Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
Pauline Duffuler,Khushwant S.Bhullar,Jianping Wu*
Department of Agricultural Food &Nutritional Science,University of Alberta,Edmonton T6G 2P5,Canada
Keywords:Dysbiosis Bone health Probiotic Prebiotic Fermented food Synbiotic
ABSTRACT Osteoporosis is the most common bone disorder,characterized by low bone mineral density and microarchitectural deterioration of the bone tissue,which increases the susceptibility to fracture.In the past decade,emerging research findings reported the implication of gut microbiota on bone health and osteoporosis pathology.Osteoporotic patients or individuals with a lower bone mineral density exhibit an alteration of the gut microbiota at several taxonomic levels.Additional reports demonstrate that gut microbiota regulates bone metabolism through the modulation of the gut function (mineral availability and absorption,gut integrity),the immune system,and the endocrine system.Thus,based on the vital role of gut microbiota on bone health,it has emerged as a novel therapeutic target for the prevention of bone loss and the treatment of osteoporosis.Microbial-based functional food ingredients,such as probiotics,prebiotics,synbiotics,and fermented foods,have been developed to alter the gut microbiota composition and function and thus,to provide benefits to the host bone health.Despite promising initial results,microbial-based therapies are still under investigation.Moreover,additional animal studies and clinical trials are needed to understand the interactions between gut microbiota and bone metabolism before further applications.
1. Osteoporosis,a worldwide public health issue
Bones are dynamic organs that are continuously remodeled primarily by specialized bone cells called osteoblasts and osteoclasts[1].Osteoblasts promote bone formation whereas osteoclasts dissolve the bone matrix (“bone resorption”).The balance between bone formation and bone resorption is essential for the maintenance of bone and overall health[2,3].When a disequilibrium between the activity of these two bone cells occurs,the human body may experience bone disorders,such as osteoporosis which occurs when the osteoclasts’ activity exceeds that of the osteoblasts[4].
According to the World Health Organization (WHO),osteoporosis affects 200 million people worldwide[5],including 1.5 million Canadians[6],10 million Americans[7],and 32 million Europeans[8].Additionally,the healthcare expenses of osteoporosis,particularly the treatment of osteoporosis and fractures cost around $2.3 billion to Canadian Healthcare system[9].Consequently,osteoporosis has been defined as a major worldwide public health issue.According to WHO,osteoporosis is defined as “a multifactorial systemic skeletal disease characterized by low bone density and microarchitectural deterioration of bone tissue”.Osteoporosis drastically compromises bone strength and architecture,leading to an increase in bone fragility and susceptibility to fracture[5].Osteoporotic patients usually don’t realize that they have osteoporosis until the first fracture occurs.As bone loss happens progressively and is painless,osteoporosis is also described as a “silent killer” or an “iceberg disease”.Osteoporosis results in 8.9 million fractures every year worldwide,mostly occurring in the spine,hips,and forearms[10].Osteoporosis also reduces the life quality of patients and impacts the mental health and overall well-being.Indeed,bone fractures often lead to severe pains,loss of independence,height loss,deformity,a hunched posture,and morbidity.Two primary risk factors are associated with osteoporosis development: age and gender.Women are more likely to develop osteoporosis compared to men.Indeed,one in three women and one in five men over 50 years old will experience osteoporosis once in their life[11,12].Moreover,the risk of osteoporosis increases with the age as after 50 years,osteoporosis becomes an important health risk for about 55% of the population[11].
There are numerous factors associated with bone health,such as diet,genetic determinants,metabolic health,micronutrients,and environmental factors.However,in the past decade,emerging research findings have reported the crucial implication of gut microbiota alterations in the pathogenesis of osteoporosis.
2. Interactions between gut microbiota and bone metabolism
2.1 Introduction to gut microbiota
Gut microbiota is a complex and dynamic community of living microorganisms (bacteria,fungi,viruses) that inhabits the gastrointestinal tract.Gut microbiota contains more than 1 000 different microbial species and more than a trillion microorganisms[13].Gut microbiota contains collectively 100-fold more genes than the human genome[13].Acquired at birth,the gut microbiota is shaped by birth methods and infant feeding methods and reaches a steady-state at the age of three years old[3].Then,the composition and abundance of the gut microbiota are shaped depending on a variety of host factors (gender,age,genetics)and environmental factors (diet,lifestyle,medications,antibiotics,environment,diseases)[14].Gut microbiota interacts with the host and exerts a marked influence on many functions of the host,including gut physiology and function[15],endocrine system[16],production[17]and absorption of nutrients[18-20],energy extraction[21,22],modulation of the immune function[23],and acts as a protective barrier against pathogens[23].
When the gut microbiota is altered,it loses its protective functions.Alteration of gut microbiota,also known as dysbiosis,is characterized by a decrease inα-diversity and a decrease in abundance of the commensal bacteria[24].In the past decade,emerging studies reported that gut dysbiosis has been linked with numerous diseases and disorders including obesity[25],Parkinson’s disease[26],Alzheimer’s disease[25],autism spectrum disorder[27,28],metabolic syndrome[29],inflammatory bowel diseases[30],type 2 diabetes[31],cardiovascular diseases[32],cancers[33]and osteoporosis[34,35].
2.2 Interactions between gut microbiota and bone metabolism
The impact of gut microbiota on bone health was investigated for the first time in 2001 when Distefano et al.[36]reported that small intestinal bacterial overgrowth was associated with a lower bone mineral density (BMD) in humans[36,37].Several additionalin vivotrials have studied the influence of gut microbiota on bone metabolism.Sjögren et al.demonstrate for the first time that gut microbiota regulates bone mass in mice[34].The authors reported that germ-free mice have a higher bone mass and a higher BMD compared to conventionalized mice[34].These results were associated with an alteration of the immune system leading to a reduction in osteoclast number and thus,a decrease in overall bone resorption[34].However,when germ-free mice were colonized with healthy gut microbiota,their bone mass and their immune system normalized and were comparable with conventionalized mice[34].Moreover,Yan et al.[38]demonstrated that gut microbiota influences both bone formation and bone resorption.Indeed,colonization of germ-free mice leads to bone loss in the short term.However,in the long term,colonization of germ-free mice increases bone mass and longitudinal and radial bone growth by both promoting bone resorption and bone formation[38].On the opposite side,Schwarzer et al.observed that germ-free mice possessed weaker bones with a lower femur length and cortical thickness[39].However,these contradictions with other studies might be explained by the different animal models used (gender,breed)[39].Therefore,gut microbiota regulates bone homeostasis.However,dysbiosis has also been considered as a risk factor for metabolic bone diseases,including osteoporosis[40].Indeed,Li et al.[41]demonstrate the critical role of gut microbiota in post-menopausal osteoporosisin vivo.In case of estrogen deficiency,gut microbiota modulates the immune system to induce bone loss[41,42].Finally,Wang et al.demonstrate that gut dysbiosis induced by the feces transfer from senile osteoporosis rats to young health rats induces osteoporosis[43].This last study demonstrates one more time the essential role of gut microbiota in bone health.As gut microbiota regulates bone metabolism and dysbiosis induces bone loss,it is gaining acceptance as an underlying factor in patients with a low BMD compared to the healthy individuals.
