz Biotechnology Co.,Ltd.,Ningbo,China).Then the homogenates were centrifuged at 10 000 ×gfor 30 min at 4 °C and the supernatants were collected for analysis of pH and salt content.The pH was measured using an electronic pH meter (Mettler Toledo Instruments Co.,Ltd.,Shanghai,China) at room temperate.The salt content of the supernatants was measured using a CON 2700 conductivity meter (Thermo Eutech,CA,USA),and the salt content of ham was expressed as the percentage of the measured salt content to the dry matter of the ham sample.After 5 g of the stranded sample was dried to constant weight at 105 °C,the moisture content was indicated as the percentage of the weight difference before and after drying to the weight before drying of the ham samples.awwas measured at room temperate with a water activity meter (Shuo Guang Electronic Technology Co.,Ltd,Shanghai,China).All indictors were measured with 6 ham samples of each group.
2.4 Total volatile basic nitrogen (TVBN) determination
The TVBN values were estimated according to methods of Xu et al.[23].Ten grams of samples were taken into a conical flask with 100 mL distilled water,and immersed for 30 min with shaking.Next,10 mL of the solution filtered through the filter paper was added 5 mL of 10 g/L magnesium oxide and distilled for 5 min using an automatic Kjeldahl Apparatus (Hanon Instruments Co.,Ltd.,Jinan,China).The distillate was absorbed by 10 mL of 20 g/L boric acid,using 50 μL mixed indicator solution (1 g/L methyl red ethanol solution:1 g/L bromocresol green ethanol solution=1:5),then titrated to purplish red with 0.1 mol/L hydrochloric acid.The TVBN values was measured with 6 samples of each group and expressed with a unit of mg/100 g.
2.5 Total aerobic bacterial count
In sterile ultra clean bench,the samples ofbicepsfemorisandsemimembranosuswere treated separately.The surface of samples was disinfected,and then the samples were collected from the maximum depth of 2 mm on the surface using a sterile knife;after cutting and shredding,an appropriate amount of mixed sample was taken into sterilized frozen storage tube and immediately stored at−80 °C for bacterial 16S rDNA sequencing.At the same time,10 g of mixed samples were immediately taken for the determination of total aerobic bacterial count.
Total aerobic bacterial count was carried out by methods of Aksu et al.[17],in sterile ultra clean bench,10 g of mixed samples was homogenized in 90 mL sterile 0.9% saline solution in a sterile homogeneous bag for 90 s.Appropriate dilutions (10−1−10−4) were made with sterile 0.9% saline solution and 1 mL dilutions were plated onto the Plate Count Agar (PCA,Oxoid,Unipath,Basingstoke,UK)by coating method,then inverted incubated for 72 h at 37 °C.The colonies were counted by Colony Meter (Xun Minor Technology Co.,Ltd.,Hangzhou,China) and expressed as CFU/g of sample.Six ham samples were measured in each group,then all samples were tested three times.
2.6 Determination of the microbial communities of Rugao ham
2.6.1 DNA extraction
According to Lu et al.[24],total genome DNA was extracted from 6 ham samples of each group using CTAB method.20 μL of lysozyme was added into 1 000 μL of CTAB lysis buffer (0.05 mol/L CTAB,1.4 mol/L NaCl,0.1 mol/L Tris-HCl,20 mmol/L Na2EDTA),and then an appropriate amount of sample was added.The mixture was incubated for 2−3 h at 65 °C.950 μL of the supernatant was extracted with an equal volume of phenol:chloroform:isoamylalcohol (25:24:1) and centrifuged for 10 min at 12 000 r/min.Then the supernatant was added an equal volume of chloroform:isoamylalcohol (24:1) and centrifuged for 10 min at 12 000 r/min.0.75 volume of isopropanol was added to the supernatant and incubated for 10 min at −20 °C.After centrifugation for 10 min at 12 000 r/min,the supernatant was discarded.The precipitate was washed twice with 1 mL of 75% ethanol.The precipitate was dried at room temperature and dissolved in 51 μL ddH2O.RNA in the DNA solution was degraded by 1 μL of 10 mg/mL RNase A (Qiagen,German) for 15 min at 37 °C.DNA concentration and purity were monitored on 1% agarose gels.DNA concentration was adjusted to 1 ng/μL with sterile water.
2.6.2 PCR amplification of bacterial 16S rDNA genes
According to the method of Wang et al.[19],16 S rDNA genes (V3-V4) were amplified by primer 5’-CCTAYGGGRBGCASCAG-3’ and primer 5’-GGACTACNNGGGTATCTAAT-3’.All PCR reactions were carried out in 30 μL solution contained 15 μL of Phusion®High-Fidelity PCR Master Mix (New England Biolabs,USA),1.5 μL of each primer (0.2 μmol/L),10 μL template DNA and 2 μL of ddH2O,and cycling conditions consisted of a first denaturation step at 98 °C for 1 min,followed by 30 cycles at 98 °C (10 s),50 °C (30 s) and 72 °C (30 s) and a final 5 min extension at 72 °C.PCR products were performed electrophoresis on 2% agarose gel and purified by using GeneJET Gel Extraction Kit (Thermo Scientific,USA).Sequencing libraries were generated with TruSeq DNA PCRFree Library Preparation Kit (Illumina,USA).The library quality was evaluated on the Qubit@ 2.0 Fluorometer (Thermo Scientific,USA) and Agilent Bioanalyzer 2100 system.Finally,the library was sequenced on an Illumina NovaSeq 6000 platform and 250 bp pairedend reads were generated.
