The eff iciency and safety evaluation of hemoglobin hydrolysate as a non-heme iron fortif ier
Dejing Xue,Shui Jing,Mio Zhng,Ki Shn,René Lmetsch,Chuno Li,
a Key Laboratory of Meat Processing and Quality Control, Ministry of Education, Key Laboratory of Meat Processing, Ministry of Agriculture and Rural Affairs, Jiangsu Collaborative Innovation Center of Meat Production, Processing and Quality Control, College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
b Department of Food Science, University of Copenhagen, Frederiksberg C DK-1958, Denmark
Keywords: Hemoglo bin hydrolysate Non-heme iron Absorption Liver homeostasis Hepcidin
ABSTRACT Hemoglobin hydrolysate is derived from the enzymatic degradation of hemoglobin.This work aimed to evaluate whether hemoglobin hydrolysate promotes the absorption of non-heme iron and the safety of absorbed iron in mice by analyzing the iron binding content,iron circulation,and liver homeostasis.We found that hemoglobin hydrolysate promoted the absorption of non-heme iron with high eff iciency in duodenum by spontaneously binding non-heme iron during digestion,and increased hepatic iron content by up-regulating divalent metal transporter 1,zinc transporter 14,but hepatic iron content only increased at 3 weeks.Duodenal iron entered the blood through ferroportin without restriction at 3 weeks,and excessive iron entered the liver and then affected the hepatocyte membranes permeability and lipid synthesis through oxidative stress.With the prolongation of dietary intervention,the up-regulated hepcidin acted on the ferroportin to restrict excess iron from entering the blood,and then the hepatic homeostasis recovered.In addition,hemoglobin hydrolysate enhanced the hepatic antioxidant capacity.Taken together,hemoglobin hydrolysate has a strong ability to promote the absorption of non-heme iron in vivo,and the absorbed iron is relatively safe due to the regulation of hepcidin.
1.Introduction
In recent years,with the increasing demand for meat,the production of slaughtering by-products has also increased[1].By 2017,the production of animal blood has exceeded 19.8 million tons[2-3].Animal blood,which is composed of plasma (60%) and blood cells(40%),is a high-quality protein resource.The protein accounts for more than 80% of the dry weight of animal blood[4].Hemoglobin is the most abundant protein in the blood (more than 60%),which provide essential amino acids and iron for the body[5].However,hemoglobin is often discarded or used as additives of animal feedstuff due to consumers’ religious beliefs and the heme of hemoglobin that impart a dark-red color and metallic flavor to the product,which caused huge economic losses[5-6].At present,enzymatic hydrolysis is an important method to enhance the commercial value of hemoglobin and separate unpleasant heme from hemoglobin[7].After hydrolysis,active peptides with antioxidant,anti-inf lammatory and antibacterial properties exposed from hemoglobin[7-8].Meanwhile,hemoglobin hydrolysate promoted the absorption of heme-iron[9].However,it is still unknown whether hemoglobin hydrolysate has other biological functions.
Currently,the iron supplementation has become particularly important with more than 3.5 billion people suffering from iron def iciency worldwide[10-11].Compared with non-heme iron,heme iron has higher absorption efficiency[12].However,recent studies have shown that dietary heme (0.25 µmol/g) damage the colon barrier and even induce colorectal cancer in mice[13-14].Therefore,there are safety risks in dietary supplementation with heme iron.In view of the low absorption efficiency of non-heme iron,protein hydrolysates have been widely studied as a non-heme iron fortifier due to their strong binding capacity of non-heme iron and biological safety[15].The carboxyl,hydroxyl and amino groups exposed after protein hydrolysis spontaneously combined with non-heme iron through ionic bonds,hydrogen bonds,or hydrophobic interactions,enhanced the solubility of non-heme iron,and then promoted the absorption of non-heme iron in the duodenum[16],which has been proven in the hydrolysate of animal proteins such as pork and Antarctic krill[17-18],and plant proteins such as chickpea and lentil[19-20].In previous studies,hemoglobin produced more than 50% of small peptides (< 0.5 kDa)after protease A hydrolysis,and these small peptides contained a large number of amino,carboxyl and hydroxyl groups[21].The structure of hemoglobin hydrolysate is similar to the peptides that promote non-heme iron absorption[17,19].Thus,hemoglobin hydrolysate has a potential to be developed as a non-heme iron fortifier.However,it is still unknown whether hemoglobin hydrolysate promotes the absorption of non-heme ironinvivo.