Several studies demonstrate various significant taxonomic differences between the gut microbiota of osteoporotic patients and healthy controls (Table 1)[44-47].First,gut microbiota alterations were observed in osteoporotic patients at a phylum level.The human gut microbiota contains four main phyla: Bacteroidetes,Firmicutes,Proteobacteria,and Actinobacteria.More than 90% of healthy gut microbiota is composed of species within two main phyla Bacteroidetes and Firmicutes.The individual with a low BMD has a lower Firmicutes/Bacteroidetes ratio compared to healthy people[45].Moreover,Actinobacteriawas also positively correlated with BMD whereasProteobacteriawas negatively correlated with bone mass[45,48](Table 1).Additional gut microbiota alterations were observed at the genera and family taxonomic levels.The abundanceof Bacteroidetes,Lactobacillus,Eggerthella,Dialister,Rikenellaceae,Enterobacter,Klebsiella,Citrobacter,Pseudomonas,Succinivibrio,Desulfovibrio,andEisenbeigiellahas been positively associated a lower BMD,bone loss,and/or with osteoporosis risk[47-51].On the opposite,Bifidobacterium,Lachnospiraceae (includingClostridiumcluster XIVa genera),Veillonella,andBlautiawere found in lower abundance in individuals with osteoporosis[44-46,48,50,52].
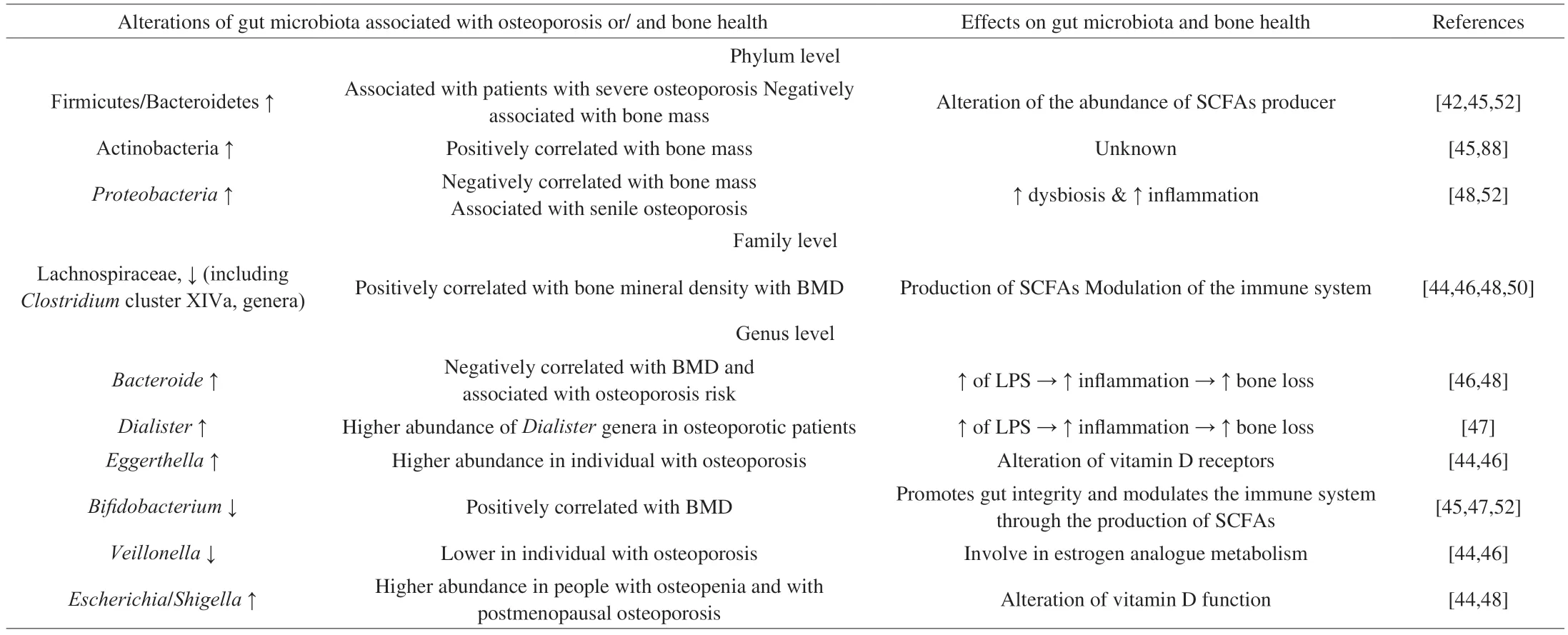
Table 1 Effects of gut microbiota alterations on bone metabolism and osteoporosis.SCFAs: short-chain fatty acids.
In summary,numerous studies compared the gut microbiota of osteoporotic individuals with those of healthy individuals.These clinical trials showed that alterations in the bacterial abundance at several taxonomic levels are observed in osteoporotic individuals with lower BMD (Table 1).However,it is important to note that these studies are preliminary,and additional clinical studies are needed in order to confirm these observations and to establish the association between the members of the gut microbiota and bone health.Future studies are also necessary to better understand the interactions between gut microbiota and bone.
3. Gut microbiota and bone homeostasis: proposed mechanisms
Gut microbes regulate the bone metabolism of the host through the modulation of gut function,immune system,and endocrine system.
3.1 Gut microbiota modulates bone metabolism through host metabolism
The gut barrier modulates essential functions of the host metabolism,including nutrient digestion and absorption,and defense against potentially harmful bacteria and metabolites.Indeed,a healthy gut microbiota promotes gut epithelium function.However,dysbiosis can lead to the alteration of gut barrier functions and could trigger bone catabolism or resorption (Fig.1).
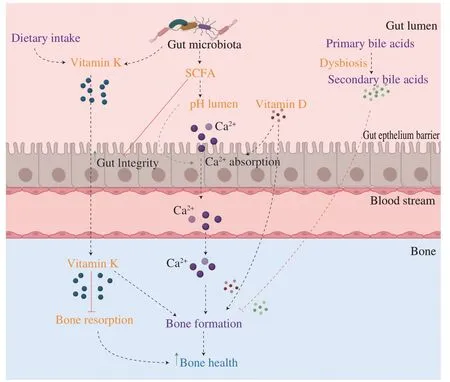
Fig.1 Gut microbiota modulates bone metabolism through the host metabolism.(1) Gut microbiota produces SCFAs that lower the pH of the gut lumen and thus,enhance calcium absorption.By promoting calcium absorption,gut microbiota promotes bone mineralization and bone formation.(2) Vitamin D promotes calcium absorption and stimulates directly bone formation.Dysbiosis can alter vitamin D metabolism.(3) Vitamin K can be produced by gut microbiota.Vitamin K inhibits bone resorption and promotes bone formation.(4) Dysbiosis leads to the production of secondary bile acids which,inhibits bone formation and thus,negatively impacts bone health.SCFA: short-chain fatty acids.
