Akkermansia muciniphila-directed polyphenol chlorogenic acid intervention for obesity in mice
Xiaoxiang Gao,Chenbo Yue,Ruocen Tian,Leilei Yu,Fengwei Tian,Jianxin Zhao,Wei Chen,c,Qixiao Zhai,*
a State Key Laboratory of Food Science and Technology,Jiangnan University,Wuxi 214122,China
b School of Food Science and Technology,Jiangnan University,Wuxi 214122,China
c National Engineering Research Center for Functional Food,Jiangnan University,Wuxi 214122,China
Keywords:Akkermansia muciniphila polyphenol chlorogenic acid obesity
ABSTRACT Akkermansia muciniphila play an important in ameliorating obesity but is not allowed for direct consumption in most countries.To date,microbiota-directed foods selectively promote the targeted human gut microbes,providing a strategy for A. muciniphila enhancement.Multiple studies have indicated the potential regulation of the polyphenol on A. muciniphila.Therefore,a polyphenol screening based on A. muciniphila upregulation was performed in mice.Chlorogenic acid (CGA) exhibited a greater response to A. muciniphila upregulation.Furthermore,we found that CGA did not directly promote A. muciniphila growth or mucin secretion.Microbiome and metabolomics revealed that the increased abundance of A. muciniphila resulted from the inhibition of CGA on Desulfovibrio and Alistipes and the influence of docosahexaenoic acid,β-hydroxybutyrate,and N-acetyl-lactosamine.Finally,to confirm the regulation of CGA on A. muciniphila under disease conditions,high-fat diet-fed mice were established.The results showed CGA promoted A. muciniphila growth,and we expectedly found that CGA suppressed the augment in body weight of mice,significantly attenuated adipose tissue abnormality,provided liver protection and improved gut barrier integrity.These results suggest that CGA inhibits the development of obesity.Overall,our results indicate that microbiota-directed food is a promising approach for the treatment of obesity.
1. Introduction
Obesity refers to metabolic dysfunction as a consequence of unbalanced energy intake and expenditure,which can lead to complications,such as hypertension,diabetes mellitus,and cancer[1].Obesity is accompanied by changes in the microbial profile,and the recovery from microbiota disorders contributes to the disease treatment[2,3].Microbiota disorders,detected in most obese patients,are most commonly caused byAkkermansia muciniphilaorBacteroides[4].
A.muciniphilais a commensal in the intestinal mucus layer,on average accounting for 1%–5% and up to 60% of the microbial community,secreting enzymes related to mucus degeneration,thereby processing carbon or nitrogen sources for other microbes[2,5].A.muciniphilaexhibits healthy regulation of intestinal homeostasis under conditions of disease,with reports of multiple clinical studies showing a negative relationship betweenA.muciniphilaand diseases such as metabolic diseases,including obesity and diabetes,genetic diseases such as Hutchinson-Gilford syndrome,and immunological diseases[2,6,7].In addition,A.muciniphilasupplementation contributes to the remission of obesity and related gut barrier dysfunction,with the latter being true becauseA.muciniphilaaffects the thickness of the mucus layer[4,8].Furthermore,active substances on the surface,such as Amuc_100 and metabolites ofA.muciniphila,provide probiotic benefits to humans[6].However,A.muciniphilais not approved as a direct food supplement in many countries besides the United States,indicating that it is difficult to directly supplementA.muciniphila.
Directed regulation ofA.muciniphilathrough food is suggested as an impactful method for obesity treatment.Anhe et al.[9]demonstrated that a polyphenol-rich cranberry extract inhibited the development of obesity inA.muciniphila-dependent manner.Dietary factors,such as polyphenols,dietary fiber,and polysaccharides,respond toA.muciniphilaabundance,and polyphenols are commonly used inA.muciniphila-directed modulation[3,10,11].Polyphenols are known to be difficult to digest and absorb in the intestine compared to other carbohydrates[12].Approximately 90% of polyphenols arrive at the colon and directly interact with microbiota[12].Among these,caffeic acid (CA),chlorogenic acid (CGA),epigallocatechin gallate (EGCG),procyanidine (PA),puerarin (Pue),resveratrol (Res),ferulic acid (FA),and genistein (Gen) are associated withA.muciniphilaregulation[13].