At present,protein hydrolysates have been studied as a non-heme iron fortifier in three aspects,including the preparation of peptide-iron complex,digestive stability of the complex,and the iron absorption efficiency in duodenum (invitroandinvivo)[15-16].However,it remains unknown whether protein hydrolysate efficiently promotes the absorption of non-heme ironinvivowithout the preparation of hydrolysate-iron complexinvitro.In addition,the iron excretionin vivois considered passive and unregulated,and occurs at a basal rate irrespective of iron deficiency or excess[22-23].Thus,it is necessary to carefully consider whether the body’s iron content is at a safe level in terms of iron fortifiers.However,the safety of absorbed iron content has not been evaluated in the existing studies on promoting iron absorption by dietary protein hydrolysates.Therefore,the purpose of this study was to explore whether hemoglobin hydrolysate promote the absorption of non-heme iron in mice without the preparation of hydrolysate-iron complexinvitroand evaluate the safety of absorbed iron in mice.The findings provide a guidance for the development of hemoglobin hydrolysate as iron fortifier and high-value utilization of animal blood.
2.Materials and methods
2.1 Preparation of hemoglobin hydrolysate and heme
Hemoglobin hydrolysate and heme were prepared as described by Fu et al.[21].Porcine hemoglobin was dissolved in 2 volumes of water,and then the pH was adjusted to 6.8.Protease A (Amano,Nagoya,Japan) was added to the hemoglobin solution at a weight basis of 0.5% (m/m),and was incubated at 50 °C with continuous shaking for 4 h.The enzyme was inactivated by raising the temperature to 95 °C for 20 min.After the mixture was cooled to room temperature,the pH was adjusted to 4.0 with food grade H2SO4,and the mixture was centrifuged (8 000 ×g,15 min).The precipitate was spray dried (inside 180 °C,outlet 80 °C) to obtain heme,and the supernatant was spray dried (inside 180 °C,outlet 80 °C) to obtain hemoglobin hydrolysate.
2.2 Non-heme iron binding capacity analysis of hemoglobin hydrolysate in vitro
2.2.1 Non-heme iron binding activity
The determination of non-heme iron binding activity of hemoglobin hydrolysate was referred to Wu et al.[24]with minor modifications.Briefly,1 mL hemoglobin hydrolysate (0.5,1,2,4,8,16 mg/mL) was mixed with 20 µL ferrous sulfate (2 mmol/L),and incubated at 37 °C for 1 h.Then the mixture was centrifuged to obtain the supernatant (10 000 ×g,5 min).The 50 µL ferrozine (2 mmol/L)was added to the supernatant,mixed gently,stood for 10 min and the absorbance was measured at 562 nm.The non-heme iron binding capacity was calculated by equation (1).
WhereA0is the absorbance of water without hemoglobin hydrolysate;A1is the absorbance of hemoglobin hydrolysate.
2.2.2 Determination of iron content in digestive products
The digestion of hemoglobin hydrolysate referred to the method of Jiang et al.[25].Briefly,the hemoglobin hydrolysate (5 g) was mixed with ferrous sulfate (75 mg).The simulated salivary fluid (SSF) was added to the mixture,and incubated at 37 °C for 2 min.The simulated gastric fluid (SGF) and CaCl2were added to the mixture,and the pH was adjusted to 3.0 with 6.0 mol/L HCl,and then the pepsin was added to the solution and incubated at 37 °C for 2 h.The pH of the mixture was adjusted to 7.0 with 6.0 mol/L NaOH to inactivate pepsin.The simulated intestinal fluid (SIF),CaCl2and pancreatin were added to the mixture,and incubated at 37 °C for 2 h,and the reaction was stopped at 95 °C.The liquids of gastric and intestinal digestion were collected,and the peptides were extracted with anhydrous ethanol and dried for subsequent determination of iron content.