3.1.1 Gut microbiota modulates the absorption of minerals and the production of vitamins essential for bone homeostasis
Gut microbiota can modulate the absorption of micronutrients and the production of vitamins essential for bone health.Calcium is one of the main nutrients involved in bone homeostasis.99% of the body’s calcium is found in the bones[53].Calcium deficiency leads to severe bone loss and triggers the development of osteoporosis[54].The gut microbiota enhances calcium absorption through the production of specific metabolites such as short-chain fatty acids[55].Short-chain fatty acids (SCFAs;including butyrate,propionate,and acetate),are metabolites derived from the fermentation of dietary fibers by symbiotic bacteria (e.g.,Bifidobacterium).SCFAs provide benefits to host health as SCFAs are also involved in motility and the gut mucosal barrier.Moreover,the SCFAs butyrate is an energy source for colonocytes and exhibits anti-inflammatory properties in the gut[56,57].The abundance of SCFAs has been correlated with an increased bone mass and prevention of bone lossin vivo.On one hand,a high abundance of SCFAs in the gut lowers the pH in the intestinal lumen which increases mineral solubility and inhibits the formation of calcium complexes (calcium phosphate)[55].Thus,SCFAs improve calcium availability and increase its absorption[55].On the other hand,SCFAs also increase paracellular calcium transportation through the intestinal epithelium[18].By enhancing calcium absorption,gut microbiota promotes bone mineralization and thus,bone formation[18,55].
The abundance ofBifidobacteriumwas positively correlated with BMD[58].Bifidobacteriumis a symbiotic bacterium of gut bacteria that plays an important role in promoting intestinal health and gut barrier functions through the production of SCFAs[59].Indeed,these bacteria promote gut integrity and prevent the translocation of pathogens and antigens into the systemic circulation[59].Alteration ofBifidobacteriumabundance could decrease the production of SCFAs,alter the gut function barrier and thus,reduce calcium absorption and induce bone loss in osteoporotic patients.
Clostridiumcluster XIVa is a genus derived from the Lachnospiraceaefamily.The abundance ofClostridiumcluster XIVa,and Lachnospiraceae was also positively correlated with BMD[46,48,50,51].Moreover,people with a low abundance of Lachnospiraceae possess a higher risk of fracture[48,50].As Lachnospiraceae produce SCFAs,a decrease in the abundance of this bacterium decreases the abundance of SCFAs and all its beneficial effects[60].
Vitamin D is an essential vitamin for bone health.Calcium is absorbed in the intestine only in the presence of vitamin D active form.Indeed,vitamin D (25 hydroxyvitamin D) enhances absorption of calcium and thus,maintains calcium homeostasis[53].Vitamin D can also directly bind to the vitamin D receptors (VDR) present on the surface of osteoblasts and promote bone formation[61].On the opposite,vitamin D deficiency increases bone loss and the risk of fracture[62].Bora et al.[19,20]demonstrate that gut microbiota regulates vitamin D metabolism through fibroblast growth factor 23 (FGF23).The authors demonstrated that dysbiosis and inflammation in the colon increase level of FGF23 and thus,decrease vitamin D availability[19,20].However,the detailed mechanisms behind these interactions are still unknown.Gut microbiota can also modulate vitamin D metabolism via secondary bile acids[63].Primary bile acids are molecules derived from cholesterol,produced in the liver,and secreted in the small intestine to promote the absorption of dietary fats.90%–95% of primary bile acids are reabsorbed in the intestine.However,excess bile acids are sent to the colon where gut microbiota converts primary bile acids into secondary bile acids through deconjugation.Primary and secondary bile acids can both enter the systemic circulation and modulate bone metabolism.Whereas primary bile acids promote bone formation and inhibit bone resorption through the activation of Farnesoid X receptor (FXR)[64];secondary bile acids,such as lithocholic acid (LCA),can impair the metabolism of vitamin D by binding to vitamin D receptors (VDR),which inhibit the beneficial action of vitamin D on osteoblasts and bone formation[63].
Alterations of specific gut microbes in osteoporotic patients were correlated with vitamin D metabolism.On one hand,the abundance ofEggerthellawas higher in people with osteoporosis[46,51].The authors hypothesized that the abundance ofEggerthellaincreases in the absence of vitamin D receptors[51].First,the number of vitamin D receptors has been positively correlated with bone formation and negatively correlated with bone resorption.Moreover,vitamin D receptor polymorphism significantly decreases BMD and increases the risk of osteoporosis[65-67].Downregulation of vitamin D receptors could be associated with bone loss and thus in the development of osteoporosis.Thus,increasing the abundance ofEggerthellamay reduce the number of vitamin D receptors and cause bone loss associated with osteoporosis[46,51].On the other hand,the abundance ofEscherichiaandShigella,two bacteria belonging to the phylumProteobacteria,was higher in people with osteopenia and with postmenopausal osteoporosis[48,51].Bashir et al.have previously reported that the abundance ofEscherichia/Shigellawas negatively correlated with vitamin D concentration[68].Thus,the higher abundance ofEscherichia/Shigellacould be related to a vitamin D deficiency,leading to bone loss[51].
Gut microbiota can also modulate the production of one vitamin essential for bone health quality: vitamin K[17].Vitamin K stimulates bone formation through the promotion of osteoblast differentiation and the decrease of osteoclast differentiation[53,69,70].Moreover,vitamin K is a cofactor in the production of essential bone-specific proteins (osteocalcin,gla protein).50% of our vitamin K intake is produced by our gut microbes,such asBacteroides[17].Thus,alteration of gut microbiota could also impair the production of vitamin K and thus,bone homeostasis.However,no studies are available on this thematic yet.
3.1.2 Gut microbiota modulates the intestinal barrier integrity
The intestinal epithelial barrier is the first line of defense of the host against potentially harmful pathogens and antigens.Healthy gut microbiota helps to maintain gut integrity.However,dysbiosis can alter gut barrier function.The epithelial cells are sealed together with the tight junctions’ proteins.Gut microbiota can also alter the expression and the distribution of tight junctions’ proteins (occludin,claudin and ZO-1),leading to the pathogenesis of osteoporosis[43].This alteration increases the permeability of the gut,leading to a dysfunction known as “leaky gut”[71].A leaky gut enhances the translocation of harmful microorganisms and microbial components in the systemic circulation,and therefore,they can reach other organs such as bone and modulate their functions.For example,lipopolysaccharides (LPS) are a major microbial component found in the outer cell membrane of gram-negative bacteria.LPS is one of the potent bacterial toxins that can translocate from the intestinal tract to the blood circulation and induce bone loss[72].The bacterial toxin,LPS,can induce inflammation and stimulate the production of pro-inflammatory cytokines (macrophage colony-stimulating factors (M-CSF),tumor necrosis factor TNF-α,interleukin (IL)-1,IL-6)[73].Inflammation has been associated with bone loss in osteoporosis.Indeed,these pro-inflammatory cytokines stimulate bone resorption[74]: IL-6 enhances osteoclast formation by upregulating the receptor activator of nuclear factor-κB ligand (RANKL)activities[75]whereas M-CSF and RANKL both induce osteoclast differentiation[76].Additional studies also demonstrate that LPS can stimulate bone loss by promoting osteoclast survival[72,77].Therefore,in the case of dysbiosis,harmful microorganisms and toxins,such as LPS,can translocate into the circulation to reach the bone,causing inflammation and inducing bone loss which increases the risk of developing osteoporosis.
Bifidobacteriumpromotes intestinal integrity and prevents the translocation of LPS into the systemic circulation.The abundance ofBifidobacteriumwas positively correlated with BMD[58].Alteration ofBifidobacteriumcould alter the gut function barrier in osteoporotic patients.On the opposite,the abundance ofBacteroidesandDialistergenera was negatively correlated with the BMD of lumbar spines.A higher abundance ofBacteroideswas associated with osteoporosis risk[46-48].AsBacteroidesandDialisterare gram-negative bacteria[78],the abundance of LPS will be higher in osteoporotic patients.LPS will initiate inflammation response in the body through the release of pro-inflammatory cytokines.Thus,LPS stimulates bone loss through inflammation[79-82].