2.6.3 Bioinformatics analysis
Paired-end reads from the original DNA fragments are merged by using FLASH,and assigned to each sample according to the unique barcodes.Sequences were analyzed and filtered using QIIME software package (Quantitative Insights Into Microbial Ecology).Sequences with ≥ 97% similarity were assigned to the same operational taxonomic units (OTU).The representative sequence for each OTU was further annotated taxonomic information by the RDP classifier.The functional composition of communities was described using the statistical software PICRUSt and annotated to their biological function according to Kyoto Encyclopedia of Genes and Genomes(KEGG) (http://www.kegg.jp/kegg/pathway.html).
2.7 Biogenic amine analysis
Biogenic amine standards (tryptamine,phenylethylamine,putrescine,cadaverine,histamine,tyramine,spermidine,and spermine) were purchased from Sigma Chemical (Sigma Chemical Co.,St.Louis,MO,USA).Standard solutions (0.5,1,2,5,20,50,100 μg/mL) of each biogenic amine were prepared in 0.4 mol/L perchloric acid.Biogenic amines were determined by the method described by Alfaia et al.[25]with modification.Five grams of samples(n=6) were homogenized with 20 mL of 0.4 mol/L perchloric acid at 12 000 r/min for 30 s in the XHF-D high-speed dispensator(Scientz Biotechnology Co.,Ltd.,Ningbo,China),then centrifuged at 5 000 ×gfor 10 min.The extraction process was repeated twice under the same conditions.The supernatants were combined and adjusted to 50 mL with 0.4 mol/L perchloric acid.Next,1 mL of the extract or standard solution was mixed with 200 μL of 2 mol/L sodium hydroxide,300 μL of saturated sodium bicarbonate and 2 mL of 10 mg/mL dansyl chloride reagent to incubate at 40 °C for 30 min in the dark.Finally,it was added 100 μL of 25%ammonium hydroxide to stop the reaction,and then adjusted to 5 mL with acetonitrile.The mixture was filtered through a 0.22 μm organic membrane filter for high performance liquid chromatography (HPLC)analysis.HPLC was carried out with a ZORBAX XDB-C18column(4.6 mm × 250 mm,5 μm,Agilent,Santa Clara,CA,USA) at 254 nm.The elution was performed with solvent A (water) and solvent B (acetonitrile) using the following gradient elution program:0 min,65% B;5 min,70% B;20 min,100% B;25 min,65% B;30 min,65% B.The flow rate was 1 mL/min,injection volume was 20 μL,and column temperature was 40 °C.
2.8 Statistical analysis
All the data were expressed as mean ± standard deviation (SD).Levene’s test was used for homogeneity and one-way analysis of variance (ANOVA) was used to evaluate the data of sensory scores,physicochemical parameters,microbial counts,and biogenic amines at a significance level of 5% using SAS 8.1 (SAS Institute Inc.,Cary,NC).The comparison of means was assessed by Duncan’s Multiple Range Test also at a significance level of 5%.The correlation of microbial community with their biological function,TVBN and biogenic amines was analyzed by Pearson correlation analysis and visualized by the network using Cytoscape (v.3.4.0).
3. Results and discussion
3.1 Sensory analysis
The color,texture,odor,taste and overall acceptance scores of thebiceps femorisandsemimembranosussamples were shown in Fig.1.In thebiceps femorissamples (Fig.1A),significant changes in sensory scores were observed among different groups (P<0.000 1),samples with potassium lactate had the higher values of color,texture,odor,taste and overall acceptance than those of the control (0% potassium lactate);the highest sensory scores were shown in the group with 2%potassium lactate addition.Similarly,the significantly high sensory scores were also observed in thesemimembranosussamples with the increase of potassium lactate (P<0.01) (Fig.1B),where the sample supplemented with 2% potassium lactate showed the highest values of color,texture,odor,taste and overall acceptance among four groups.The above results could indicate that the addition of potassium lactate improved the quality of Rugao ham,which were in agreement with the results reported by Fulladosa et al.[16],who observed significant improvements in color,texture and flavor of restructured dry-cured hams in the treatment of potassium lactate.

Fig.1 Sensory evaluation of the biceps femoris (A) and semimembranosus (B) samples,TVBN values (C) and total aerobic bacterial count (D) among 44 groups.BF represents biceps femoris samples and SM represents semimembranosus samples.a-d Different lowercase letters in the same sample mean significant difference among 44 groups (P <0.05).