This study was designed to explore the polyphenol with tremendous potential forA.muciniphilaupregulation in mice,as well as the underlying mechanisms.In a validation model,CGA efficiently promotedA.muciniphilain high-fat diet (HFD)-fed mice and was significantly associated with obesity remission by analyzing diseaserelated characteristics.Our results further reveal the relationship between polyphenol andA.muciniphilaabundance and suggest the possibility of disease regulation through specific microbe.
2. Material and methods
2.1 Study design and sample collection
All experiments involving mice were performed using protocols approved by the Jiangnan University Animal Studies Committee,Wuxi,China (JN.No20201130c0880125[350] and JN.No20211130c0500215[486]).Ninety male C57BL/6J mice (Vital River,Beijing,China),6 weeks of age with at least 20 g of weight,were housed with no more than five mice per cage under a 12 h light cycle and were fed distilled water and normal chow ad libitum.After 1-week of acclimation,mice in different group were administered intragastrically with 1 mmol/L distilled water,CA,CGA,EGCG,PA,Pue,Res,FA,or Gen daily for a month,respectively.All sources of polyphenols are listed in Supporting Information Table S1.
To confirmA.muciniphilaupregulation of CGA in the disease model,we placed another 50 male C57BL/6J mice (Vital River,Beijing,China) under the same conditions at Jiangnan University Animal Center.The mice were randomly grouped into normal fat diet(NFD) (10% energy from fat,71% from carbohydrates,and 19% from protein),HFD (60% energy from fat,21% from carbohydrates,and 19% from protein),CGAL (0.5 mmol/L CGA),CGAM (1 mmol/L CGA),and CGAH (2 mmol/L CGA) for a period of 3 months after 1-week acclimation.The detailed ingredients of the NFD and HFD are listed in Supporting Information Table S2.All the animal feed were purchased from Trophic Animal Feed High-Tech Co.,Ltd (Jiangsu,China).During the experiment,the weight,food intake,and energy intake of mice were recorded weekly.
At the end of the experiment,the blood,feces,and livers of the mice were collected after sacrifice using isoflurane.The organs,including subcutaneous fat and epididymal fat without spermaducts,were collected for weight measurement.
2.2 Histological evaluation
For histological examination,colons were collected in 10%carnoy fixative,while liver and subcutaneous fat were stored in 4% paraformaldehyde (Beyotime,Shanghai,China).After paraffin preparation,colon tissues were stained with Periodic acid-Schiff(PAS) (Beyotime,Shanghai,China) and Alcian blue-PAS (AB-PAS)(Beyotime,Shanghai,China) to observe the mucin layer.Hematoxylin and eosin (H&E) (Beyotime,Shanghai,China) staining was used for the histopathology of the liver and fat.Changes in lipid droplets were observed using an Oil Red O Staining Kit (Beyotime,Shanghai,China).All positive areas were quantified using ImageJ 6.0 software.
2.3 Oral glucose tolerance test (OGTT) and Assays for basic index
Before sacrifice,the mice were fasted for 12 h.Fasted blood glucose was measured using an Accu-Chek guide blood glucose meter(Roche,Grenzach-Wyhlen,Germany).For the OGTT,the mice were administered 2 g/kg glucose (Thermo Scientific,CA,USA).Blood was collected on test paper at different time points (15,30,60,90,and 120 min).The area under the curve (AUC) was calculated at the indicated time points[14].
Raw plasma was diluted three times for total cholesterol (TC),low-density lipoprotein cholesterol (LDL-C),triglyceride (TG),alanine aminotransferase (ALT),aspartate aminotransferase (AST),alkaline phosphatase (ALP),and glutamyltransferase (GGT),and was then detected using a BS-480 biochemical analyzer (Mindray,Shenzhen,China) using kits (Thermo Scientific,CA,USA).
2.4 Akkermansia muciniphila culture
A.muciniphilaATCC BAA-835 used in the study was purchased from the American Type Culture Collection (Gaithersburg,MD,USA).A.muciniphilawas activated and cultured in standardized brain heart infusion (BHI) (Haibo,Shandong,China).After purification three times,the bacteria were transplanted into 96 plates containing modified BHI with sterile CGA (0,50,100,200,400,and 800 mg/L).Following incubation in an automatic monitoring microplate reader Tecan Infinite F50 (Mendov,Switzerland) at 37 °C for 60 h,a growth curve was generated according to the OD600.