3.2 Gut microbiota modulates bone metabolism through the immune system
Gut microbiota closely interacts with the immune system of the host.Gut microbiota is involved in the maturation of the immune system and the protection of the host against infectious agents[83].Sjögren et al.reported for the first time that gut microbiota modulates bone mass via the immune system[34].Indeed,in the absence of gut microbiota,germ-free mice have an altered immune system with a lower expression of pro-inflammatory cytokines (TNF-α,RANKL,IL-6) and a decrease in the number of CD4+T cells in bone compared to conventional mice.This imbalance led to a decrease in osteoclastogenesis and thus,in bone resorption.Then,the immune system and the bone mass both normalize after the recolonization of the gut[34].On the opposite,dysbiosis is known to disrupt immune tolerance and has been linked with inflammatory diseases including osteoporosis disorder (Fig.2)[84].
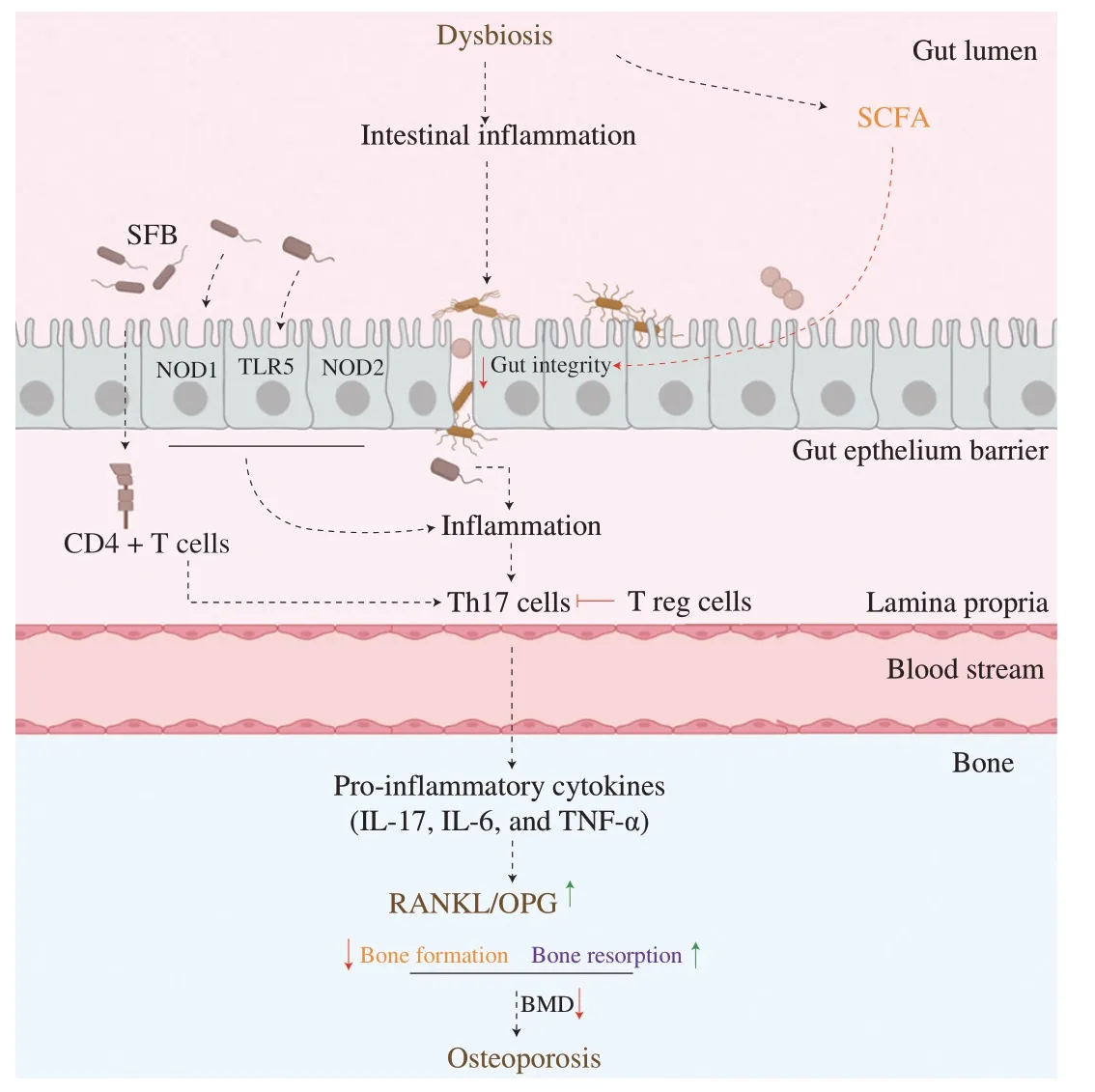
Fig.2 Gut microbiota modulates bone health through the immune system.Dysbiosis induces intestinal inflammation and alters gut integrity.A“leaky gut” enhances the translocation of harmful pathogens and microbial components into the systemic circulation,which initiates inflammation.Pattern recognition receptors (TLR-5,NOD-1&2) recognize pathogens and induce a pro-inflammatory response in the organisms.Dysbiosis also modulates the production of immune cells: inflammation activates Th17 cells which induce the production of pro-inflammatory cytokines.These inflammatory cytokines will inhibit bone formation and stimulate bone resorption.BMD: bone mineral density;CD4+ T cells: T helper cells;IL: interleukin;NOD: nucleotide-binding oligomerization;OPG: osteoprotegerin;RANKL: receptor activator of nuclear factor-κB ligand;SCFA: short-chain fatty acid;SFB: spore-forming bacteria;Th17 cells: T helper 17 cells;T reg cells: regulatory T cells;TLR5: toll-like receptor 5.
3.2.1 Gut microbiota induces inflammation through the activation of pathogen recognition regulators
Gut microbiota influences bone homeostasis through the modulation of different components of the innate immunity.Pathogen recognition regulators (PRRs) are the first line of defense of the host through the recognition of pathogen-associated molecular patterns(PAMPs).
Among PRRs,Toll-like receptors 5 (TLR5),located in the epithelial cells,can indirectly modulate bone metabolism.TLR5’s function is to recognize flagellin,a protein expressed by some pathogenic bacteria such asSalmonella,Pseudomonas.In vivo,activation of TLR5 enhances RANKL/OPG ratio in osteoblasts,which stimulates osteoclast formation and thus,promotes bone resorption.Kassem et al.demonstrate that deletion of TLR5 alters the gut microbiota of mice by increasing the abundance ofProteobacteriaand flagellated bacteria,which induces inflammation and bone loss[85].
Nucleotide-binding oligomerization domain proteins (NOD) 1 &2 are other PRRs,located in the cytoplasm.NOD 1 &2 bind to peptidoglycans situated on the surface of gram-negative bacteria and of a few gram-positive bacteria (Bacillus,Listeria).NOD2 deficiency mice have lower bone resorption compared to conventional mice,which demonstrates the function of NOD2 in bone metabolism.When bacteria bind to NOD,it induces inflammation and stimulates the receptor activator of the nuclear factor kappa-B (NF-κB) signaling pathway through receptor-interacting protein 2 (RIP2)[86].Activation of the NF-κB signaling pathway induces a pro-inflammatory response,which activates the RANKL,a promoter of bone resorption[86-89].