Color,texture,odor,taste and overall acceptance are important sensory parameters to evaluate the quality of dry-cured ham.Color of dry-cured ham is related to the state of myoglobin and its content.Myoglobin gives red color in dry-cured meat products,when it forms a metmyoglobin with a brown color due to oxidation.Myoglobin oxidation could be slowed down at higher pH,but microbial growth may promote it[26].In the present study,with the increase of potassium lactate levels,pH increased and microorganisms decreased significantly.Therefore,potassium lactate could protect myoglobin from rapid oxidation by increasing pH and reducing microbial growth.Color of dry-cured ham is also mainly due to the presence of nitrosylmyoglobin,which is a stable red component formed by the reaction of NO with myoglobin[27].It was found that the addition of potassium lactate in dry-cured meat products could reduce metmyoglobin to deoxy myoglobin by regenerating reduced nicotinamide adenine dinucleotide and accelerate the conversion of nitrite and NO,which may contribute to the forming of nitrosylmyoglobin[9].These may be the reasons for the higher color score of Rugao ham treated with potassium lactate.The development of texture is closely related to the degree of proteolysis.During the maturation of dry-cured ham,myofibrillar and sarcoplasmic proteins are intensively degraded,which contributes to the texture and final quality of the ham[28].Taste and odor are also related to the proteolysis and lipid degradation,which are main biochemical reactions generating taste and volatile compounds of dry-cured ham[29].However,excessive degradation of protein could result in excessive softness and excessive accumulation of bittertasted peptides and amino acids,resulting the textural defects and bitterness of the products[30].Excessive lipid degradation also may result in rancid flavor and undesirable color of meat products[29].Proteolysis and lipid degradation of dry-cured ham are mainly influenced by endogenous enzymes and microorganisms[31],and it has been known that abnormal growth of microorganisms could lead to abnormal proteolysis,resulting in deterioration of texture,taste and flavor,and consumer dissatisfaction[22,32,33].Potassium lactate is a flavor enhancer and could delay the development of sour and offflavors[10],and it inhibited the growth of microorganisms to prevent excessive degradation of proteins and lipids,which could contribute to the better sensory quality of the final products.Aksu et al.[17]has confirmed that potassium lactate could prevent the lipid excessive oxidation and protein excessive degradation,resulting in the increase ofa* value,and the development of gummy/tight structure.These characteristics further affected palatability of meat products[9,16].Thus,the improvement of the palatability and stability could be responsible for higher scores in sensory of the ham with potassium lactate in this study.
3.2 Physicochemical analysis
The variation in pH,aw,moisture and NaCl content was shown in Table 1.In thebicepsfemorisandsemimembranosussamples,the pH value of the treatment of 2% potassium lactate was significantly higher than those of the control (0% potassium lactate) (P<0.05),while the NaCl content decreased significantly when 2% potassium lactate was added (P<0.01).The content of moisture significantly decreased from 44.15% to 41.13% when the addition of potassium lactate increased from 0% to 2% in thesemimembranosussamples(P<0.01).The treatment of 0% potassium lactate also showed the highest moisture content (48.50%),and the significant decrease in moisture content was observed in the treatment of 0.5%,1% and 2%potassium lactate in thebicepsfemorissamples (P<0.05) compared with the control (0% potassium lactate).Theawsignificantly decreased with the increase of potassium lactate from 0% to 2% insemimembranosusandbicepsfemorissamples.These results were consistent with previous research by Fulladosa et al.[16]and Aksu et al.[17],who found that potassium lactate could contribute to the increase of pH and the decrease ofaw,moisture and NaCl content.
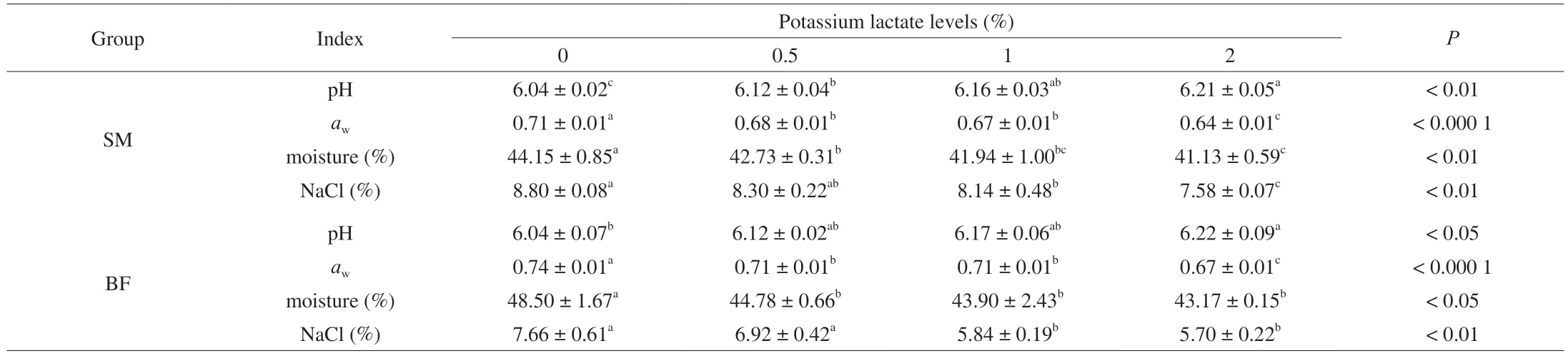
Table 1 Physico-chemical parameters of Rugao ham.