The iron contents in digestive products were determined as described by Ajsuvakova et al.[26]with minor adjustments.Briefly,the digestive products were mixed with nitric acid (16 mol/L) at a ratio of 25:1 (m/m),and reacted in a microwave digester for 30 min (Milestone,Lab Tech,Italy).Then,the mixtures were made up to 20 mL with ultrapure water.The iron content was measured by an inductively coupled plasmamass spectrometer (ICP-MS,NexION 2000,PerkinElmer,USA).The iron content was calculated according to the standard curve.
2.3 Antioxidant capacity of hemoglobin hydrolysate in vitro
2.3.1 DPPH radical scavenging activity
The DPPH scavenging activity of hemoglobin hydrolysate was determined by the method of Chen et al.[27].Briefly,0.5 mL DPPH(0.2 mmol/L) was added to a series of 0.5 mL hemoglobin hydrolysate(1,2.5,5,7.5 and 10 mg/mL).After mixing,the solution stood in the dark for 30 min.Then,the absorbance of mixture was measured at 517 nm.The DPPH radical scavenging activity was calculated by equation (2).
WhereA0is the absorbance of water without hemoglobin hydrolysate;A1is the absorbance of hemoglobin hydrolysate.
2.3.2 Superoxide anion radical(·)scavenging activity
WhereKCis the slope of water without hemoglobin hydrolysate;KSis the slope of hemoglobin hydrolysate.
2.3.3 Hydroxyl free radical(·OH)scavenging activity
The ·OH scavenging activity of hemoglobin hydrolysate was determined according to the method of Chen et al.[27].Briefly,1 mL ferrous sulfate (2 mmol/L) and 1 mL hydrogen peroxide (H2O2,0.3%) were added to 1 mL hemoglobin hydrolysate (1,2.5,5,7.5 and 10 mg/mL).The solution was mixed and incubated at 37 °C for 10 min.After mixing with 1 mL salicylic acid (2 mmol/L),the solution was mixed,incubated at 37 °C for 30 min and centrifuged at 6 000 ×gfor 10 min.The absorbance of supernatant was measured at 510 nm.The ·OH scavenging activity was calculated by equation (4).
WhereA0is the absorbance of water without hemoglobin hydrolysate;A1is the absorbance of hemoglobin hydrolysate.
2.4 Animals study
2.4.1 Animal feeding
Healthy male C57BL/6J mice (age: 6 weeks,80 in total) were obtained from Hangzhou Ziyuan Experimental Animal Technologies Co.,Ltd.(Hangzhou,China).Before diet intervention,mice were acclimatized to the new environment with the AIN 93G diet (Trophic,Nantong,China).Then mice were randomly assigned to one of four diet groups (2 mice per cage,20 mice per group) for 8 weeks,i.e.,casein diet (14% casein,Con),hemoglobin hydrolysate diet(3.39% hemoglobin hydrolysate+10.61% casein,HHb),heme diet(0.12% heme+13.88 % casein,Heme) and intact hemoglobin diet(3.39% intact hemoglobin+10.61% casein,Hb).Casein was purchased from a commercial company (Ruian,Shanghai,China).Ferric citrate was added in Con and HHb groups to make the iron content in the diet to be 100 µg/g,and no iron was added in Heme and Hb groups.
The diets were prepared according to the AIN-93M diet formulation (Trophic,Nantong,China),which includes 14%protein,46.06% cornstarch,15.33% dextrin,9.89% sucrose,3.96%soybean oil,4.95% cellulose,3.46% minerals,0.25% choline bitartrate,0.99% vitamins,0.18%L-cystine,and 0.0008% tertbutyl hydroquinone[29].All mice lived in a specific-pathogen-free environment((20.0 ± 0.5) °C,(60 ± 10)% of humidity,12 h light cycle).All experimental operations were in accordance with the relevant provisions of the Ethical Committee of Experimental Animal Center of Nanjing Agricultural University (SYXK (Su) 2011-0037,Nanjing,China).