3.2.2 Gut microbiota influences bone metabolism through the activation of T cells
Among immune cells,the lymphocytes T helper 17 cells and the regulatory T cells can also influence indirectly bone metabolism.The lymphocytes T helper (Th) 17 cells are a subset of T helper cells,mostly situated in the gut,and involve in maintaining gut barrier integrity and protecting the host against pathogens.
Th17 cells activation can trigger bone loss in postmenopausal osteoporosis.Indeed,postmenopausal osteoporosis occurs due to estrogen deficiency.In the case of estrogen deficiency,the permeability of the gut increases leading to the translocation of harmful pathogens and the production of antigens (LPS) in the systemic circulation.An increase in inflammation enhances the production of the lymphocyte’s Th17 cells.However,activation of Th17 cells also promotes osteoclastogenesis[90].Indeed,Th17 cells migrate to the bone matrix to induce local bone inflammation through the production of pro-inflammatory cytokines (IL-17,IL-1,and TNF-α) which stimulates the expression of RANKL[90].An increased expression of RANKL promotes osteoclasts differentiation which enhances bone resorption and causes bone loss[87,90-95].
Gut microbiota modulates the activation of Th17 cells.In conventional mice,Th17 cells have been associated with an increase in osteoblast proliferation and differentiation and with a decrease in BMD[96].However,in germ-free mice,Th17 cells are absent.The expression of Th17 cells can be modulated by certain gut microbes such as segmented filamentous bacteria (gram-positive and sporeforming bacteria).The colonization of the gut with spore-forming bacteria activates CD4+T helper cells to enhance the production and the differentiation of Th17 cells as well as the production of the proinflammatory cytokines IL-17 and IL-22 into the lamina propia[97].Thus,the activation of Th17 cells will induce local bone inflammation and stimulate bone loss[91,94,95].
Zaiss et al.demonstrated that regulatory T cells (Treg cells)also indirectly influence bone metabolismin vivo[98].Treg cells are immune cells,whose function is to suppress immune activation.Treg cells inhibit the production of Th17 cells,through the cytotoxic T lymphocyte-associated protein 4 (CTLA-4) signaling pathway.Indeed,activation of CTLA-4 decreases the secretion of RANKL and thus,inhibits bone resorption by decreasing osteoclastogenesis[98].Treg cell activation also promotes bone formation.Indeed,activation and proliferation of Treg cells stimulate the proliferation of CD8+T cells which activate the Wnt/β-catenin signaling pathway.Activation of Wnt/β-catenin signaling pathway stimulates osteoblast differentiation and bone formation[99].Some metabolites derived from gut microbiota fermentation,such as the SCFA butyrate,can stimulate the production of Treg cells and induce bone formation[99,100].For example,Clostridiumcluster IV andXIVa produce SCFAs which enhance the proliferation and accumulation of the Treg cells[101,102].
3.3 Gut microbiota modulates bone metabolism through the endocrine system
The endocrine system is also an important regulator of bone metabolism.Gut microbiota can indirectly impact bone metabolism through the modulation of the endocrine system.Different hormones involved in the gut-bone axis are currently studied (Fig.3).
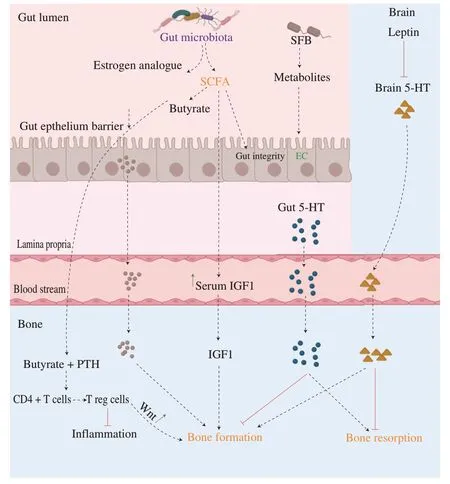
Fig.3 Gut microbiota modulates bone health through the endocrine system.Dysbiosis modulates the concentration of several hormones involved in bone metabolism.(1) Butyrate,an SCFA,in combination with PTH inhibit inflammation and promote bone formation through the activation of the Wnt signaling pathway.(2) Estrogen analogues,produced by gut microbiota,can bind osteoblasts to promote bone formation.(3) SCFAs increase the concentration of serum IGF-1 and thus,promote bone formation.(4)Depending on its production site,5-HT can modulate bone health differently.On one hand,gut 5-HT inhibits bone formation and promotes bone resorption.On the other hand,brain 5-HT,which can be modulated by leptin,promotes bone formation,and inhibits bone resorption.5-HT: serotonin;CD4+ T cells:T helper cells;EC: enterochromaffin cells;IGF1: insulin-like growth factor 1;PTH: parathyroid hormone;SCFA: short-chain fatty acids,SPF: spore-forming bacteria;T reg cells: regulatory T cells;Wnt: Wnt signaling pathway.
3.3.1 The role of serotonin (5HT) and leptin in bone metabolism
Serotonin (5-hydroxytryptamine,or 5-HT) is a hormone and a neurotransmitter that plays an essential role in several functions of the host,including mood,behavior,immune system,cardiovascular function,blood regulation as well as bone homeostasis[103].First,Lavoie et al.[104]report that gut-derived serotonin contributes to bone loss induced by colitisin vivo[104].Then,Sjögren et al.demonstrate that germ-free mice,which have a higher bone mass compared to conventional mice,also,exbibit lower serum levels of serotonin[34].On the opposite,mice with a higher level of serum serotonin have a lower BMD.These findings were further confirmed in clinical trials where high serotonin levels were inversely associated with BMD in elderly men[105]and post-menopausal women[106].According to the authors,serotonin levels could predict an increased risk for osteoporotic fractures: patients with high serotonin levels have three times increased risk for hip fracture[105].Therefore,scientists start to study the mechanisms behind the interactions between serotonin and bone metabolism.
Serotonin is mainly synthesized by two different sites in the body: the brain and the gut[107].Depending on the site of production,serotonin modulates differently bone metabolism.90%of the serotonin of the human body is synthesized in the colonic enterochromaffin cells of the gut[103].This reaction is catalyzed by the rate-limiting enzyme tryptophan hydroxylase 1 (Tph1)[108,109].Once synthesized,gut-derived serotonin translocates into the system circulation and is transported to the bone cells where this neurotransmitter binds to serotonin receptors 5-HT1B,expressed in the surface of osteoblasts and osteocytes[110,111].Gut-derived serotonin has a negative impact on bone formationin vivo.According to Yadav et al.gut-derived serotonin reduces osteoblast proliferation and inhibits bone formation leading to a decrease in BMD and a higher risk of osteoporosis[112].On the opposite,Chabbi-Achengli et al.reported that a decrease in serotonin level has been associated with a decrease in osteoclastogenesis,indicating the role of the site in serotonin’s impact on bone mass accrual[113].Some gut microbes can modulate the level of gut-derived serotonin.For example,Yano et al.reported that some spore-forming bacteria produce metabolites that stimulate the biosynthesis of 5-HT by colonic enterochromaffin cells[110].