pH,aw,moisture and NaCl content are the basic physical properties that may affect quality of meat products.In terms of pH,Gou et al.[34]also reported that potassium lactate had a negative effect on the decrease of pH value of fermented sausage.Although high pH value will increase the risk of spoilage,the main factor affecting microorganisms in dry-cured ham isawat the end of processing,while pH value has little effect on product stability[16].In addition,during the processing of meat products,some microorganisms can produce acid,thus reducing the pH value[35].However,Fulladosa et al.[4]detected lower aerobic total count and lactic acid bacteria in restructured dry-cured hams with the addition of potassium lactate than the control group,which indicated that potassium lactate inhibited the growth of these microorganisms and reduced the production of acidic substances[8].Thus,the increase of pH value in this study might be related to the inhibition of microorganisms by potassium lactate in the production process.awand moisture content in dry-cured ham are essential to ensure good storage characteristics,and lower moisture is beneficial for microbial stability[36].Generally,awwas positively associated with moisture content,but negatively correlated with salt content[28].However,aw,moisture and salt content decreased with the addition of potassium lactate in this study,which may be attributed to the fact that potassium lactate is hygroscopic and can bind water in meat reducing the available water[37],resulting in the decrease of moisture content andaw[38].Thus,potassium lactate played a more critical role in reducing moisture content andawin this study,which further affected the microorganism community structure of Rugao ham.Meanwhile,the salt content was lowest in ham with 2% potassium lactate,in part because of the potassium lactate addition effect on the dry-matter basis[2].Thus,the ham with 2% potassium lactate may have the best quality due to its lowestaw,moisture and salt content.
3.3 TVBN and total aerobic bacterial count
As is shown in Fig.1C,the TVBN values of thebicepsfemorisandsemimembranosussamples significantly decreased with the increase of potassium lactate (P<0.000 1).In Fig.1D,it can be observed that with the increase of potassium lactate from 0% to 2%,the total aerobic bacterial count of thesemimembranosussamples sharply decreased from 4 783.33 CFU/g to 116.67 CFU/g (P<0.01),and the total aerobic bacterial count of thebicepsfemorissamples sharply decreased from 2 833.33 CFU/g to 83.33 CFU/g (P<0.000 1).The reduction of total aerobic bacterial count also observed in restructured dry-cured hams with potassium lactate by Fulladosa et al.[4].
TVBN is one of the most widely used quality indicators in meat products and is highly consistent with the degree of spoilage in meat products[39].Therefore,the lower TVBN values may contribute to the improvement of sensory score of Rugao ham supplemented with potassium lactate.TVBN compounds are the sum of volatile amines and toxic nitrogen-containing compounds,including ammonia(NH3),trimethylamine (TMA) and dimethylamine (DMA).These compounds are produced by the degradation of proteins and other nitrogen-containing compounds,which are closely related to the activity of endogenous enzymes and the metabolism of spoilage bacteria,such as Enterobacteriaceae andMicrococcus[39-41].It has been confirmed that TVBN could be produced by Enterobacteriaceae[41],Bekhit et al.[39]found that TVBN also had a positive correlation with total aerobic bacterial count,which was similar with our result that TVBN and total aerobic bacterial count both significantly decreased with the increase of potassium lactate from 0% to 2%.Gelabert et al.[8]and Aksu et al.[17]also found that potassium lactate reduced total viable count and the growth of Enterobacteriaceae in fermented sausages and pastırma.These results suggested that the decrease of TVBN values was related to the inhibition of microbial growth and the prevention of excessive proteolysis.Meanwhile,the decrease of total aerobic bacterial count may be attributed to the antibacterial ability of potassium lactate.In fact,potassium lactate has the ability of weak lipophilic acid (such as lactic acid),it could pass across the cell membrane in undissociated form,then dissociate within the cell to release protons and acidify the cell interior,resulting in requiring more cell energy to maintain the constant pH and reducing the growth of the cell,which further affected the normal proton gradient that could damage the function of the cell[11].Moreover,potassium lactate also reducedawin this study,which increased the antibacterial ability of potassium lactate itself[42].
3.4 Comparison of microbial communities between different treatments
There was no significant difference in sensory and physicochemical properties between the groups of 0.5% and 1%potassium lactate (Figs.1A,B and Table 1).Therefore,in order to evaluate the changes of microbial community structure influenced by the addition of potassium lactate,the microbial species richness of ham samples manufactured with 0%,1% and 2% potassium lactate was analyzed by HTS of bacterial 16S rDNA gene.
In allsemimembranosusandbicepsfemorissamples,the bacterial community was composed of 37 phyla,and top 10 abundances phyla were showed in Fig.2A.Proteobacteria and Firmicutes were found as the most abundant microbial phyla with a total relative abundance over 70% in allsemimembranosusandbicepsfemorissamples.In thebicepsfemorissamples,the abundance of Proteobacteria was the highest in the control (0% potassium lactate),up to 45.94%,and the least in the ham samples with 2% potassium lactate,accounting for 36.73%;while the abundance of phylum Firmicutes increased with the increase of potassium lactate,and the highest abundance was observed in the group of 2% potassium lactate,up to 39.28%.In thesemimembranosussamples,with the increase of potassium lactate,the abundance of Proteobacteria also showed a downward trend(from 51.64% to 40.77%),while phylum Firmicutes increased from 41.94% to 50.83%.Above all of these suggested that Proteobacteria and Firmicutes play a vital role in Rugao ham,which also occupied the main status in Panxian ham[18]and Jinhua ham[19].The variation of Firmicutes and Proteobacteria may further affect their related genera,such asHalomonasbelonging to Proteobacteria,andStaphylococcusbelonging to Firmicutes.

Fig.2 The relative abundance of bacteria at the phylum (A) and genus (B) level,and evolutionary branch map of the biceps femoris (C) and semimembranosus (D)in the different samples.BF-0%,BF-1% and BF-2% represent the biceps femoris samples with 0%,1% and 2% potassium lactate,respectively;SM-0%,SM-1%and SM-2% represent the semimembranosus samples with 0%,1% and 2% potassium lactate,respectively.(C,D) The circle radiating from the outside to inside represented the classification level from the phylum to the species.The different species biomarkers follow the group for coloring.