2.4.2 Sample collection
Mice were killed after 3 and 8 weeks of diet intervention.Blood was collected and centrifuged (3 000 ×g,30 min) to obtain serum(the supernatant).The serum was kept at -80 °C for determining aspartate aminotransferase (AST) and alanine aminotransferase(ALT).The liver was weighed and divided into large lobe (used in all experiments) and small lobe.The large lobe was cut into pieces(5 mm × 5 mm × 5 mm) for histological analysis.The duodenum was weighed and divided into upper (used in all experiments) and lower parts.The remaining liver and duodenum were stored at -80 °C for further analyses.
2.4.3 Sample preparations
The liver and duodenum samples (20 mg) were homogenized in 180 µL of physiological saline solution (0.86% NaCl) at 4 °C,8 000 r/min for 30 s and repeated for 3 times (Precellys Evolution,Bertin,French).The supernatant was obtained for further analyses after centrifugation at 5 000 ×g.
2.4.4 Serum basic indexes
The AST and ALT activities in serum were measured by commercial kits (C009-2-1,C010-2-1,Jiancheng,Nanjing,China).Briefly,AST reacted withα-ketoglutarate and aspartate for 30 min to produce glutamate and oxaloacetate that were automatically decarboxylated to pyruvate.ALT reacted with alanine andα-ketoglutarate for 30 min to generate pyruvate and glutamate.Then,2,4-dinitrophenylhydrazine (DNPH) reacted with pyruvate to generate phenylhydrazone that appeared reddish-brown color under an alkaline condition.The AST and ALT activities were calculated according to the absorbance at 505 nm.
The hepcidin and unsaturated iron binding capacity (UIBC)in serum were measured by enzyme-linked immunosorbent assay(ELISA) (RD-RX21431,Ruidahenghui,Beijing,China).Briefly,the serum and the standard substance respectively were added to the microplates that were pre-coated with the antibody of target substance,and then HRP-labeled secondary antibodies were added to the microplates.Then the reacting solutions were incubated at 37 °C for 60 min.The microplates were washed with the detergent for 1 min and repeated for 5 times.Then,3,3’,5,5’-tetramethylbenzidine(TMB) and H2O2were added to microplates,and the mixtures stood at 37 °C for 15 min.During incubation,TMB reacted with H2O2and catalase,and were converted into yellow color.The level of UIBC and hepcidin was calculated according to the absorbance at 450 nm.
2.4.5 Determination of iron content in tissues
The iron contents in duodenum and liver were determined with ICP-MS.The specific operation was described in subsection 2.2.2.
2.4.6 Determination of divalent metal transporter 1(DMT1)and ferroportin(FPN)
The DMT1 content in liver samples and the FPN content in duodenum samples were determined with the ELISA method(Jiancheng,Nanjing,China).The specific principle and operation steps were described in subsection 2.4.4.
2.4.7 Hepatic oxidative stress indexes
2.4.8 Hepatic lipid metabolism indexes
The contents of triglycerides (TG),total cholesterol (T-CHO),low density lipoprotein (LDL),cholesterol ester (CE) and phosphatidylcholine (PC) in liver samples were determined by commercial kits (A110-1-1,A111-1-1,A113-1-1,ELISA,Jiancheng,Nanjing,China).Briefly,the TG in liver samples was transformed to H2O2after reacting with lipase,glycerol kinase and glycerol 3-phosphate oxidase.The CHO in liver samples produced H2O2after the reaction with cholesterol oxidase.The LDL in liver samples were converted into H2O2under surfactant,cholesterol esterase and cholesterol oxidase.H2O2reacted with 4-aminoantipyrine,phenol and peroxidase to generate red quinoids.The contents of TG,T-CHO and LDL in liver samples were calculated according to the absorbance at 510 nm.The CE and PC contents were determined by ELISA.The specific principle and operation steps were the same as the hepcidin and the UIBC in serum.