10% of the serotonin is generated in the central nervous system and catalyzed by the rate-limiting enzyme tryptophan hydroxylase 2(Tph2)[108].In opposition to gut-derived serotonin,brain-derived serotonin benefits bone health by decreasing the inhibition of osteoblasts and thus,promoting bone formation[114].Recently,studies demonstrated that the hormone leptin could also regulate bone homeostasis through the modulation of brain-derived serotonin levels.Leptin is an adipocyte-derived hormone initially recognized for its function on energy expenditure and homeostasis[115].Leptin binds to its receptor expressed in the brain,leading to the inhibition of serotonin receptors and a decrease in brain-serotonin levels[116].The beneficial effect of brain-derived serotonin on bone mass is thus inhibited by leptin[117].Gut microbiota can modulate the level of leptin: leptin serum level was positively correlated with the abundance ofBifidobacteriumandLactobacilluswhereas leptin serum level was negatively correlated withClostridium,Bacteroidesand,Prevotella[118].However,the mechanism behind these correlations still remains unknown.
In summary,dysbiosis contributes to bone loss via the modulation of serotonin synthesis.On one side,gut-derived serotonin is inversely associated with BMD.Knowing that a low BMD is the main risk factor of osteoporosis,high levels of gut-derived serotonin could contribute to the development of this bone disorder.On the other side,gut microbiota can inhibit the beneficial effect of brainderived serotonin through the modulation of the leptin hormone.Therefore,decreasing the levels of gut-derived serotonin and leptin by modulating the gut microbiota,could be a viable approach to treat and prevent osteoporosis[107].
3.3.2 Parathyroid hormone (PTH)
Parathyroid hormone (PTH) is an essential hormone for skeletal development and bone homeostasis,produced by parathyroid glands.PTH regulates the calcium serum level but also promotes bone resorption and induces bone formation depending on the bone cells targeted and the level of hormones released.Li et al.demonstrated that butyrate produced by gut microbiota was the key modulator responsible for the anabolic activity of PTH in bonein vivo[119].When gut microbiota was depleted in mice,the level of butyrate and PTH decreases as well as bone formation and bone mass.On the opposite,when microbiota was restored,the levels of butyrate and PTH normalized,and bone homeostasis was re-established[119].The following mechanism was proposed by the authors.Butyrate and PTH act jointly to produce and differentiate the naïve CD4+T cells into Treg cells in the bone marrow.These Treg cells stimulate bone marrow CD8+T cells to activate the Wnt signaling pathway and enhance the production of osteoblast precursors and osteoblast proliferation and thus,promoting bone formation[119].Thus,if the gut microbiota is imbalanced,the level of butyrate is altered which would negatively impact the PTH action on bone formation.
3.3.3 Insulin growth factor-1
Gut microbiota can stimulate bone formation via the synthesis of insulin-like growth factor 1 (IGF-1).IGF-1 is a growth hormone,mainly synthesized by the liver after growth hormone stimulation.Severalin vivostudies demonstrate that gut microbiota modulates the level of IGF-1 in the host and thus indirectly influences bone homeostasis[38,120-122].Germ-free mice have a lower level of IGF-1,and a lower bone mass compared to conventional mice[39].However,the IGF-1 level increases after microbial colonization as well as bone mass.On the opposite,a low level of IGF-1 has been correlated with osteoporosis[123].The mechanisms are not yet fully understood.However,scientists hypothesize that gut microbiota modulates host IGF-1 levels through the production of SCFAs.Indeed,the serum level of IGF-1 has been correlated with the abundance of cecal SCFAs.According to the authors,SCFAs stimulate the production of IGF-1 in the liver and thus,increase the serum level of IGF-1[124-126].Then,IGF-1 binds the IGF receptor present in osteoblast/osteoclast resulting in stimulating bone formation through the promotion of osteoblast differentiation and mineralization[38,120-122].
3.3.4 Estrogen
Estrogen is a sex steroid hormone that plays a crucial role in bone development and disorders.In postmenopausal women,estrogen deficiency leads to a decrease in bone mass and is the main driver of postmenopausal osteoporosis[127].Recent advances demonstrate that gut microbiota can modulate the estrogen level of the organism via the estrobolome activity.Estrobolome is defined as “the aggregate of enteric bacterial genes whose products are capable of metabolizing estrogens”[128].Gut bacteria that possessβ-glucuronidase and/orβ-galactosidase activity can metabolize estrogen.Free estrogens are then absorbed and enter the bloodstream.As they are structurally similar to estrogen,they can bind to estrogen receptors(ERα/ or ER β) and thus,exert their bioactivity[129].In the human gastrointestinal tract,several bacterial genera have been identified with these enzymes,includingBifidobacterium,Faecalibacterium,Lactobacillus,Veillonella[130].Clinical studies demonstrate that the abundance of the genusVeillonellaandBifidobacteriumis lower in an individual with osteoporosis compared with normal BMD[46,51,58].Das et al.hypothesized that a decreased abundance inVeillonellais associated with lower production of estrogen analogs such as equol.A lower abundance of estrogen in the body would then lead to bone loss[51].
Li et al.demonstrate that sex steroid deficiency induces bone loss through the modulation of the gut microbiota[41].Dysbiosis can alter estrogen metabolism.Dysbiosis decreases estrogen metabolizing bacteria,leading to a reduction of estrogen levels in the blood circulation.Dysbiosis could then trigger the development of estrogendriven diseases.Emerging studies started to evaluate the link between gut estrobolomes and estrogen deficiency-related diseases such as breast cancer[129],obesity[130],type 2 diabetes[131],non-alcoholic fatty liver diseases[131],etc.However,despite the critical role of estrogen in osteoporosis,no study regarding the link between estrobolomes and osteoporosis is available yet.
Estrogen metabolites have positive effects on gut intestinal barrier function.Indeed,estrogen metabolites promote the expression of tight junction protein and decrease intestinal permeability.On the opposite,steroid deficiency provokes an alteration of gut intestinal barrier integrity and promotes gut permeability.These phenomena induce pro-inflammatory responses and bone loss increases[41].Moreover,estrogen directly modulates the level of vitamin D,an essential nutrient for bone health[132].
In summary,numerous studies reveal the close link between gut microbiota and bone health.To our best knowledge,the gut microbiota seems to modulate bone health through three main axes:the host system,the endocrine system,and the immune system(Figs.1-3).Despite a trending topic,most of the mechanisms of actions,underlying the action of gut microbiota on bone health,remain poorly understood and signalling pathways are not yet elucidated.Nevertheless,scientists have started to study the potential applications of microbial-based therapies for the management of osteoporosis and the promotion of bone health.
4. Microbial-based therapy for the prevention of bone loss
Two main therapeutic strategies are currently used to counteract osteoporosis: anabolic agents and antiresorptive agents[133].Antiresorptive agents promote osteoclast apoptosis,reduce bone resorption,and then enhance bone strength.The most common antiresorptive agents already used for osteoporotic patients are bisphosphonates (alendronate,risedronate),estrogen,and hormone replacement therapies (ERT,HRT),raloxifene,calcitonin,and others[133].A few limited treatments target the stimulation of bone formation.These anabolic agents aim to increase the activity of osteoblasts and thus,promote bone formation.The most common anabolic agent used is the parathyroid hormone (PTH)[133].However,these treatments exhibit a long list of severe side effects that such as nausea,dizziness,irregular heartbeat,loose bowel movement,development of cancer,an increase in the risk of cardiovascular diseases,etc.Moreover,none of these treatments can reverse completely the bone loss and the efficiency of these drugs remains debatable[134,135].Thus,scientists are looking for efficient alternatives to traditional therapies for the prevention and treatment of osteoporosis.Knowing the importance of gut microbiota on bone metabolism,gut microbiota has become a research spotlight for the prevention and treatment of osteoporosis.In this past decade,several microbial-based functional foods emerge to promote bone health,including prebiotics,probiotics,synbiotics,and fermented foods (Fig.4).