In allsemimembranosusandbicepsfemorissamples,total 656 genera were detected and top 10 abundances genera were showed in Fig.2B.A large proportion of microbial species wereStaphylococcus,Halomonas,PsychrobacterandCobetia.Especially,in thebiceps femorissamples,Staphylococcusaccounted for 23.08%,24.52% and 35.87% of the bacteria in the group of 0%,1% and 2% potassium lactate,respectively,while the relative abundance ofHalomonasdecreased from 32.07% to 27.24% with the increase of potassium lactate from 0% to 2%.Similarly,in thesemimembranosussamples,Staphylococcuswas most (48.69%) in the treatment of 2% potassium lactate and lowest (37.82%) in the control (0% potassium lactate),while the abundant ofHalomonasdecreased from 38.62% to 23.88%with the increase of potassium lactate.Staphylococcushas been reported to be the dominant genus in the resting,ripening stages and final products of dry-cured ham,such as Panxian ham[18]and Jinhua ham[19],andHalomonasalso was most abundant genus in Rugao ham (2-year fermentation period) detected by Zhu et al.[20],which was in the line with our results thatStaphylococcusandHalomonaswere the two most abundant genera.These indicated thatStaphylococcusandHalomonasmay be competitive in Rugao ham.Due to the influence of potassium lactate,Staphylococcuswas more dominant and gradually became the bacterium with the largest proportion,whileHalomonaswas suppressed to a certain extent.In thesemimembranosusmuscle samples treated with 2% potassium lactate,the proportion ofStaphylococcuswas more than twice that ofHalomonas.It reported thatHalomonashad a high amylase activity existed in fermented food and was related to the formation of flavor substances,such as 1-penten-3-ol,hexanal,3-methylbutyric acid and 2-butanone[43].Therefore,Halomonasstill contributed to the quality of Rugao ham,because it still accounted for a large proportion of microbial flora in ham.Staphylococcushad proteolytic,lipolytic and nitrate reductase enzymes activity,which could enhance the degradation of fat and protein to contribute to the flavor[18,19,44]and promote the formation of nitromyoglobin to provide the desired color of ham products[45].Thus,Staphylococcuswas the main microorganism responsible for the improvement of ham quality by potassium lactate.
LDA effect size (LEfSe) analysis could find key microbes with statistical differences between groups[46].In this study,bacteria with LDA value greater than 4 were regarded as key microbes to improve the quality of Rugao ham,LDA scores histogram was shown in supplementary (Fig.S1).In thebicepsfemorissamples,LEfSe detected 11 bacterial clades (Fig.2C).It was found that the most important contribution to the samples with 1% potassium lactate was Bacteroidota at the phylum level,whileStaphylococcuswas regarded as the most important genus contributed to the quality of the samples with 2% potassium lactate;the samples with 0% potassium lactate were missing in the figure,this may because distances among intrasample in the samples with 0% potassium lactate were larger than the equivalent distances among other groups,which leaded to a lack of taxa common to the samples with 0% potassium lactate.Therefore,no taxa were determined by LEfSe to be characteristic of the samples with 0% potassium lactate[46].In thesemimembranosussamples (Fig.2D),it revealed that there were 10 microbes with LDA scores over 4,showing significant differences among the three groups.The most important contribution to the samples with 0% potassium lactate of thesemimembranosussamples was Proteobacteria (at the phylum level),while Firmicutes was the most important contribution to the group of 1% potassium lactate,andStaphylococcusplayed a major role in improving the quality of the samples with 2% potassium lactate.Staphylococcusgenus had a very high LDA score (near 5 orders of magnitude) in thebicepsfemorisandsemimembranosussamples with 2% potassium lactate,reflecting its high abundance in the treatment of 2% potassium lactate.These results further indicated that potassium lactate had a great effect onStaphylococcus,which may be a main genus related to the quality improvement of Rugao ham.
To investigate the differences of microbial community in the different samples of Rugao ham,cluster analysis was performed on the top 35 species and their relative abundances in all samples ofbiceps femorisandsemimembranosuswere shown as clustering heatmap(Fig.3).In thebicepsfemorissamples,the results showed that the samples with 0% and 1% potassium lactate were clustered into one group,and these samples with 2% potassium lactate were also formed into one group.In thesemimembranosussamples,two obvious clusters also were observed,the first cluster was the samples with 0% and 1% potassium lactate,while the samples with 2% potassium lactate were divided into another cluster.These results showed that both inbicepsfemorisandsemimembranosussamples,the microbial composition at the species level between the samples with 0% and 1% potassium lactate was not difference,while the samples with 2% potassium lactate were different from these groups (0% and 1% potassium lactate).Specifically,inbicepsfemorissamples,Halomonassubglaciescolawas higher abundant in the samples with 0% potassium lactate than the group of 2% potassium lactate,whileStaphylococcusequorumandLactobacillusfermentumin the samples with 2% potassium lactate were more abundant than that of the samples with 0% and 1% potassium lactate.In thesemimembranosussamples,the samples with 2% potassium lactate were also detected lower abundantH.subglaciescola,but more abundantS.equorumandL.fermentumthan the control group (0% potassium lactate).Xu et al.[23]found that the inoculation ofL.fermentumin fermented sausage improved the color,taste,flavor and overall acceptability of the sample sausages by its metmyoglobin reductase activity and ability of preventing excessive changes including fat oxidation and protein degradation[47].In addition,Wang et al.[19]found thatS.equorumcould produce aldehydes,which were the major flavor compounds in Jinhua ham,such as hexanal,nonanal,benzaldehyde and 2-heptenal associated with sweet,fruity and floral odors[44].Dias et al.[48]also found the application ofS.equorumas a starter in traditional drycured sausage did not adversely affect its sensory acceptability.In our study,the higher content ofL.fermentumandS.equorumin the ham samples with 2% potassium lactate could be responsible for its higher scores in sensory evaluation.The characteristics ofH.subglaciescolahave not been reported.However,as one of the most abundant species in Rugao ham in this work,it was likely to affect the formation of microbial community and ham quality.These results were consistent with LEfSe analysis (Figs.2C,D and S1),in thebicepsfemorissamples,S.equorumwas also considered to play a major role in the improvement of quality in the treatment of 2% potassium lactate,which had a high LDA score (4.72),while the most important contribution to the sample with 0% potassium lactate ofsemimembranosuswasH.subglaciescola,and the LDA score was 4.84.Therefore,it could be considered that the key species affected by potassium lactate wereS.equorum,H.subglaciescolaandL.fermentumwhich could contribute to improving the quality of Rugao ham.

Fig.3 Clustering heatmap of the changes in the microbial communities whose relative abundances were in the top 35 at the species level of Rugao ham.BF-0%,BF-1% and BF-2% represent the biceps femoris samples with 0%,1% and 2% potassium lactate,respectively;SM-0%,SM-1% and SM-2%represent the semimembranosus samples with 0%,1% and 2% potassium lactate,respectively.
3.5 Biogenic amines analysis
Table 2 showed the changes of biogenic amines concentrations in ham samples with different levels of potassium lactate.In allbiceps femorissamples,histamine and spermine were the main amines produced among 4 groups (0%,0.5%,1% and 2% potassium lactate),followed by phenylethylamine,cadaverine and spermidine.Similarly,histamine and spermine also were main biogenic amines detected in allsemimembranosussamples,followed by phenylethylamine and spermidine.
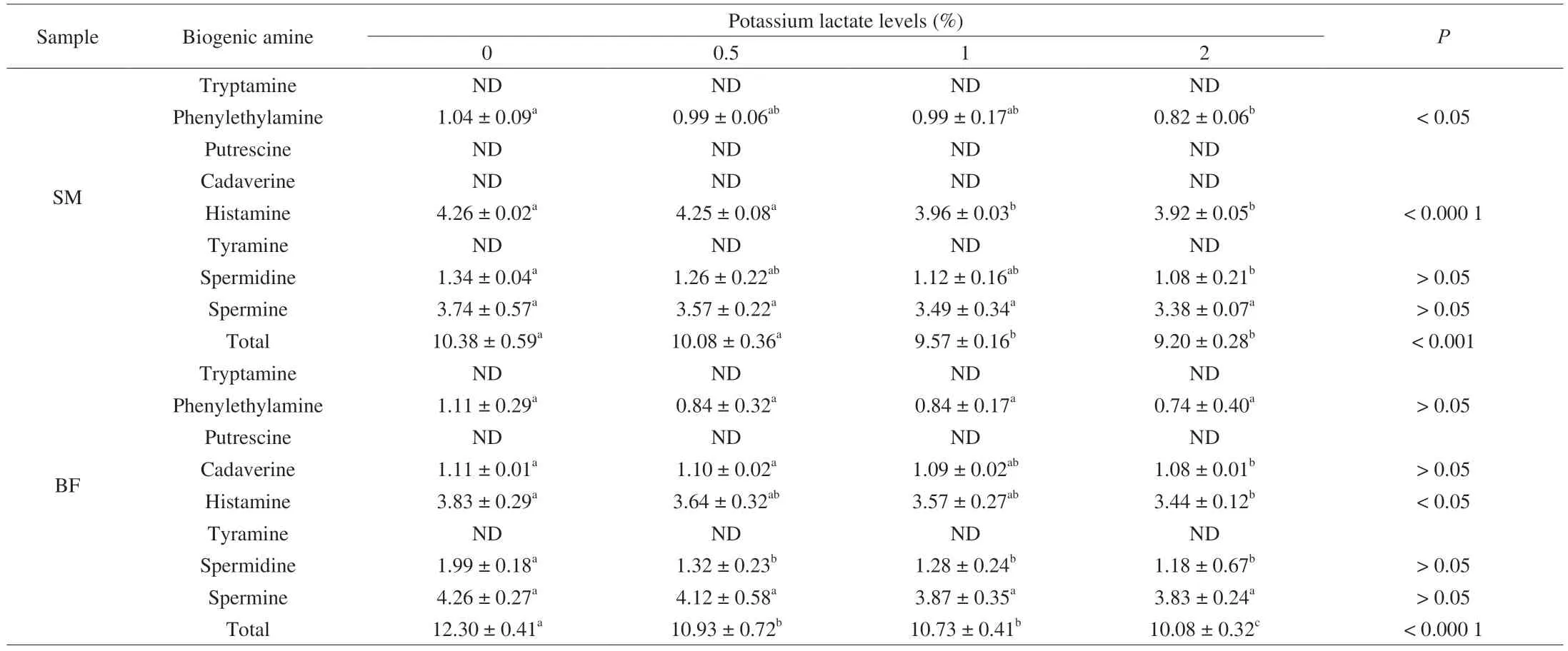
Table 2 Biogenic amine levels (mg/100 g) of Rugao hams.