2.4.9 Quantitative real-time polymerase chain reaction(RT-qPCR)
RT-qPCR was operated according to the method of Shi et al.[30].The extraction of total RNA and the reverse transcription of RNA were performed with commercial kits (Vazyme,Nanjing,China).The mRNA levels of target genes were quantified with a ChamQ SYBR qPCR Master mixture on a real-time PCR system (QuantStudio 6 Flex,Applied Biosystems,Foster,CA).The target genes includeDMT1,zinc transporter 14 (ZIP14),transferrin receptor 1 (TFR1),transferrin receptor 2 (TFR2),poly(C)-binding protein 1 (PCBP1),ferritin,ceruloplasmin (CP),and hepcidin.Primers are listed in Table 1.The Con group was set as control,and the relative abundance of each mRNA was analyzed using the 2-ΔΔCtmethod withβ-actin for normalization.

Table 1 The sequence of the primers in RT-qPCR analysis.
2.4.10 Hematoxylin-eosin(H&E)staining
H&E staining was manipulated according to the method of Xie et al.[31].The liver samples were fixed in 4% paraformaldehyde buffer for 24 h,then embedded in paraffin and cut into sections(5 µm) by a microtome (RM2016,Leica,Germany).The sections were deparaffinized and rehydrated with xylene and ethanol,then stained with hematoxylin solution for 4 min,differentiated with hydrochloric acid ethanol solution (1%) for 30 s,and rinsed with tap water for 30 s.Then sections were dehydrated with alcohol for 5 min,stained with eosin for 5 min,and dehydrated with anhydrous ethanol for 3 times(5 min each) and xylene for 2 times (5 min each).Finally,images were obtained with a light microscope (BX51,Olympus,Tokyo,Japan).
2.4.11 Oil red staining
Oil red staining was conducted according to the method of Hao et al.[32]with minor modification.The liver samples were embedded with optimal cutting temperature compound (OCT),and cut into sections(10 µm) on a microtome cryostat (CM 1900,Leica,GER).The sections were fixed in paraformaldehyde for 30 min,standing in 60%isopropyl alcohol for 3 min,and then reacting with oil red O dye for 5 min.After that,the sections were cleaned in 60% isopropyl alcohol for 30 s,deionized water for 10 s,and then dried naturally.The sections were observed under a light microscope (BX51,Olympus,Tokyo,Japan) and images were analyzed by the Image-Pro Plus software (version 6.0,Rockville,MD,USA).
2.5 Statistical analysis
The effects of diet type and feeding time on the variables were evaluated by factorial analysis of variance (ANOVA).Duncan’s multiple-range test was performed to compare the least squares means(P< 0.05) by SAS software (version 8.0,Cary,NC,USA).Figures were generated with GraphPad Prism software (version 8.0.1,San Diego,CA,USA).
3.Results
3.1 Hemoglobin hydrolysate exhibited strong non-heme iron binding capacity in an in vitro condition
Hemoglobin hydrolysate had a strong ability to bind non-heme ironinvitro,which was proportional to the hemoglobin hydrolysate concentration (P< 0.05,Fig.1A).During simulated digestion,hemoglobin hydrolysate could bind non-heme iron.Compared with undigested HHb (0.51 µg/mg),the non-heme iron binding content of HHb respectively increased to 5.89 and 7.59 µg/mg during gastric and intestinal digestion (P< 0.05,Fig.1B).

Fig.1 The non-heme iron binding capacity of hemoglobin hydrolysate in vitro.(A) The iron binding activity.(B) The iron binding content in simulated digestion.a-f,indicate differences among samples (P < 0.05);n=10 in each group for panels A-B.
3.2 Hemoglobin hydrolysate had antioxidant capacity in an in vitro condition
Hemoglobin hydrolysate showed good antioxidant capacityin vitro.With the increased concentration of hemoglobin hydrolysate,the DPPH radical scavenging activity increased from 26.22% to 64.94%,the O2-· scavenging activity increased from 26.70% to 87.37%,and the ·OH scavenging activity increased from 10.88% to 70.80% (P< 0.05,Fig.2).