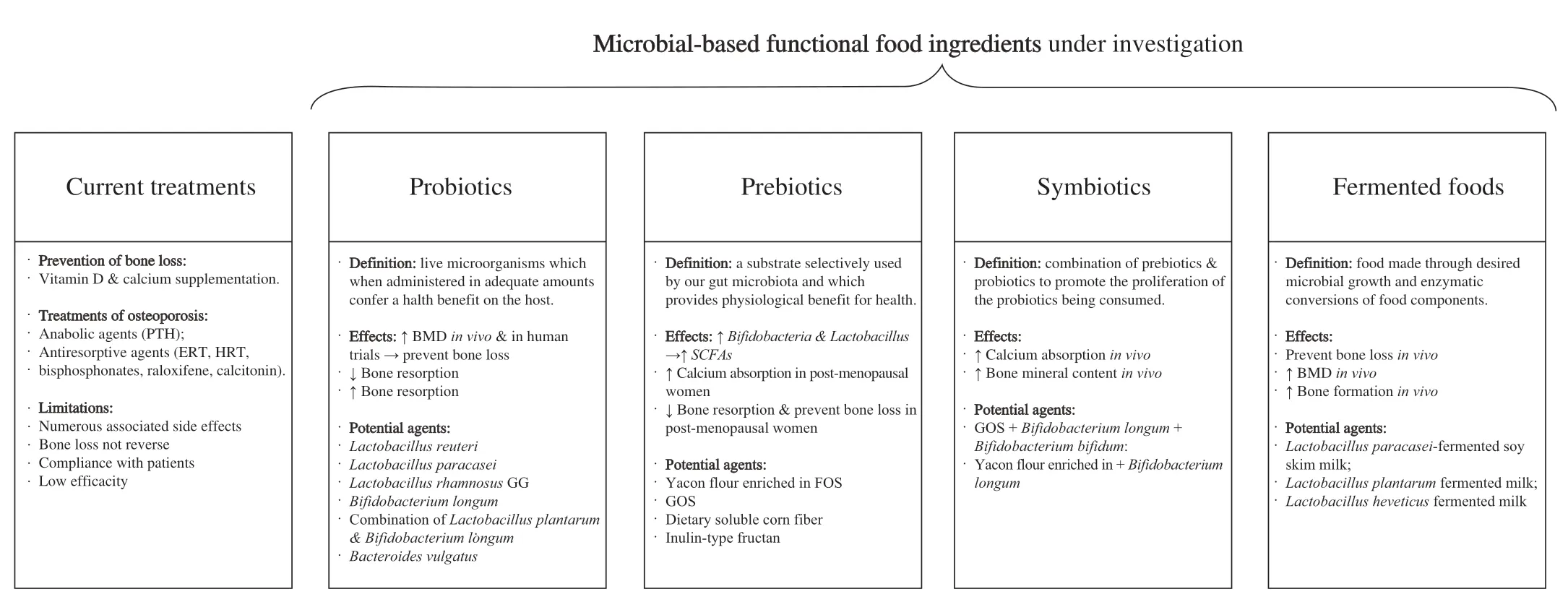
Fig.4 Current treatments and potential microbial-based strategies for the prevention of bone loss and the treatment of osteoporosis.BMD: bone mineral density; ERT: estrogen replacement therapies; FOS: fructooligosaccharides;GOS: galactooligosaccharides;HRT: hormone replacement therapies;PTH:parathyroid hormone;SCFAs: short-chain fatty acids.
4.1 Probiotics
Probiotic is defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host”[136].Knowing the role of gut microbiota on bone health,the use of probiotics could benefit bone health.
The most common probiotics studied for the promotion of bone health,belong to the genusLactobacillus.Reports showed thatLactobacillus-derived probioticscould prevent bone loss by altering the immune system.The probioticL.reuteriis widely recognized for its anti-inflammatory properties.Supplementation withL.reuteridecreases intestinal inflammation[137].Four weeks of supplementation ofL.reuteridecreases the production of pro-inflammatory cytokines TNF-α,which reduces bone resorption and prevents the suppression of Wnt10b involved in bone formationin vivo[137-139].In vivo,supplementation withL.reuteriprevents trabecular bone loss associated with estrogen deficiency[137,138],glucocorticoid treatment[140],and type 1 diabetes[139].These results were further confirmed in the human trial where daily oral administration ofL.reuteri,for 12 months,prevents bone loss in older women (75–80 years old) with low BMD[141].
Additionally,otherLactobacillusstrains demonstrate strong potential as a probiotic for bone health.Indeed,six weeks of supplementation with the probioticLactobacillus paracaseiprevents cortical bone loss in ovariectomized mice by reducing the production of two pro-inflammatory markers (TNF-α,IL-1β) and inhibiting osteoclastogenesis[142].The same effects were reported with the strainLactobacillus brevis[143].Moreover,oral administration ofLactobacillus rhamnosusGGreduces gut permeability by promoting the expression of tight junction proteins and preventing the production of pro-inflammatory cytokines involved in bone resorption[41].Thus,supplementation withL.rhamnosusGG reduces inflammation induced by estrogen deficiency and prevents bone loss[41].On the other hand,L.rhamnosusGG increases the production of SCFAs,which benefits bone formation[99].
Additionally,lactic bacteria also promote bone health by modulating calcium and vitamin D metabolism.On one hand,administration ofLactobacillus acidophilus,Lactobacillus casei,andBifidobacteriumsignificantly increased the level of serum calcium[144].On the other hand,the level of serum 1,25-(OH)2-vitamin D significantly increases in response to oral supplementation ofL.casei,Bacillus coagulansandLactobacillus reuteri[144]and protects bone loss in ovariectomized rats.Oral supplementation withL.reuteri,also increases the serum level of 25-hydroxyvitamin D in humans[145].
In addition to lactic bacteria,other’s probiotics were studied for the prevention of bone loss.Supplementation ofBifidobacterium longum(16 weeks;108–109colony-forming units/mL) in ovariectomized rats prevents bone loss induced by estrogen deficiency,by enhancing bone formation and inhibiting bone resorption[146].Additional studies demonstrate thatB.longumsupplementation significantly improves bone strength and BMDin vivo[147].Moreover,the probioticBifidobacterium lactisBL-99 prevents osteoporosis caused by colitisin vivo[148].CombiningLactobacillus plantarumandB.longumprevents bone loss in ovariectomized mice.Indeed,the combination of these two probiotics promotes serum level of calcium,phosphorus,and osteocalcin,reduces the expression of TNF-α and,downregulates the production of microbial LPS.The authors suggest that the combination ofL.plantarumandB.longumprevents bone loss by enhancing bone formation[149].Yuan et al.investigated the oral administration of the probioticBacteroides vulgatuson ovariectomized mice[150].The authors reported thatB.vulgatussupplementation minimizes dysbiosis,downregulates markers of inflammation such as TNF-α,reduces microbial production of LPS,leading to an improvement of the structure and the strength of the bones[150].Oral supplementation ofBacillus subtilus(24 weeks),in postmenopausal women,significantly inhibits bone resorption and increases BMD by decreasingFusobacteriumabundance and increasing the abundance ofBifidobacterium[151].
4.2 Prebiotics
Prebiotics are defined as non-digestible food ingredients that promote the growth and/or activity of one or several specific bacteria and that benefit the health of the host[152].The majority of known prebiotics are complex and non-digestible carbohydrates;that are synthetic compounds or naturally present in plant-based foods.Prebiotics are resistant to enzymatic digestion in the stomach and the small intestine,and go to the colon where they are fermented by gut microbiota.Prebiotics promote the proliferation of symbiotic microorganisms such asBifidobacteriaandLactobacillus,two probiotics well recognized for providing health benefits to the host.These bacteria enhance the production of SCFAs,mineral bioavailability and absorption,decrease inflammation and thus,benefit bone health[153].
Supplementation with the prebiotic galactooligosaccharides(GOS) promotes the growth ofBifidobacteria[153]which increases calcium absorption and prevents bone loss in OVX rats[154,155],and enhances calcium absorption and inhibits bone resorption in postmenopausal women[156].Then,four weeks of supplementation with yacon flour,enriched in fructooligosaccharides (FOS) improved bone strengthin vivo[147].Additionally,dietary soluble corn fiber significantly increases bone calcium absorption in postmenopausal women[157].Soybean whey is a tofu by-product enriched in nondigestible oligosaccharides (NDO),composed of GOS stachyose and inulins.NDO was reported to promote the growth of beneficial lactic bacteria in the gut microbiota and the production of SCFAs,which increases calcium absorption in the gut and thus,benefits bone health[158].Finally,inulin-type fructan improves mineral absorption and decreases markers of bone resorptionin vivo[159]and in postmenopausal women[160].
4.3 Synbiotics
Synbiotics are the combination of prebiotics and probiotics to promote the proliferation of the probiotics being consumed[161].Due to the beneficial effects of both probiotics and prebiotics on bone health,scientists also studied the effectiveness of synbiotics.Galactooligosaccharides combined withB.longumandBifidobacterium bifidumincrease calcium absorptionin vivo[162,163].Moreover,yacon flour,enriched in fructooligosaccharides and combined with the probioticB.longumincreases bone mineral content and bone strength compared to rats fed only with the prebiotic[147].Adoplphi et al.reported that 2 weeks supplementation of fermented milk supplemented with prebiotics (inulin-type fructan) and fortified with calcium reduces bone resorption and increases calcium absorption in postmenopausal women[164].Previous studies demonstrate that synbiotics were more efficient than the separate use of probiotics and prebiotics[147].
4.4 Fermented foods
Fermented foods have the potential to be alternative strategies for the treatment of osteoporosis and the prevention of bone loss.Fermented foods are defined as “food made through desired microbial growth and enzymatic conversions of food components”[165].
Recent studies reported thatLactobacillus-fermented products can benefit bone health.Indeed,eight weeks of supplementation withLactobacillus paracasei-fermented soy skim milk prevents bone loss in ovariectomized mice[166].Fermented milk withLactobacillus plantarumpromotes bone health in ovariectomized rats compared to sham rats[167].Supplementation withLactobacillus helveticusfermented milk enhances bone formation by increasing BMD in hypertensive rats and promoting calcium metabolism in postmenopausal women[168-170].
Additionally,other fermented dairy products also exhibit osteoprotective properties.Clinical studies report that incorporating milk and yogurts into the diet of post-menopausal women could decrease the markers of bone resorption as well as increase BMD and thus,prevent bone loss[171-175].Moreover,supplementation of soft cream cheese in elderly women’s diet led to a decrease in bone resorption and reduced fracture incidence[176].However,it remains unclear if the benefices of fermented dairy products are attributed to the increased calcium intake,a shift in gut microbiota or due to the synergic effects of these two.Oral supplementation of kefir fermented peptides (8 weeks),in vivo,prevents bone loss induced by estrogen deficiency via altering positively the gut microbiota[177]and suppressing inflammation[178].
Other fermented foods such as natto and kimchi,also present some bone health properties.Natto is a traditional Japanese dish,obtained after the fermentation of soybeans withBacillus subtilis[179].Intake of natto has been associated with a lower bone loss at the hip and femoral neck,and a reduced risk of osteoporosis in Japanese postmenopausal women[179-181].Kimchi is a traditional fermented cabbage widely consumed in Korea.The impact of kimchi on bone health remains poorly studied and results are controversial.On one hand,a diet composed of “white rice,kimchi and seaweed” diet negatively influence the bone health of post-menopausal women by enhancing the risk of osteoporosis[182].On the opposite,Shin et al.demonstrated that a diet rich in rice and kimchi was positively associated with BMD in women and men[183].These positive results could be explained by the presence ofL.brevis,a lactic acid bacteria involved in kimchi fermentation,which demonstrates bone healthpromoting properties[143].However,due to the conflicting results,additional studies are essential to understand the real impact of kimchi intake on bone health.
5. Conclusions and perspectives
Although the etiology of osteoporosis and bone loss is multifactorial,recent discoveries highlight the critical role of gut microbiota in bone metabolism.Numerous studies demonstrate the implication of dysbiosis on bone loss and,bone disorders through the modulation of the host metabolic system,immune system,and endocrine system.The close physiological relationship between gut and bone health has increasingly gained attention in recent years.Despite numerous advances,the scientific community needs to further explore the gut-bone axis to clarify and elucidate the underlying interactions and cross-talk between bone and gut microbiota.Despite this knowledge gap,the gut microbiota is a promising target to manage osteoporosis and related bone disorders.Different strategies including microbial-based therapies could be used as an early intervention to promote healthy gut microbiota and thus,prevent the development of bone disorders due to dysbiosis.Further,microbialbased therapies could be also used as an osteoporosis treatment to reverse dysbiosis and restore a healthy and stable microbiome in osteoporotic patients or people at risk of bone disorders (postmenopausal women,elderly population).Despite the great promises of these microbial-based therapies,several challenges need to be addressed before further commercial and clinical applications.Most importantly,a limited number of clinical data is available,which limits their clinical acceptance and eventual commercialization.Therefore,additional clinical trials are necessary to obtain an indepth understanding of the effects of these novel therapies on bone loss in genetically diverse group(s) of individuals and to provide robust evidence of the clinical efficacity.However,there are still many questions to be answered.The key questions include: Is their efficacity comparable with the approved treatment options in the market? Are there any adverse effects associated with their long-term use? Should microbial-based therapies target a specific commensal bacterium (ex:Bifidobacterium,Lactobacillus),or promote the overall gut microbe’s community? In summary,targeting the gut microbiome is an attractive alternative therapy for bone disorders but underlying mechanisms along with clinical outcomes remain unclear and need to be studied further.
Disclosure statement
Jianping Wu is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
- 食品科学与人类健康(英文)的其它文章
- Modifications in aroma characteristics of ‘Merlot’ dry red wines aged in American,French and Slovakian oak barrels with different toasting degrees
- Effect of different drying methods on the amino acids,α-dicarbonyls and volatile compounds of rape bee pollen
- Dynamic changes in physicochemical property,biogenic amines content and microbial diversity during the fermentation of Sanchuan ham
- A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:antioxidant and bile acid-binding capacity
- Yolk free egg substitute improves the serum phospholipid profile of mice with metabolic syndrome based on lipidomic analysis
- Underlying anti-hypertensive mechanism of the Mizuhopecten yessoensis derived peptide NCW in spontaneously hypertensive rats via widely targeted kidney metabolomics