2.5 qRT-PCR
Colon tissue (50 mg) was dissolved in TRIzol (Tiangen,Beijing,China),and total RNA was extracted using the Tissue Total RNA Isolation Kit (Vazyme,Nanjing,China).cDNA was reversely transcribed from total RNA using the iScript One-Step RT-PCR Kit(Bio-Rad,CA,USA).Quantitative RT-PCR was performed on an ABI7300 (Applied Biosystems,CA,USA) to measure Muc2,ZO-1,Klf4,and occludin,whose primers are listed in Supporting Information Table S3,using SYBR-green.The difference in mRNA expression was quantified by normalizing each amplicon to that ofβ-actin.
2.6 DNA extraction and 16S rRNA gene sequencing
Fresh fecal samples were collected from healthy mice and weighed after pre-freezing at–80 °C.Mouse DNA was extracted using an MP kit (MP Biomedicals,Hessen,Germany),following the manufacturer’s instructions.The 16S rRNA gene was amplified in triplicate using the 341F/806R (V3-V4 regions) barcoded primer pairs (F:5’-CCT AYG GGR BGC ASC AG-3’;R:5’-GGA CTA CNN GGG TAT CTA AT-3’),with reaction conditions: 95 °C for 5 min,30 cycles of 95 °C for 30 s,52 °C for 30 s,and 72 °C for 30 s,an extension for 7 min at 72 °C,and sequenced using the Illumina Miseq PE300 at Jiangnan University,Wuxi.The raw sequencing data were demultiplexed and quality-filtered according to a previously described method in Qiime2 (v2018.8).
Microbial data with a quality score of <30 were excluded.After processing,high-quality data with >97% similarity were classified into operational taxonomic units.All microbiota-related plots were generated using MicrobiomeAnalyst (https://www.microbiomeanalyst.ca/) or R package ggplot2 (v3.3.2).
2.7 Short chain fatty acid (SCFA) quantitation
The SCFA (acetate,propionate,butyrate,isobutyric acid,valeric acid,and isovaleric acid) of dry cecal contents (30–50 mg)were extracted using absolute ether and were measured using a gas chromatography-mass spectrometer (GC-MS) (Thermo Fisher Scientific,CA,USA) with an Rtx-Wax column (30 m × 0.25 μm,0.25 μm) according to a previously described method[15].The parameter settings for temperature were as follows: the initial temperature was 100 °C,which was increased to 140 °C at 7.5 °C/min;the temperature was required to be held for 3 min after increasing to 200 °C at 60 °C/min.The injector and detector temperatures were 220 °C and 250 °C,respectively.
2.8 Metabolites extraction and Chromatography-Mass Spectrometry (LC-MS) analysis
Changes in the metabolic environment of the mouse fecal samples were determined using liquid LC-MS (Thermo Fisher Scientific,CA,USA).(20 mg) were diluted in 200 μL water and 800 μL methanol and acetonitrile (1:1) for protein elimination.After ultrasound for 10 min and incubation at–20 °C for 1 h,the sample was centrifugated at 15 000 ×gfor 20 min and then dried until water was removed.The dried sample was mixed with 200 μL of 80% methanol solution.After centrifugation at 15 000 ×gfor 15 min,the supernatant was filtered using a 0.22 mm filter for spectrum acquisition.Specific parameters were used in LC-MS according to Li et al[16].After spectrum detection,the results were analyzed using MetaboAnalyst (version 5.0;https://www.metaboanalyst.ca/)[16].Pearson’s correlation was employed and visualized using the stats package (version 3.5.0) to explore the relationship between microbiota and metabolites.
2.9 Quantification and statistical analysis
Statistical significance was analyzed using GraphPad Prism software (v8.0.2) or R software (v4.0.2) using an unpaired Student’st-test for two-group comparisons or ANOVA for multiple comparisons.All data are presented as mean ± SEM.Statistical significance was considered at *P<0.05,**P<0.01,***P<0.001,and ****P<0.000 1,and ‘n.s.’ represents no significance.
3. Results
3.1 Screening of eight polyphenols based on the regulation of A.muciniphila abundance
Eight polyphenols with clear structure were employed to identify which grow factor has a better ability forA.muciniphilaupregulation[17-22].According to the qPCR results,A.muciniphilaexhibited notable responses to CA,CGA,EGCG,PA,and Pue,rather than Res,FA,and Gen,compared with the control group (1.78 ± 0.87)% (Fig.1a).CGA administration increased the relative abundance ofA.muciniphilato nearly 10%.Similar results were obtained in the quantitative analysis ofA.muciniphilausing 16S rRNA (Fig.1b).Overall,these results suggest that CGA is the most promising candidate forA.muciniphiladirected regulation (P<0.000 1).

Fig.1 CGA indirectly enhances the abundance of A.muciniphila in healthy mice.(a and b) Relative abundance of A.muciniphila measured using qPCR and 16S rRNA.(c) Growth situation of A.muciniphila through continuous measurement for 30 h.(d) mRNA expression of colonic ZO-1,Muc2,Occludin,and Klf4.(e) Representative PAS-stained pictures of colonic goblet cells and relative number of goblet cells (n =5 images per mouse).Scale bars,50 μm.(f) AB-PASstained image of colonic mucus and mean area of mucus (n=5 images per mouse).Scale bars,50 μm.Number of mice per group in (a),(b),and (d) are ten.Compared with control group,n.s.>0.05;*P <0.05;**P <0.01;***P <0.001;****P <0.000 1.

Fig.1 (Continued)
3.2 CGA neither directly promotes A.muciniphila growth nor mucin secretion
To explore the direct effect of CGA onA.muciniphilain vitro,we transplantedA.muciniphilainto media containing different concentrations of CGA;CGA (50,100,200,and 400 μg/mL) did not promote the growth ofA.muciniphilain vitroat OD600nm,and it inhibited the growth ofA.muciniphilaat a concentration of 800 μg/mL (Fig.1c).
Next,we investigated whether CGA enhanced the abundance ofA.muciniphilain the colonic mucin layer by promoting mucin secretion.The increase in mucin is a major cause of goblet cell differentiation.PAS staining,which revealed the number of goblet cells,indicated no association between CGA and goblet cells(Fig.1e,P>0.05).Moreover,AB-PAS staining,which directly showed the characteristics of mucin,indicated that CGA did not lead to an increase in mucin,which was similar to our results in mRNA expression related to colonic goblet cells and mucin secretion(Figs.1d-f,P>0.05).The above results indicated that CGA had no direct effect onA.muciniphilaregulation and failed to promote mucin secretion.
3.3 CGA enhanced the abundance of A.muciniphila in a microbiota-depended manner
Interspecific competition and cooperation play potential roles inA.muciniphilapromotion.16S rRNA gene sequence-based surveys revealed that CGA did not affect the complexity of the microbial community because there were no differences in Shannon,Simpson,and Invsimpson indices between the CGA and control groups(Fig.2a,P>0.05),whereas the PCoA ascertained a clear separation between the CGA and control groups (Fig.2b),indicating that CGA intake did not lead to changes in microbiota diversity but in species composition.At the phylum and genus level,a similar increase inA.muciniphilawas found in Verrucomicrobia and Akkermansia,further confirming the significant improvement inA.muciniphilaafter CGA administration (Fig.2c).To explore the potential link betweenA.muciniphilaand other microbes,a correlation analysis was applied.Strong co-presence or competitive relationships were observed betweenA.muciniphilaandAlistipes,Desulfovibrio,Clostridium sensu stricto,Lachnoclostridium,RuminococcaceaeUCG-014,Turicibacter,orEubacterium(Fig.2d).LefSe,for further distinguishing biomarkers,was used to assess which microbiota(from phylum to genus levels) was important post CGA treatment(Fig.2e).CGA showed an obvious increase inA.muciniphilawithin Verrucomicrobia and the bacteria in Cyanobacteria,and a decrease in Desulfovibrio and Alistipes,which was the same as the results of the correlation analysis (Fig.2f).