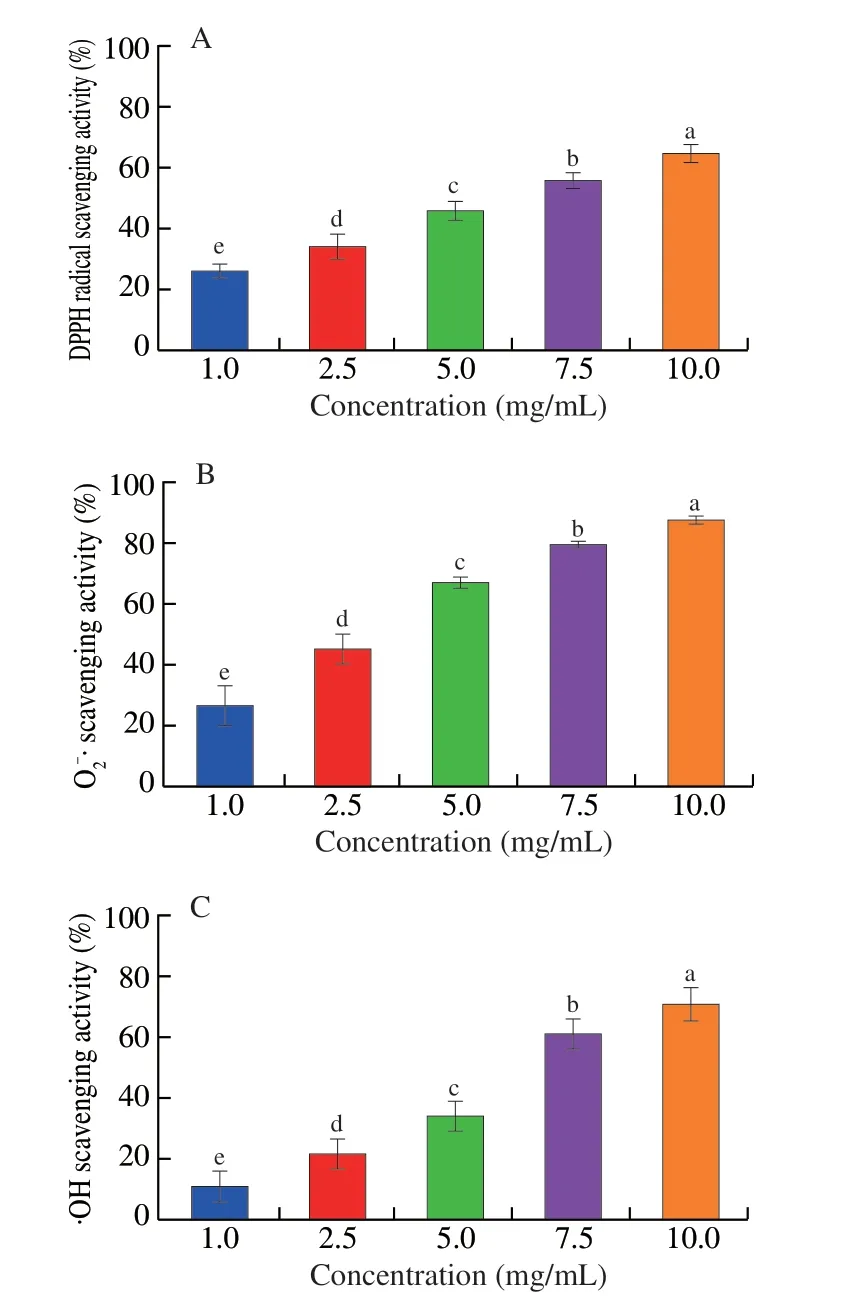
Fig.2 The antioxidant capacity of hemoglobin hydrolysate in vitro.(A) The DPPH radical scavenging activity.(B) The O-2· scavenging activity.(C) The·OH scavenging activity.a-e,indicate differences among samples (P < 0.05);n=10 in each group for panels A-C.
3.3 Hemoglobin hydrolysate diet highly increased the iron level in mice
The diet type and feeding time showed significant impacts on the iron content in liver and duodenum,UIBC in serum,and significant interactions were also observed in hepatic iron content (P< 0.05,Table 2).Compared with the Con group,the iron content in duodenum and liver significantly increased in the HHb,Heme and Hb groups at 3 weeks and the HHb group showed the greatest value in hepatic iron content (P< 0.05,Figs.3A,C).The UIBC level in serum lower in the HHb,Heme and Hb groups than the Con group (P< 0.05,Fig.3B).These results indicated that diet type affected the iron absorption at 3 weeks.As feeding time extended,the duodenal iron significantly increased in the HHb,Heme and Hb groups (P< 0.05,Fig.3A),and the hepatic iron content significantly decreased in the same groups(P< 0.05,Fig.3C),the UIBC level in serum increased in the Heme and Hb groups (P< 0.05,Fig.3B).Meanwhile,there was no significant difference in hepatic iron and UIBC between HHb and Con groups at 8 weeks.
3.4 Hemoglobin hydrolysate affected iron circulation in liver and duodenum

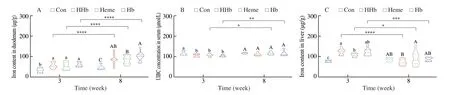
Fig.3 The effect of hemoglobin hydrolysate on body iron level.(A) The iron content in duodenum.(B) The UIBC concentration in serum.(C) The iron content in liver.Con: casein group;HHb: hemoglobin hydrolysate group;Heme: heme group;Hb: intact hemoglobin group.a-c,indicate differences among samples at 3 weeks (P < 0.05);A-C,indicate differences among samples at 8 weeks (P < 0.05);n=10 in each group for panels A-C.*P < 0.05,**P < 0.01,***P < 0.001,****P < 0.000 1.

Fig.4 The effect of hemoglobin hydrolysate on liver iron circulation.(A,C-H) The mRNA level of genes involved in iron circulation.(B) The DMT1 content.Con: casein group;HHb: hemoglobin hydrolysate group;Heme: heme group;Hb: intact hemoglobin group.a-c,indicate differences among samples at 3 weeks(P < 0.05);A-C,indicate differences among samples at 8 weeks (P < 0.05);n=10 in each group for panels A-H.*P < 0.05,**P < 0.01,***P < 0.001,****P < 0.000 1.
The diet type affected the abundances of iron circulation-related genes and proteins in liver (Table 2).The abundances of iron inputrelated genes includingDMT1,ZIP14,TFR1andTFR2in the HHb group were higher than the Con group at 3 weeks (P< 0.05),and the mRNA levels ofZIP14,TFR1andTFR2were not different among HHb,Heme and Hb groups (Figs.4A,C-E).Nevertheless,the mRNA and protein levels of DMT1 were higher in the HHb groups than the Con,Heme and Hb groups during the whole feeding period (P< 0.05,Figs.4A-B).These results indicated that HHb diet increased hepatic iron content by upregulating the expression of iron transporters.The abundances of iron storage-related genes includingPCBP1,ferritinandCPin the HHb group were higher than the Con group,but lower than the Heme and Hb groups at 3 weeks (P< 0.05,Figs.4F-H).
Additionally,feeding time had a significant impact on the hepcidin levels in liver and serum and the FPN level in duodenum(P< 0.05,Table 2),which showed a time-dependent increase for hepcidin and decrease for FPN during feeding in the HHb group (Fig.5).
3.5 Hemoglobin hydrolysate affected oxidative stress and antioxidant capacity in the liver
The MDA content in liver was higher in the HHb,Heme and Hb groups than the Con group at 3 weeks (P< 0.05,Fig.6A),but no significant difference was found in the MDA content among HHb,Heme and Hb groups (P> 0.05,Fig.6A).Feeding time had a significant impact on the MDA content in liver (P< 0.05,Table 2).As feeding time extended from 3 weeks to 8 weeks,the MDA content in liver was significantly reduced in the HHb,Heme and Hb groups(P< 0.05,Fig.6A).Additionally,the diet type significantly affected the CAT activity,O2-· scavenging activity and ·OH scavenging activity in liver (P< 0.05,Table 2).The CAT activity,O2-· scavenging activity and ·OH scavenging activity of the HHb group were higher than Con,Heme and Hb groups during the whole feeding period (P< 0.05,Figs.6B-D).These results suggested that the liver of HHb group appeared oxidative stress at 3 weeks,but the oxidative stress disappeared at 8 weeks.And the antioxidant capacity of HHb group was significantly enhanced during dietary feeding.