The content of histamine in the treatment of 2% potassium lactate significantly decreased compared with the treatment of 0% potassium lactate in thebiceps femorisandsemimembranosussamples(P<0.05),which indicated that the addition of 2% potassium lactate had a significant inhibitory effect on the formation of histamine.Histamine was the major causes for food poisoning by biogenic amines,and it was considered that histamine level in food should not exceed 100 mg/kg[7].All ham samples met the safety requirements in this study.Histamine is formed by decarboxylation of histidine(substrate) under the action of microorganisms[7].However,it was also found that someLactobacillusstrains could degrade histamine and someStaphylococcusstrains were negative for histidine decarboxylase activity[49-51].Thus,the formation of histamine was related to the content of histidine and the growth of these microorganisms.Aksu et al.[17]had demonstrated that the dry-cured meat product treated with the salt mixture including potassium lactate significantly reduced the histidine content than that of control group,which may partly contribute to the reduction of histamine.Moreover,L.fermentumdetected in this study has been confirmed to inhibit the production of histamine by Xu et al.[23].Dias et al.[48]also found thatS.equorumpromoted the reduction of histamine on traditional drycured sausage.These results indicated that the highest amount ofS.equorumandL.fermentumin the samples with 2% potassium lactate may be the main reason for its lowest content in histamine.
In thebiceps femorisandsemimembranosussamples,spermine was no obvious change among 4 groups,and spermidine contentswas low and slight reduction with the increase of potassium lactate.Studies reported that spermine and spermidine initially present in meat used as raw material and their formation was not affected by the curing process or microbial activity[52],but they could be utilized as a nitrogen source by some microorganisms and degraded by bacteria in dry-cured meat products[24].Dias et al.[48]demonstrated thatS.equorumhas showed a great potential to reduce spermidine and spermine.Therefore,the slight reduction of spermine and spermidine could be attributed to the activities ofS.equorumin this study.
The content of phenylethylamine inbiceps femorissamples had no significant change among 4 groups (P>0.05),and the samples with 2% potassium lactate showed obviously lower content in phenylethylamine than that of the control group insemimembranosussamples (P<0.05).Cadaverine was only detected inbiceps femorissamples and its content was the lowest in the group of 2% potassium lactate than other groups.Phenylethylamine and cadaverine are formed by decarboxylation of their substrate (phenylalanine and lysine) by microbial decarboxylase[7],while the formation of phenylethylamine and cadaverine was inhibited byL.fermentumandS.equorum[23,48].Therefore,the decrease of phenylethylamine and cadaverine in the treatment of 2% potassium lactate could be explained by the lack of decarboxylase activity of these two microorganisms and their degradative activities for biogenic amines[50,51].
In this study,tryptamine,putrescine and tyramine were not detected in allbiceps femorisandsemimembranosussamples,while the low content of tryptamine,putrescine and tyramine was detected in Iberian hams[25].In fact,tryptamine,putrescine and tyramine could be produced by Enterobacteriaceae,which have high decarboxylase activity[7].However,the low level of Enterobacteriaceae was detected in this study,which may be a reason for no detection of tryptamine,putrescine and tyramine in all tested samples.
The total biogenic amine contents were calculated and shown in Table 2,it was obvious decreased with the increase of potassium lactate,this because biogenic amines detected in ham were affected by microorganisms and show varying degrees of reduction.Both insemimembranosus(P<0.001) andbiceps femorissamples (P<0.000 1),the total biogenic amine contents in the treatment of 2% potassium lactate were significantly lower than the control group (0% potassium lactate),which indicated that the ham with 2% potassium lactate could have the better safety quality.
3.6 Correlation network of bacterial community with TVBN and biogenic amines and metabolic pathway analysis
Predicted functional analysis showed that these microorganisms had biological functions including arginine and proline metabolism,histidine metabolism,lysine degradation,phenylalanine metabolism,tryptophan metabolism,tyrosine metabolism and nitrogen metabolism.These different metabolic pathways together contributed to the quality and safety development of the dry-cured ham.In order to evaluate to relationship between bacterial community,and TVBN and biogenic amines,the correlation network between the 35 core bacterial species and top 10 biological functions,biogenic amines and TVBN was established,and the significant correlations (|r| >0.5,P<0.05) were visualized in Figs.4A and B.Biogenic amines and TVBN detected in the ham samples were matched with known compounds in the KEGG to identify the metabolic pathways,which were summarized and presented in Fig.4C.

Fig.4 Correlation network diagram of the microbial community with their biological function,biogenic amines and TVBN of biceps femoris (A) and semimembranosus (B) and the metabolic pathway network (C) of biogenic amines and TVBN produced through microorganism in Rugao ham based on KEGG.(A,B) The middle circle represents the main species;the left-hand circle represents the biological function,and the right-hand circle represents TVBN and biogenic amines.(C) Yellow represent metabolic pathway,red represent the substances and key species detected.
As is shown in Fig.4C,potassium lactate in Rugao ham affected the formation of biogenic amines,which are produced by decarboxylation of free amino acids by microbial decarboxylase[7].Aksu et al.[17]found the addition of potassium lactate significantly reduced the content of histidine,lysine and phenylalanine.Dabadé et al.[53]noticed that the significantly positive correlations were present between histamine and histidine and betweenβ-phenylethylamine and phenylalanine.Therefore,the reduced production of histidine by potassium lactate may be partly responsible for the lower histamine levels.Moreover,it has been found thatL.fermentumdisplayed a great capability to inhibit the formation of biogenic amines[50],whileS.equorumdid not contain decarboxylase genes that contributed to the formation of biogenic amines[51].Xu et al.[23]also found that there was not phenylethylamine and lower histamine detected in sausage usingL.fermentumincubation.Dias et al.[48]found thatS.equorumpromoted the reduction of biogenic amines in traditional dry-cured sausage.Similarly,in our study,the most amount ofS.equorumandL.fermentumand the lowest content of histamine were detected in the 2% potassium lactate treatment.Meanwhile,L.fermentumwas negatively correlated with 4 kinds of biogenic amines (cadaverine,spermidine,spermine and histamine) inbicepsfemorissamples,and it also had a negative correlation with phenylethylamine of thesemimembranosussamples;S.equorumhad a negative correlation with phenylethylamine inbicepsfemorisand histamine insemimembranosussamples,shown in Figs.4A and B.We also found thatS.equorumhad negative correlation with lysine degradation and phenylalanine metabolism in thesemimembranosussamples,and their correlation value was more than 0.8,which indicated that this species played a key role in reducing the level of cadaverine and phenylethylamine,respectively (Fig.4C).These results indicated that potassium lactate reduced the production of biogenic amines mainly by affecting the activities ofS.equorumandL.fermentum.
TVBN was formed through nitrogen metabolism and was also associated withStaphylococcusandLactobacillus[39].In thebiceps femorissamples,L.fermentumwas negatively correlated with TVBN (Fig.4A),which was similar with the results reported by Xu et al.[23],and they found thatL.fermentumused in fermented sausage could prevent protein excessive decomposition from microorganisms and endogenous enzymes,resulting in the decrease of TVBN values.Moreover,S.equorumexhibited bacteriocin activity[51],which may inhibit the growth of microorganisms that can produce TVBN[39].In our study (Figs.4A and B),there were also negative correlations betweenS.equorumand TVBN in thebicepsfemorisandsemimembranosussamples,andS.equorumwas negatively related with nitrogen metabolism insemimembranosussamples,which further inhibited the formation of TVBN.These findings were supported by the obvious increase ofS.equorumandL.fermentumin the samples with 2% potassium lactate and the sharp decrease of TVBN value.
In addition,H.subglaciescolahad a positive correlation with arginine and proline metabolism,histidine metabolism,lysine degradation,nitrogen metabolism,tryptophan metabolism and tyrosine metabolism.As is shown in Fig.4A,in thesemimembranosussamples,H.subglaciescolahad a positive correlation with histamine,phenylethylamine,spermidine and TVBN,which was consistent with the research result of Zhang et al.[54]in a traditional pickled mustard tuber.However,it had no significant correlation with biogenic amines in thebicepsfemorissamples (Fig.4B).Similarly,Wang et al.[55]found thatHalomonasapparently degraded biogenic amines rather than synthesized them in Chinese traditional fish sauce.Jiang et al.[56]also found that amine oxidase on the membrane ofHalomonasspecies played a role in reducing biogenic amines.It maybe because that the function of this microorganism changed due to different raw materials,resulting in inconsistent results.In addition,due to the effect of potassium lactate,theHalomonaswas inhibited to a certain extent,which was similar to the change of biogenic amines,whileStaphylococcusgradually became the dominant bacterium with the largest proportion and may play a more important role thanHalomonasin reducing biogenic amines.This may also be the reason why our results are inconsistent with previous researches.Therefore,the specific effect ofH.subglaciescolaon the formation of biogenic amines and TVBN in dry-cured ham needs to be further studied.
As a consequence,S.equorumandL.fermentumwere main species to promote the decrease of biogenic amines and TVBN;amino acids metabolism and nitrogen metabolism were the main metabolism pathways affected by potassium lactate,which could be responsible for the decrease of biogenic amines and TVBN in Rugao ham.
4. Conclusions
The present study clearly proved that the addition of 2%potassium lactate improved the sensory properties,decreased TVBN values and total aerobic bacterial count,and changed microorganism community of Rugao ham than that of the control.The treatment of 2% potassium lactate increased the abundance ofStaphylococcusand decreased the content ofHalomonasin comparison with control group.L.fermentumandS.equorum,confirmed by the analysis of LEfSe and correlations could be the key species to improve sensory attributes,and to decrease biogenic amines and TVBN content in the treatment of 2% potassium lactate.Amino acids metabolism and nitrogen metabolism could be the main metabolic ways,which were further responsible for the decrease of TVBN and biogenic amines in the treatment of 2% potassium lactate.
Declaration of conflicting interest
The authors declare that there is no actual or potential conflict of interest.
Acknowledgement
This work was supported by National Natural Science Foundation of China (32 022066;32101975),Zhejiang Province Natural Science Foundation (LQ22C200017),China Postdoctoral Foundation(2020M 681806;2021T140348),and Science and Technology Programs of Ningbo (202003N4130;202002N3067).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250017.
- 食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics