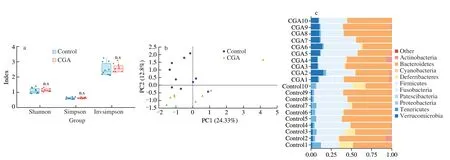
Fig.2 CGA enhances A.muciniphila abundance by enrichment of other microbes.(a) Alpha diversity measured by Shannon,Simpson,and Invsimpson index.(b) Beta diversity measured by the Bray-Curtis Index,represented by PCoA between CGA and the control group.(c) Distribution of microbiota at the phylum level after CGA treatment.(d) Network diagram of correlative microbiota according to A.muciniphila relative abundance.(e and f) Linear discriminant analysis (LDA)effect size (LEfSe) of intestinal microbiota between the control and CGA group.Each group had ten mice.Compared with control group,n.s.>0.05.

Fig.2 (Continued)
3.4 Microbiota and Metabolomics revealed the effect of CGA on A.muciniphila
Metabolites could yield important mechanistic insights intoA.muciniphilaupregulation.By determining the SCFA,we observed that CGA exposure only resulted in a significant increase in butyrate,suggesting that the effect of CGA on the growth ofA.muciniphilawas not dependent on SCFA (Figs.S2a-g).To identify metabolites that potentially affectedA.muciniphila,we first profiled the metabolic network post CGA treatment.A significant difference was observed between the control and CGA groups according to the orthogonal partial least squares discriminant analysis (OPLS-DA),indicating a significant effect of CGA on microbial metabolites (Fig.3a).Variable importance in projection (VIP) scores determine the importance of each variable in the projection used in a PLS model and are applied in variable prediction.To determine the potential metabolites after CGA treatment,a VIP plot with VIP >1,P<0.05,and fold change(FC >1.2 or <0.8) was employed,and 56 metabolites were considered (Fig.3b);among these,15 unique metabolites were found to be significantly decreased in CGA group compared with control group while 41 differentially increased metabolites in CGA group were identified.
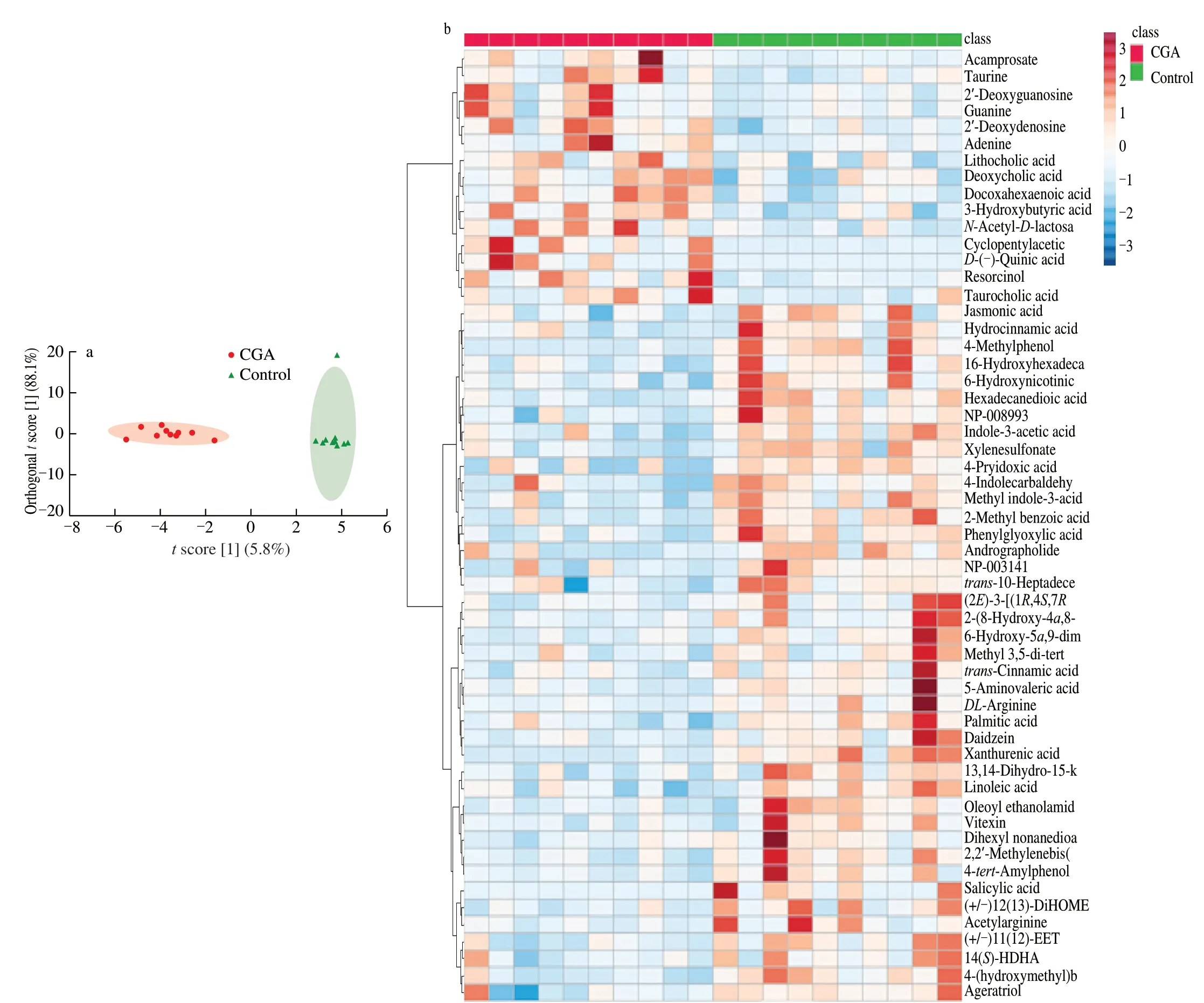
Fig.3 CGA enhances A.muciniphila abundance through metabolites in the stool.(a) OPLS-DA according to difference in metabolites,each dot indicates an individual mouse.(b) The VIP analysis of differential metabolites in the CGA and control groups.(c) The association picture between A.muciniphila and related microbiota and metabolites.Each group had ten mice.Compared with control group,* P <0.05;** P <0.01;*** P <0.001.

Fig.3 (Continued)
Gut microbiota constitute a complex ecosystem where certain species are not only affected by other microbes within the community but also by metabolites.To explore the important relationship between microbiota and 56 metabolites that responded toA.muciniphila,Spearman correlation analysis was performed using a heatmap,in whichA.muciniphilaresponded to multiple metabolites(Fig.3c).Nine metabolites (N-acetyl-d-lactosamine,docosahexaenoic acid,β-hydroxybutyric acid,deoxyguanosine,quinic acid,resorcinol,cyclopentylacetic acid,acamprosate,and deoxyadenosine) potentially play a dominant role inA.muciniphilaregulation.
3.5 CGA intake restored HFD-induced obesity
To verify theA.muciniphilaupregulation by CGA in abnormal conditions,we further selected an HFD-induced mouse model with daily oral administration of CGA for 3 months.Subsequently,HFD exhibited a decrease inA.muciniphila,in contrast to mice receiving CGA,where an increased abundance ofA.muciniphilawas observed(Fig.4a,P<0.000 1).Moreover,the effect of CGA on obesity development was investigated,and the results showed that CGA treatment did not significantly affect the body weight of mice within 7 weeks,after which an obvious change was found (Fig.4b,P<0.001).At the end of the experiment,CGA showed weak blood glucose improvement,suggesting that CGA ameliorated obesity,independent of blood glucose (Fig.4c;Fig.S3b,P>0.05).In addition,obesity regulation did not result in changes in food and energy intake(Fig.S3a,P>0.05).Finally,we found that CGAH treatment in HFD-fed mice significantly attenuated obesity-induced accumulation of subcutaneous fat,adipose hypertrophy,and serum lipid parameters(Figs.4d-g;Fig.S3e,P<0.05).
For liver metabolism,the HFD group exhibited higher lipidosis,which was relieved after CGA intake (Figs.4h–i;Fig.S3d,P<0.000 1).Histopathological examination revealed HFD-induced hepatocyte steatosis,vacuole formation in the cytoplasm (black arrow),and focal infiltration of inflammatory cells (red arrow).In contrast,the livers in the CGAH group showed few hepatocyte steatosis,circular vacuoles (black arrow),and few inflammatory cell infiltrates (Fig.S3f).CGA had no effect on plasma ALT,AST,ALP,and GGT levels,suggesting that there might be no liver injury.(Fig.S3h–k,P>0.05).Similarly,we found that CGA resulted in an increase in colonic goblet cells and the percentage of mucus,suggesting that CGA could improve the impairment of the intestinal barrier (Fig.4j–k;Fig.S3g,P<0.05).Overall,these results indicate that CGA effectively inhibits the development of obesity.
4. Discussion
Although an increase inA.muciniphilaabundance contributes to the alleviation of obesity,A.muciniphilais still not permitted for direct administration or as a dietary supplement[23].Our approach provides great guidance for the regulation ofA.muciniphilathat cannot be directly consumed,which is also applied on other microbes.In the present study,eight polyphenols were screened based on the regulation ofA.muciniphilaabundance in mice and CGA was identified as the most efficient growth factor.We also analyzed the mechanism by which CGA regulatesA.muciniphila,combined with mucin secretion,SCFA,microbiome,and metabolomics.Finally,the effect of CGA onA.muciniphilaupregulation was verified in obese mice.
Recent evidence has suggested that several polyphenols have the potential to regulateA.muciniphilaabundance[17-22].CA and CGA have been shown to significantly increase the abundance ofA.muciniphilain colitis mouse[17,18].A notable elevation inA.muciniphilaabundance has been reported in HFD-induced mice after EGCG,PA,and Pue treatments[19,20,24].We screened polyphenols based on the modulation ofA.muciniphilagrowth and found that polyphenols,including CA,CGA,EGCG,PA,and Pue,responded toA.muciniphila.However,in this study,polyphenols including Res,FA,and Gen had no effect onA.muciniphila,which is in contrast to previous results[21].
A previous study revealed that mucin contributes toA.muciniphilagrowthin vivoandin vitro,which led to the hypothesis that CGA is a direct growth factor forA.muciniphila[25].After coculturingA.muciniphilawith CGA and assessing mucin content,no obvious results were obtained.There is little evidence supporting the phenomenon thatA.muciniphilauses polyphenols as an energy source.Li et al.[26]found that polyphenols rarely provided energy forA.muciniphilagrowth.In addition,mucin secretion is correlated with Muc2 and Klf4,which are responsible for the secretion and differentiation of goblet cells,respectively[27].Combined with the observation of mRNA expression,CGA failed to promote mucin production,because mucin secretion remains stable in healthy mice and is difficult to increase.These results reject the direct effect of CGA onA.muciniphilaand suggest that the effect of CGA on the host is more dependent on the microbiota.
Alteration of the microbiota is also dependent on other microbesmediated antagonistic action[28].Here,we aimed to functionally determine whether gut microbes play a coordinated or competitive role inA.muciniphilaimprovement after CGA treatment owing to the bacteriostatic action of CGA[29].Microbiome analysis revealed a decrease in Desulfovibrio and Alistipes attributed to the abundance ofA.muciniphila.Desulfovibrio is characterized as an anaerobic microbiota,restoring sulfate in H2S,which leads to chronic injury in enterocytes,causing intestinal sensitivity,intestinal leakage,and stomach ache[30,31].Notably,A.muciniphilaand Desulfovibrio absorb mucin for their growth,implying a competitive relation between the two[32].Another significantly decreased microbiota,Alistipes,did not have a relative report that demonstrated a relationship between the two;despite this,Alistipes shared similar glucoside hydrolases (GH)related to mucin utilization withA.muciniphila,including GH16,GH33,GH29,and GH95,indicating Alistipes exhibits a competitive ability withA.muciniphilain terms of mucin degradation[33].Therefore,to the best of our knowledge,the increase inA.muciniphilamay be due to the competition with Desulfovibrio and Alistipes.
Regarding the effect of CGA onA.muciniphilaabundance based on changes in metabolites,other studies have indicated that acetic acid is a key factor inA.muciniphilaenrichmentin vivoandin vitro[34].According to SCFA measurements,CGA did not significantly increase the levels of acetate,but the secretion of butyrate was enhanced,suggesting the benefit of CGA for inflammatory disease[35].In addition to targeted metabolomics,we found thatA.muciniphilawas strongly responsible for the three metabolites,as shown by the associated analysis of the microbiome and unoriented metabolomics.Notably,a previous study indicated that administration of a polyunsaturated fatty acid diet rich in fish oil,mainly containing DHA,increased the intestinal abundance ofA.muciniphilain mice[35,36].The ketogenic diet induces the host to use fat for energy rather than glucose through a high-fat-low-carbohydrate diet pattern,significantly enrichingA.muciniphilaand improving antiepileptic capacity in mice[37].This process is accompanied by the production of ketone bodies,β-hydroxybutyric acid being one of the three ketone bodies[38].N-acetylD-lactosamine is a disaccharide known as a substrate for galactosidase,fucosyltransferase,and sialic acid transferase,which can be absorbed byA.muciniphila[39].Other metabolites were not found to be associated withA.muciniphilain a previous study.Therefore,the effects of CGA onA.muciniphilamay result in the enrichment of docosahexaenoic acid,β-hydroxybutyrate,andN-acetyl-lactosamine.
Accumulating evidence has demonstrated that the abundance ofA.muciniphilasignificantly declines in obese people;thus,the administration ofA.muciniphilarecovers the abundance ofA.muciniphilaand alleviates obesity[2,40].CGA treatment increased the production of mucin and the abundance ofA.muciniphilain obese mice,reducing the weight of HFD-fed mice at the end of the experiment.Moreover,TC,TG,and LDL-C levels are responsible for obesity alleviation[41].CGA decreased the HFDinduced increasing levels of TC,TG,and LDL-C,suggesting that CGA plays a regulatory role in fat and blood lipid accumulation.A review of previous studies has indicated that CGA inhibits obesity development through microbiota[42].Our results further indicated that CGA directly regulated obesity,which was dependent on the increase inA.muciniphilaabundance.In addition,the improvements in metabolism of liver were observed according to ALT,AST,ALP,and GGT reflecting liver injury[43,44].Ding et al.[45]also found that CGA protects the liver,as indicated by free radicals and lipid peroxidation.Combined with histological examination,these results confirm that CGA provides protection to the liver.
Microbiota-directed food provides nutrition for the targeted microbiota,thereby alleviating disease,thus providing a reasonable treatment strategy.Gordon et al.[46]performed large-scale screening of dietary fiber to improve obesity based on obesity-related microbiota.Based on a similar experimental design,we successfully screened a polyphenol forA.muciniphilapromotion and confirmed its effect on the improvement of obesity,suggesting the potential of a microbiotadirected food for disease treatment.
5. Conclusion
We successfully screened a polyphenol,CGA,which significantly increases the abundance ofA.muciniphila.However,CGA did not directly increase the abundance ofA.muciniphilaand failed to enhance the production of mucin to promoteA.muciniphilagrowth.Rather,microbiome and metabolomics analyses revealed that CGA triggersA.muciniphilagrowth mainly via competitive inhibition of Desulfovibrio and Alistipes and accumulation of docosahexaenoic acid,β-hydroxybutyrate,andN-acetyl-lactosamine.CGA intervention inA.muciniphilain HFD-fed mice was verified.In addition to confirming the effect of CGA onA.muciniphila,we found that CGA inhibits the development of obesity and dyslipidemia,prevents adipocyte degeneration,and enhances the intestinal barrier.These results highlight the potential preventive effect of CGA against obesity via the promotion ofA.muciniphila.
6. Limitations of study
A clear limitation of our study is the lack of complete understanding of the mechanism by which CGA affectsA.muciniphilain the complex intestinal community.The detailed interaction mechanism forA.muciniphilaupregulation needs to be investigated in germ-free mice to determine the effect of microbiota and metabolites,with the use of bioinformatics approaches.Another limitation is that CGA has not been approved for human trials,which limits its extensive use.Future studies are needed to evaluate the effects of CGA-rich foods.
Conflict of Interest
Qixiao Zhai is an associate editor forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
Acknowledgement
This work was supported by the Natural Science Foundation of Jiangsu Province [BK20200084],the National Natural Science Foundation of China [No.32122067 and 32021005];and the Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Compliance with Ethics Requirement
This research has fully complied with research ethics,the principles embodied in the revised Declaration of Helsinki (2008)(59th WMA General Assembly,Seoul,Republic of Korea,October 2008).All experiments were performed in accordance with the protocols approved by the Jiangnan University Animal Studies Committee,Wuxi,China.
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250007.
- 食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics

