Effects of alcohol on digestion,absorption and metabolism of sea cucumber saponins in healthy mice
Wenxin Dng,Rong Li,Jinyue Yng,Chnghu Xue,b,Qingrong Hung,c,Yuming Wng,b,*,Tintin Zhng,*
a College of Food Science and Engineering,Ocean University of China,Qingdao 266003,China
b Laboratory for Marine Drugs and Bioproducts,Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237,China
c Department of Food Science Rutgers State University,New Brunswick,NJ 08901,USA
Keywords:Holothurin A Echinoside A Alcohol Absorption Metabolism
ABSTRACT Sea cucumber saponins have attracted more attention in recent years due to biological activities.It is a popular practice to soak sea cucumber in Baijiu at home and being applied to industrial manufacturing in China.However,knowledge of the effect of alcohol on the absorption and metabolism of sea cucumber saponins is limited.The effects of alcohol on digestion,absorption and metabolism of sea cucumber saponins in BALB/c mice were investigated after gavage and tail intravenous injection. The results showed that the content of saponins in serum and liver was significantly higher under the influence of alcohol than that in the control group after oral administration.Alcohol promoted the absorption of sea cucumber saponins prototype as well as inhibited the process of saponins being transformed into deglycositic metabolites in the small intestine.Moreover,sea cucumber saponins remained in circulation for a long time and alcohol slowed down the clearance of sea cucumber saponins under the influence of alcohol after intravenous injection.This confirmed the feasibility of marinating sea cucumber in Baijiu to improve the efficacy of saponins and provides an important theoretical basis for the utilization of sea cucumber and the development of sea cucumber liquor.
1. Introduction
Sea cucumber has been reported to contain more protein and less fat than most other foods[1].Moreover,it plays an important role in regulating the physiological functions of human beings due to the presence of bioactive compounds,such as saponins[2],chondroitin sulfate[3],fucoidan[4],peptides[5],cerebrosides[6],etc. Therefore,it is usually used as a kind of traditional tonic food and folk medicine in many Asian countries for thousands of years,especially in China[7].Saponins is a kind of important secondary metabolites for sea cucumber[1].Most of the discovered sea cucumber saponins are primarily triterpene glycosides of lanosterol type aglycone with a sugar chain linked to C-3 position[8],at least the carbohydrate chains at C-4 of the first xylose residue are sulfated[9]. Studies have shown that sea cucumber saponins have a variety of biological activities.Sea cucumber saponins exerted antimetastatic activitis by inhibiting MMP-9 and VEGF expression[10]. Moreover,dietary sea cucumber saponins could alleviate obesity,hepatic steatosis and glucose intolerance by preventing adipokine imbalance,increasing adiponectin production and decreasing tumor necrosis factor alpha level[8].Hu et al.found the lipids-lowering effect of dietary sea cucumber saponins was associated with the inhibition of SREBP-1c mediated lipogenesis and the enhancement ofβ-oxidation via PPARα activation[11].Additionally,low dose of sea cucumbersaponins could improve the humoral immunity and nonspecific immune function in mice by increasing the antibody production to promote monocyte phagocytosis and the release of cytokines[12].
Studies have shown that oral saponins hardly decompose in the stomach of rats[13].The active components of sea cucumber saponins were absorbed by intestinal epithelial cells and then entered the blood,which was collected by hepatic portal vein and then reached the liver.The active ingredients containing glycosides are usually hydrolyzed in response to intestinal flora,releasing glycosidin and then entering the entero-hepatic circulation[14].Absorption and metabolism of saponins is a complex process.Presystemic metabolism,presystemic efflux,variable gastric emptying,the “absorption window” along the gastrointestinal tract and entero-hepatic recirculation can all influence its absorption process[15].After entering the blood,saponins are transported to various tissues and organs of the body for metabolism through the systemic circulation.
It is a traditional method to process medicinal herbs by marinating in Baijiu to achieve the purpose of health care in China;it is still popular practice in China now[16].This method is being applied at home and industrial manufacturing.Baijiu is generally used to soak herbs for a period to extract the valuable compounds with health promotion effects[17].As a well-known classic recipe in Traditional Chinese Medicinal Sciences,“Gualou-xiebai-baijiu-tang” has been used for the treatment of coronary heart disease and angina pectoris for several hundred years,in which a new triterpenoidal saponin and nine known steroidal saponins were isolated[18].Songet al.found that the efficacy of Xiaojin Pills accompanied with Chinese Baijiu was better than that of water in activating the blood,anti-inflammation,analgesia and anti-mammary gland hyperplasia[19].Numerous studies have focused on the effects of active ingredients on the metabolism of alcohol[20-23],but knowledge on the effects of alcohol on the metabolism of active ingredients is limited.Chinese coastal residents always have the habit of drinking sea cucumber wine[24].Absorption and metabolism of sea cucumber saponinsin vivohave been reported[25,26],but the effect of alcohol on absorption and metabolism of sea cucumber saponins remains unknown.
In this study,the effects of alcohol on digestion,absorption and metabolism of sea cucumber saponins were investigated in BALB/c mice after oral administration and intravenous injection of Baijiu and sea cucumber saponin.The levels of sea cucumber saponins prototype and metabolites in serum,liver and small intestinal contents were determined.
2. Materials and methods
2.1 Materials
Sea cucumber (Pearsonothuria graeffei) is purchased from Nanshan Aquatic market in Qingdao.The fifty-six degrees Baijiu used in the experiment was purchased from Beijing Red Star Co.,Ltd.The pure deionized water was produced by Millipore Milli-Q Gradient system (Millipore,Bedford,MA,USA).Chloroform,methanol and ethanol (purity ≥ 99%) was purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).All other reagents used were of the highest grade available.
2.2 Preparation of sea cucumber saponins
Saponins were obtained from the sea cucumber (P.graeffei)according to the method reported in previous literature[14].Briefly,the dried body wall of sea cucumberwas ground into powder,and then the powder was extracted with 60% (V/V) ethanol at room temperature.The ethanol extract was evaporated in a vacuum at 45 °C and further extracted in water and chloroform.The water layer was extracted withn-butanol,and the organic layer was evaporated in vacuo to yieldn-butanol extracts.Thenn-butanol extract was dissolved in water and placed on a HP20 resin column,eluting with water and 80% ethanol successively.The 80% ethanol eluent was filtered and dried under a vacuum at 45 °C.The obtained 80% ethanol extract was then loaded into the normal phase silica gel column and eluted with chloroform:methanol:water (10:1:0.1−7:3:0.3,V/V)successively.The eluent at the gradient of 8:2:0.2 and 7:3:0.3 was collected and dried to get the total saponins.The content of holothurin A (HA) and echinoside A (EA) in the total saponins was 22.3% and 69.1%,respectively.The pure EA and HA were separated from the saponin extracts using ODS silica gel (YMC-Pack,Japan) column chromatography with methanol and H2O as elution.The purity of HA and EA was both above 90% determined according to the previously reported method and their structures were shown in Fig.S1[14].
2.3 Animal Treatment.
The experimental protocols were approved by the ethical committee of experimental animal care at Ocean University of China(Qingdao,China).Male BALB/c mice (18-20 g) were housed under the temperature ((23 ± 2) °C) and a 12 h light-dark cycle (from 7:30 to 19:30) with free access to water and food.
2.3.1 Effects of alcohol on digestion,absorption and metabolism of sea cucumber saponins after oral administration
After one week of adaptive feeding,the mice were divided into two groups (n=5) by weight and fasted for 10 h before the experiment.In the control group,mice were given saponins solution(60 mg/kg,dispersed in normal saline at the concentration of 10 mg/mL)by gavage.In the alcohol group,mice were given the same dose of saponins solution dispersed in Baijiu.Then the mice were sacrificed at 1,2,3,4,6,7,8 and 9 h after gavage of sea cucumber saponins,and the corresponding blood,liver (perfusion),small intestine and small intestine contents were collected,respectively.All samples were stored at -80 °C until use.
2.3.2 Effects of alcohol on pharmacokinetics of sea cucumber saponins after intravenous injection
After one week of adaptive feeding,the mice were divided into four groups (n=5),including the control group and alcohol group of HA,the control group and alcohol group of EA according to body weight and fasted for 10 hours before the experiment.In the control groups,mice were injected EA or HA at the dose of 70 μg/kg dispersed in normal saline by tail vein.In the alcohol groups,mice were firstly given 100 μL Baijiu by gavage,after 30 min,then were injected the same dose of HA or EA by tail vein.The mice were then sacrificed at 5,10,30,45,60 and 90 min after intravenous injection of sea cucumber saponins,and the corresponding blood and liver(perfusion) were collected,respectively.All samples were stored at-80 °C until use.
“Yes, there is always snow and ice,” said the reindeer; “and it is a glorious place; you can leap and run about freely on the sparkling ice plains. The Snow Queen has her summer tent there, but her strong castle is at the North Pole, on an island called Spitzbergen.”
2.4 Sample preparation and determination
The content of saponins in serum and tissue samples were determined using the previously reported method[14,27].Briefly,methanol was added to serum (serum: methanol,1:4,V/V) to remove protein.After centrifugation at 7 500gfor 15 min,the supernatant was dried with N2flow at 35 °C,and then re-dissolved with methanol.Liver homogenate and intestinal contents were extracted three times with ethyl acetate and n-butanol,respectively.All supernatant was vacuum-dried and re-dissolved with methanol after centrifugation.All samples were filtered with a 0.22 μm membrane before analysis.
Standard stock solutions of HA and EA were prepared in methanol and stored at 4 °C.Working standard solutions were serially diluted with methanol to obtain concentrations of calibration standards of HA at 0.05,0.1,0.25,0.5,1 and 5 μg/mL,and concentration of calibration standards of EA at 0.05,0.1,0.25,0.5,1 and 5 μg/mL for EA.
The saponins HA and EA have undergone three deglycosylation in the small intestine,and their process and chemical structure are shown in Fig.S1.H1,H2 and H3 are the metabolites of HA after removing 1,2 and 3 glycosyls,and E1,E2 and E3 are the metabolites of EA after removing 1,2 and 3 glycosyls,respectively.HA,EA and metabolites in serum,liver and small intestine contents were determined by liquid chromatography-tandem mass spectrometry(LC-MS/MS)[26].Chromatographic separations were achieved on an Agilent 1260 HPLC separation system (Palo Alto,CA,USA) using a Proshell 120 EC-C18(3.0 mm × 150 mm,2.7 μm).The column temperature was 25 °C.The mobile phase was composed of water(A) and acetonitrile (B).The gradient elution conditions were as follows: 0 → 6 min 20% B,6 → 20 min 30% B,20 → 25 min 45% B,25 → 45 min 55% B,45 → 50 min 20% B.The flow rate was 0.2 mL/min and the injection volume was 10 μL.Mass spectrometric experiments were performed using an Agilent G6410 Triple Quad(QQQ) tandem mass spectrometer (Palo Alto,USA) equipped with an electrospray ionization (ESI) source.The setting parameters of scan were as follows: resolution,70 000;automatic gain control (AGC),1 × 105;maximum injection time (IT),100 ms;scanning range,m/z200-2 000.During ion screening,the 5 ions with the highest abundance in the full scan were subjected to high-energy collision cracking(HCD).The dynamic removal time of the 5 ions with the highest abundance was 10 s.Data-dependent acquisition mode parameters were set as follows: resolution,17 500;AGC target,1 × 105;maximum IT,50 ms;the isolation window,m/z1.0,HCD collision energy (NCE),35%.The single ion monitoring was set asm/z=1 197.4 (C54H84O27S) for HA,m/z=1 183.5 (C54H86O26S) for EA,m/z=1 021.4 (C47H73O22S) for H1,m/z=859.4 (C41H62O17S) for H2,m/z=713.3 (C35H53O13S) for H3,m/z=1 007.5 (C47H75O21S) for E1,m/z=845.4 (C41H64O16S) for E2 andm/z=699.3 (C35H55O12S) for E3[14].MassHunter Workstation Software (Agilent Technologies,USA) was used to acquire and analyze data.
2.5 Pharmacokinetic calculations
Pharmacokinetic variables for each mouse after gavage and intravenous administration were analyzed by a noncompartmental method using WinNonlin software program (Pharsight Corporation,Scientific Consulting Inc.,North Carolina,USA).Pharmacokinetic parameters mainly included terminal elimination half-life (t1/2λz),volume of distribution at steady state (Vdss),total clearance (ClT),mean residence time (MRT0-tand MRT0-∞) and area under the plasma concentration-time curve (AUC0-tand AUC0-∞).The peak concentration (Cmax) and the time to reachCmax(Tmax) were measured directly according to the plasma concentration-time curve.The AUC were estimated using a linear/log method.
2.6 Statistical analyses
All experimental samples were in quintuplicate,and the results were expressed as mean ± standard deviation (SD).In the calculation of pharmacokinetic parameters,all values were shown as the mean ±SD.Normality of data points was tested using the Shapiro-Wilk test.If data sets were not normally distributed,then the Mann-Whitney test was performed.All statistical analyses were performed using SPSS 26.0 program (IBM Corp,Armonk,NY) with a confidence level of 95%.P<0.05 was considered as statistically significant.
3. Results
3.1 Effects of alcohol on the content of sea cucumber saponins in the serum of mice after oral administration
The concentration of HA in the serum of control mice reached the first peak of 232.16 ng/mL at 1h and decreased slowly to 184.33 ng/mL at 2 h (Fig.1A).After that,the concentration of HA began to rise and reached the second peak of 491.56 ng/mL at 3 h.The concentration of HA decreased from 3 to 6 h,which reached to 87.35 ng/mL at 6 h and then tended to be flat.Notably,the concentration of HA in the alcohol group reached the first peak of 555.97 ng/mL at 2 h,and then decreased within 1 h.HA concentration began to rise at 3 h and reached the second peak of 383.49 ng/mL at 4 h after alcohol administration.Then HA concentration decreased to 59.82 ng/mL at 6 h and tended to be flat from 6 to 7 h,which was consistent with the control group.Interestingly,the concentration of HA began to rise sharply at 7 h and reached the third peak of 904.12 ng/mL at 8 h after alcohol administration.Then the HA concentration decreased sharply and recovered to the lowest value at 9 h.

Fig.1 The effects of alcohol on the content of sea cucumber saponins in mice serum (n =5).(A) HA,(B) EA.
The change curve of EA concentration in serum was similar to that of HA both in the control and alcohol groups (Fig.1B).In the control group,the concentration of EA in serum increased steadily with the time after oral administration,and reached a peak at 4 h.Subsequently,EA concentration decreased sharply from 4 to 6 h and reached a minimum at 6 h,and then remained basically unchanged at 6 to 9 h.Notably,the difference was that EA concentration in the alcohol group reached three peaks at 2 h,4 h and 8 h,with the concentration of 943.31 ng/mL,209.62 ng/mL and 1 437.25 ng/mL,respectively.Moreover,the first peak value of EA concentration at 2 h after intragastric administration of ethanol at the same time was significantly higher than that of the first peak at 4 h in the control group.
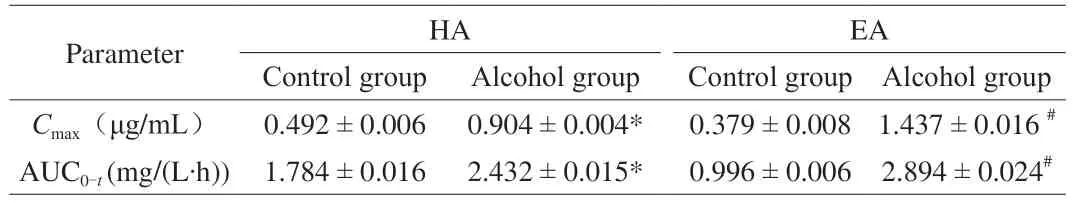
Table 1 Effects of alcohol on the pharmacokinetics parameters Cmax,AUC0-t of HA and EA after intragastric administration of sea cucumber saponins in mice.
3.2 Effects of alcohol on the content of sea cucumber saponins in the liver of mice after oral administration
As shown in Fig.2A,the content of HA in the liver of the control group gradually increased over time and peaked at 3 h,gradually decreased within 3 to 8 h,and then showed an upward trend after 8 h with the content of 251.8 ng/g at 9 h.In the alcohol group,the HA content in the liver showed three peak values with the content of 300.8,642.6 and 1 123.5 ng/g at 1,4 and 8 h,respectively.The maximum HA accumulation in the liver was reached at 8 h after alcohol administration,which was 4.46 times of that in the control group.After 8 h,the content of HA decreased sharply,and the content of HA remained at the same value with that of the control group at 9 h.
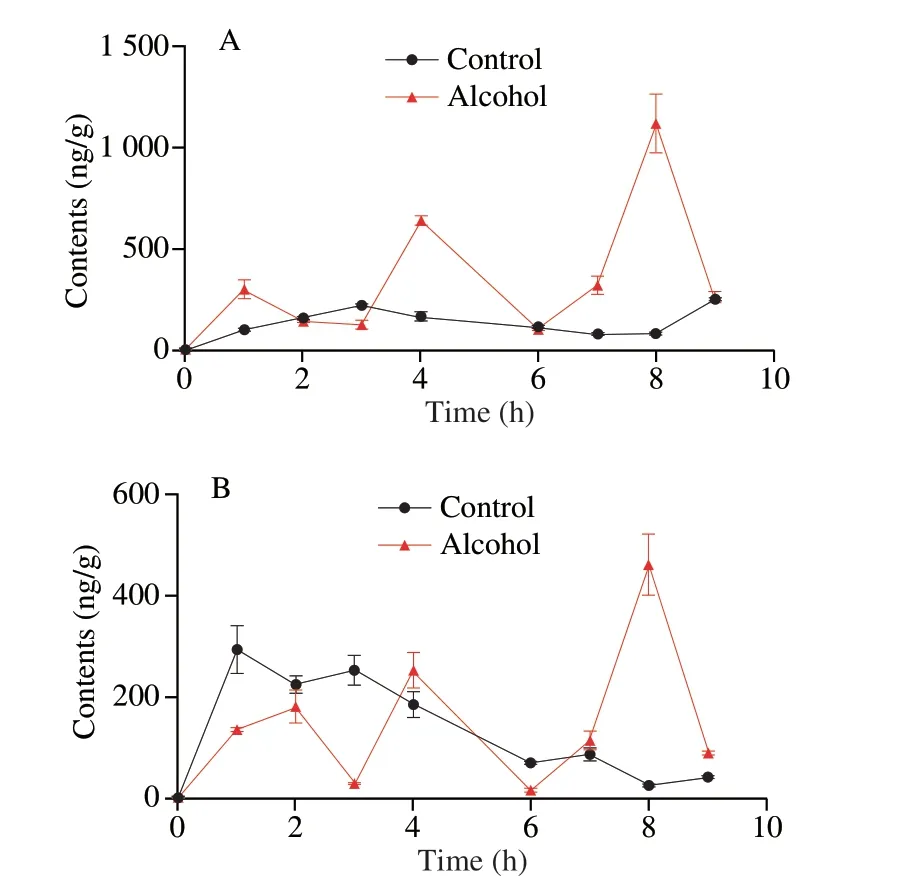
Fig.2 The effects of alcohol on the content of sea cucumber saponins in mice liver (n =5).(A) HA.(B) EA.
In the control group,the content of EA in the liver showed an upward trend from 0 to 1 h,and reached the maximum value at 1 h after oral administration.Then the EA content showed an overall downward trend with time,and reached the lowest value at 8 h and remained basically unchanged.Interestingly,in the alcohol group,the change of EA content in the liver over time was similar to that of HA and there were three peaks with the values of 181.2,252.8 and 462.5 ng/g at 2,4 and 8 h,respectively.Then the content of EA decreased sharply to 90.2 ng/g at 9 h (Fig.2B).
3.3 Effects of alcohol on the metabolism of sea cucumber saponins in small intestine
Changes in the contents of HA and its metabolite H1-H3 in intestinal contents were shown in Fig.3A-D.After oral gavage,the content of HA in the intestinal content of control mice increased sharply and reached the first peak with 5.00 μg at 2 h followed by rapid decrease from 2 to 3 h,and then remained almost constant from 3 to 6 h.After 6 h,the concentration of HA increased slowly and reached the second peak with 2.65 μg at 8 h.Notably,in the alcohol group,the content of HA in the small intestine content increased slowly within 1 to 7 h,and reached the peak value of 3.5 μg at 7 h.For metabolites H1,H2 and H3,their contents in the alcohol group were lower than that in the control group.In both groups,the metabolites with two glycogroups removed (H2) had the highest content,followed by H3 and H1.The concentration of H2 in the control group increased sharply after oral gavage,and reached the first peak at 1h (13.3μg),which was two times higher than the H2 concentration of the alcohol group at 1 h.Notably,H2 reached the first peak at 2 h with a value of 6.1 μg.The second peak value of H2 in the control group was 11.6 μg at 8 h,while the second peak value was changed to 5.0 μg at 7 h after the simultaneous gavage of alcohol.The change curve of H3 in the control group and the alcohol group was similar to that of H2.The peak values of H3 in the control group were 1.8 μg and 3.3 μg at 1 h and 8 h,respectively,while the peak value of H3 in the alcohol group was only 1.1 μg at 2 h.
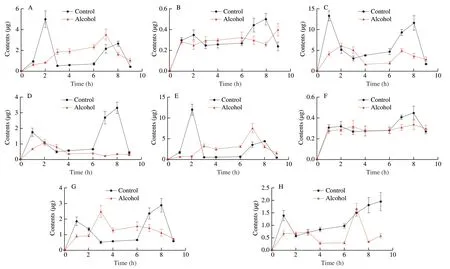
Fig.3 Effects of alcohol on the content of sea cucumber saponins HA,EA and metabolites in the small intestine (n=5).(A) HA,(B) H1,(C) H2,(D) H3,(E) EA,(F) E1,(G)E2,(H) E3.
Changes in the content of EA and its metabolites E1-E3 in intestinal content are shown in Fig.3E-H.The change curve of EA was similar to that of HA.In intestinal contents,both HA and EA reached their peak values at 2 h,when EA content was about 2.5 times that of HA.Under the influence of alcohol,HA and EA reached their peak values at 7 h,when the content of EA also was 2.5 times that of HA.Among the three metabolites,E2 exhibited the highest content,followed by E3 and E1.For E2,the change of the control group was basically the same as that of H2.In the alcohol group,the content of E2 increased gradually with time,reached the peak value at 3 h,then gradually decreased,and reached the lowest value at 9 h,keeping the same level as that of the control group.
3.4 Effects of alcohol on serum subtractive curve of sea cucumber saponins after intravenous injection
After intravenous injection of saponins,the changes of HA and EA contents in serum over time were shown in Fig.4.The concentration of HA in the serum of both the control group and the alcohol group decreased rapidly within 15 min (Fig.4A).In the control group,the concentration of HA decreased from 0.455 μg/mL at 5 min to 0.151 μg/mL at 15 min,and the concentration of HA decreased to 33.2% of the initial concentration.The concentration of HA in the alcohol group decreased from 0.547 μg/mL at 5 min to 0.243 μg/mL at 15 min,which was 44.4% of the initial concentration.Notably,within 15 min to 60 min after tail intravenous injection,the reduction rate of HA’s concentration in the alcohol group was significantly lower than that of the control group.At 60 min,the two groups decreased to the same level and kept the same trend of slow decline at 60 min to 90 min,and reached the lowest value at 90 min.

Fig.4 Effects of alcohol on the serum reduction curve of sea cucumber saponins after tail vein injection in mice (n=5).(A) HA,(B) EA.
The changes in EA content over time in the control and alcohol groups were shown in Fig.4B The results showed that the concentration of EA in the alcohol group was higher than that in the control group within 5 to 60 min after tail vein injection.Within 0 to 15 min,the concentration of EA in the control group decreased from 0.154 μg/mL to 0.040 μg/mL,which was 26.0% of the initial concentration,while in the alcohol group,the concentration of EA decreased from 0.224 μg/mL to 0.118 μg/mL,which was 52.7% of the initial concentration.After 30 min of injection,EA concentration in the control group decreased to 17.5% of the initial concentration,while EA concentration in the alcohol group decreased to 50% of the initial concentration.After 30 min,EA concentration in the alcohol group decreased at a faster rate than that in the control group.Until 60 min,the concentrations of the two groups decreased to the lowest and remained at the same level until 90 min after injection.
3.5 Effects of alcohol on the liver accumulation of sea cucumber saponins after intravenous injection
We investigated the effect of alcohol on the liver accumulation of sea cucumber saponins after tail intravenous injection in mice by detecting the content of HA and EA in the liver (Fig.5).In general,the accumulation of EA in the liver was about 3 times that of HA,and the accumulation of HA and EA in the liver of alcohol group was much greater than that of control group within 5 to 45 min after injection.The accumulation of HA in the control group and the alcohol group increased gradually with time and reached the first peak at 10 min.The accumulation of HA at 10 min in the alcohol group was 554.3 ng/g,about 8.5 times that of the control group.The HA content in the liver was decreased to 136.3 ng/g in the alcohol group and 30.6 ng/g in the control group at 15 min.Subsequently,the HA level of the two groups began to rise and the increase rate of the alcohol group was greater than that of the control group.The accumulation of HA in the control and alcohol groups reached the second peak values of 466.4 ng/g and 57.1 ng/g,respectively,at 30 min.After 30 min,the amount of HA in the liver of both groups decreased,and reached the lowest value at 45 min,and maintained the lowest level until 90 min after injection.The amount of EA in the liver changes with time was in a similar way to HA.Notably,in the alcohol group,the accumulation of EA increased slowly from 858.7 ng/g to 1 067.9 ng/g at 20 to 30 min,and the change of other time was basically consistent with that of HA.
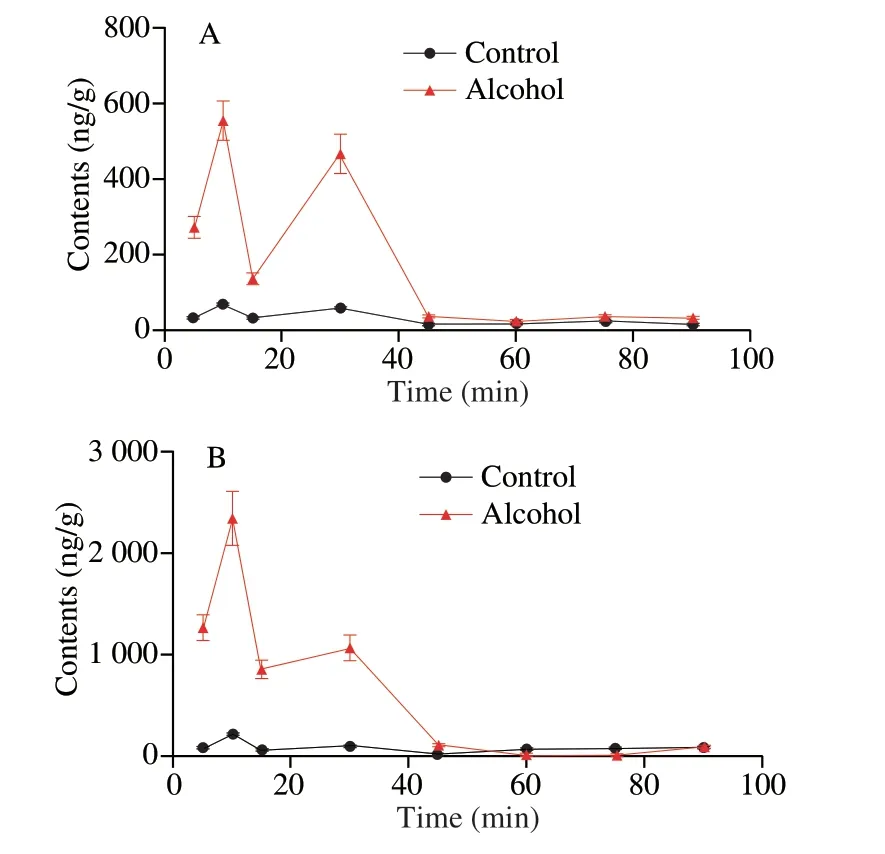
Fig.5 Effects of alcohol on liver accumulation of sea cucumber saponins after tail vein injection in mice (n =5).(A) HA,(B) EA.
3.6 Pharmacokinetic parameters
Main pharmacokinetic parameters of HA and EA in the control group and alcohol group after intravenous injection of sea cucumber saponins were calculated by WinNonlin software (Table 2).HA and EA achieved the maximum plasma concentration in half an hour after tail intravenous injection.TheTmaxof HA and EA in serum was not affected by alcohol,but theCmaxof HA and EA in serum was increased by alcohol.Both thet1/2λzof EA and ClTof HA and EA were significantly reduced in the alcohol group compared with the control group,while thet1/2λzof HA was not changed.The AUC0-tand AUC0-∞of HA and EA were significantly increased in the alcohol group compared with the control group.
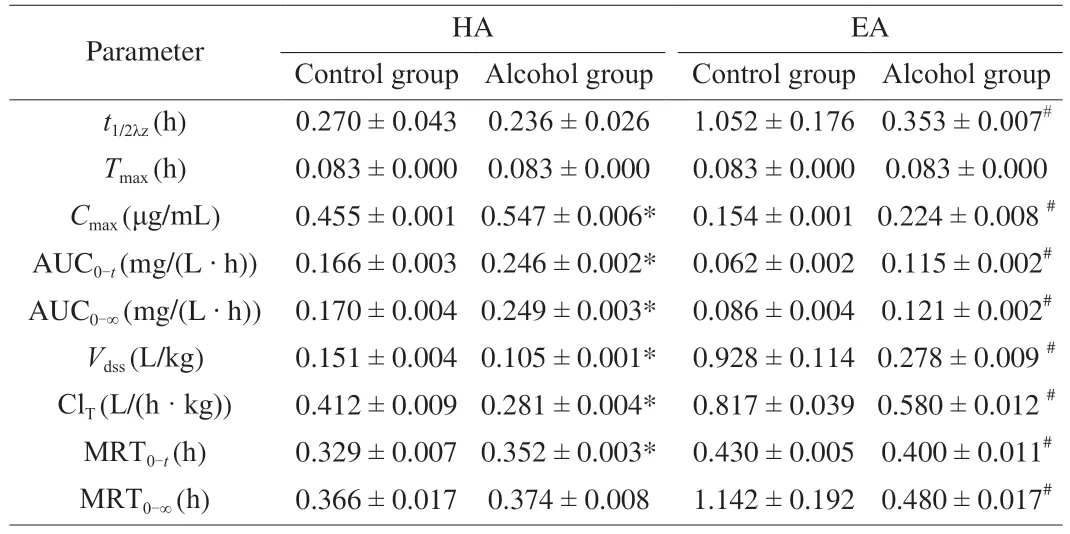
Table 2 Effects of alcohol on the pharmacokinetics of HA and EA after tail vein injection of sea cucumber saponins in mice.
4. Discussion
In the present study,the absorption curves of HA and EA showed that two peak values of HA appeared at 1 h and 3 h,and one peak value of EA appeared at 4 h in mice after gavage of sea cucumber saponins.Notably,a previous study has shown that the absorption curves of HA and EA in Wister rats after gavage of sea cucumber saponins[15].Both HA and EA showed two peak values,the peak time of HA was 3 h and 9 h,and the peak time of EA was 3 h and 7 h[15].The difference in peak number and time might be caused by different experimental animals.Moreover,the concentration and peak number of HA and EA increased significantly under the influence of alcohol,and HA and EA showed three peaks at 2,4 and 8 h,respectively.It has been reported that the plasma concentration of wine-processed Radix Achyranthis bidentatae (RAB) was higher than that of crude RAB in rats after oral administration,which was consistent with our study[28].In order to explore the effect of alcohol on saponins absorption in mice after oral administration of saponins,theCmaxand AUC of HA and EA in mice serum were obtained.The significant increases in AUC0-tandCmaxin the alcohol group indicated that alcohol promoted the absorption of saponinsin vivo.However,the exact mechanism remains unclear.
As an important metabolism organ,the liver has receptors that can recognize specific glycosylates and are selective for the accumulation of specific saponins,which may result in the different characteristics of HA and EA curves in the liver.In addition,in the process of absorption and metabolism of saponins,there are often atypical drug absorption curves,such as “double peak” or “multipeak” phenomenon[29-31].In the present study,the concentration-time curves of sea cucumber saponins in serum and liver also appeared“double peak” or “multi-peak” phenomenon.Similarly,Song et al.found that the hepatic HA concentration showed a peak at 9 h,and EA concentration showed two absorption peaks at 2 h and 9 h after gavage of sea cucumber saponins in rats[15],which was thought to be possibly caused by enterohepatic circulation,distribution and reabsorption[28].It has been reported that saponins are cleared from the body through the liver and biliary excretion[32-34],and the glycosyls in saponins obstruct the connection with biliary transporters and thus slow down biliary excretion[33].The high concentration of saponins in the liver might be attributed to the transporter-mediated uptake mechanisms[35].
As the main component of Baijiu,alcohol enters the bloodstream through the stomach and proximal small intestine[36],and then it’s distributed all over the body.The first absorption of alcohol occurs in the stomach and is absorbed faster in the small intestine than in the stomach[37].Studies have shown that the gastrointestinal tract of mammals does not contain enzymes that hydrolyze saponins[38],and the deglycosylation of saponins is mainly accomplished by intestinal flora[39,40].Song et al.further confirmed that the deglycosylation process of sea cucumber saponins was mediated by intestinal flora throughin vivoandin vitroexperiments in mice[26].The level of sea cucumber saponins and their corresponding metabolites in small intestine content was detected after oral administration.Since EA was about 3 times as much as HA in the saponins we gave mice intragastric administration,the proportion of EA and HA in the small intestine of mice was basically consistent with the proportion of the saponins sample.H2 and E2 were the major metabolites of deglycosylation for sea cucumber saponins in the small intestine,which was consistent with the previous results[26].In intestinal contents,the peak time of HA and EA in the alcohol group was significantly delayed after alcohol administration,and the contents of metabolites H1-H3 and E1-E3 in the alcohol group were lower than those in the control group,which indicated that alcohol reduced the metabolic rates of HA and EA in the small intestine.So that more sea cucumber saponin prototypes HA and EA were absorbed by the small intestine into serum and stored in the liver,which could be proved by the higher levels of sea cucumber saponin prototypes in the liver and serum of the alcohol group compared to those of the control group.At present,we have not found relevant reports on the mechanism through which alcohol inhibits the saponins metabolism in the small intestine.It is reported that the mucous side of the small intestine inhibits the active transport of macronutrients through the epithelial layer during acute exposure to alcohol[41].We also speculate that it may be because alcohol affects intestinal flora and thus affects the saponins metabolism in the small intestine.
Many studies have reported that chronic alcohol consumption could change the metabolic characteristics of many substances in the body[42,43].We found that alcohol also affected the metabolic characteristics of saponinsin vivo.Alcohol could slow down the decreased rate of blood saponins concentration after intravenous injection and inhibit their metabolic rates in the liver.The ClTof HA and EA were lower in the alcohol group than in the control group,suggesting that alcohol slowed the elimination process of HA and EA in the body.The significant increase inCmaxand AUC after alcohol administration suggested that the compound compatibility of sea cucumber saponins combined with alcohol could lead to a great circulating concentration and a reduced excretion rate.Pharmacokinetic parameters showed that HA and EA were retained within the circulation for a long time under the influence of alcohol and alcohol slowed the clearance of sea cucumber saponins in the body.Alcohol is eliminated from the body by alcohol dehydrogenase(ADH) and cytochrome P450 (CYP2E1)[44].Hepatic cytochrome P450 can also catalyze saponins metabolism[45].The main metabolic pathways of saponins were dehydrogenation,hydroxylation,carboxylation and the combination of these steps,and it has been reported that Saikosaponin D and its derivatives were converted to Phase-I metabolites produced by the CYP enzymes in rat liver microsomes[46].When mice were ingested alcohol and saponins at the same time,there might be competition between alcohol metabolism and saponins metabolism in the liver since they needed the same enzyme to be metabolized in the liver,which inhibited the metabolism of saponins and made the saponins stay in the liver for a long time,but the specific reasons need to be further explored.
5. Conclusions
In the present study,the effects of alcohol on the absorption and metabolism of sea cucumber saponins were explored and pharmacokinetic characteristics were investigated.Alcohol administration promoted the absorption of sea cucumber saponins prototype and inhibited their metabolism into deglycositic metabolites in the small intestine,which was proved by the high serum saponins prototype concentration and accumulation in the liver of the alcohol group compared to those of control group after oral administration.Moreover,the experimental results of tail intravenous injection showed that alcohol slows down the clearance of HA and EA in the serum and increases their accumulation in the liver in archetypal form.In addition,we analyzed the differences of alcohol on the pharmacokinetic characteristics of HA and EA by intravenous injection.In conclusion,the results confirmed the feasibility of marinating sea cucumber in Baijiu to improve the efficacy of the saponins of sea cucumber and also provided an important theoretical basis for the development and utilization of sea cucumber liquor.
Conflict of interest
Yuming Wang is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250011.
- 食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics