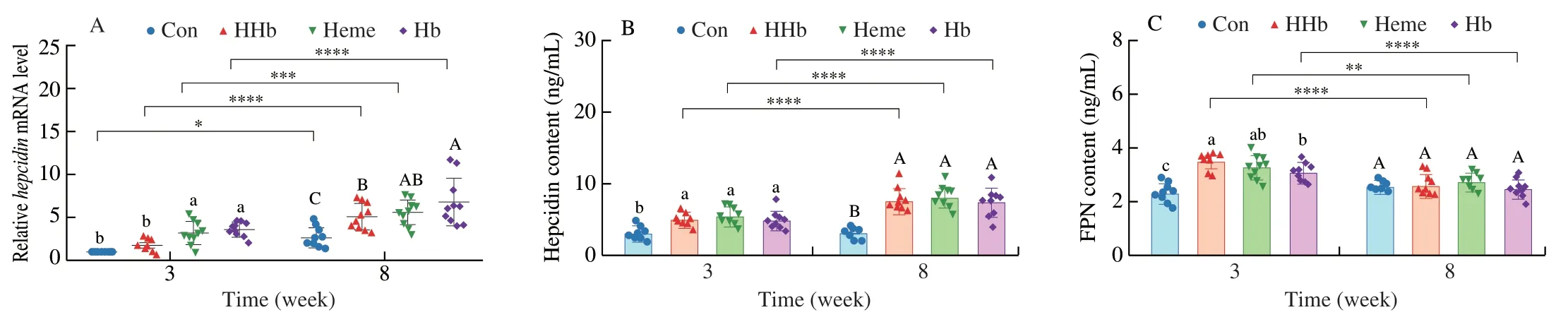
Fig.5 The effect of hemoglobin hydrolysate on hepcidin and FPN.(A) The mRNA level of hepcidin in liver.(B) The hepcidin content in serum.(C) The FPN content in duodenum.Con: casein group;HHb: hemoglobin hydrolysate group;Heme: heme group;Hb: intact hemoglobin group.a-c,Indicate differences among samples at 3 weeks (P < 0.05);A-C,Indicate differences among samples at 8 weeks (P < 0.05);n=10 in each group for panels A-I.*P < 0.05,**P < 0.01,***P < 0.001,****P < 0.000 1.
3.6 Hemoglobin hydrolysate affected hepatocyte membrane integrity
The diet type and feeding time affected the AST and ALT activities in serum,and the PC content in liver (Table 2).At 3 weeks,the AST and ALT activities of the HHb,Heme and Hb groups were higher than the Con group (P< 0.05,Figs.7A-B),which indicated that the leakage of cell membrane appeared in the HHb,Heme and Hb groups.Meanwhile,the PC content of the HHb,Heme and Hb groups was lower than those of the Con group (P< 0.05,Fig.7C).No significant difference was found in the AST and ALT activities and PC content among the HHb,Heme and Hb groups (P> 0.05,Figs.7A-C).As feeding time increased from 3 weeks to 8 weeks,the AST and ALT activities of the HHb,Heme and Hb groups were significantly decreased,and the PC contents of the HHb,Heme and Hb groups were significantly increased (P< 0.05,Figs.7A-C).These results indicated that the leakage of cell membrane disappeared in the HHb,Heme and Hb groups at 8 weeks.The similar hepatocyte changes were also observed on H&E staining.The H&E staining showed that cell swelling and slight vacuoles appeared in liver tissues of the HHb,Heme and Hb groups at 3 weeks.Nevertheless,cell swelling and slight vacuoles disappeared at 8 weeks (Fig.7D).
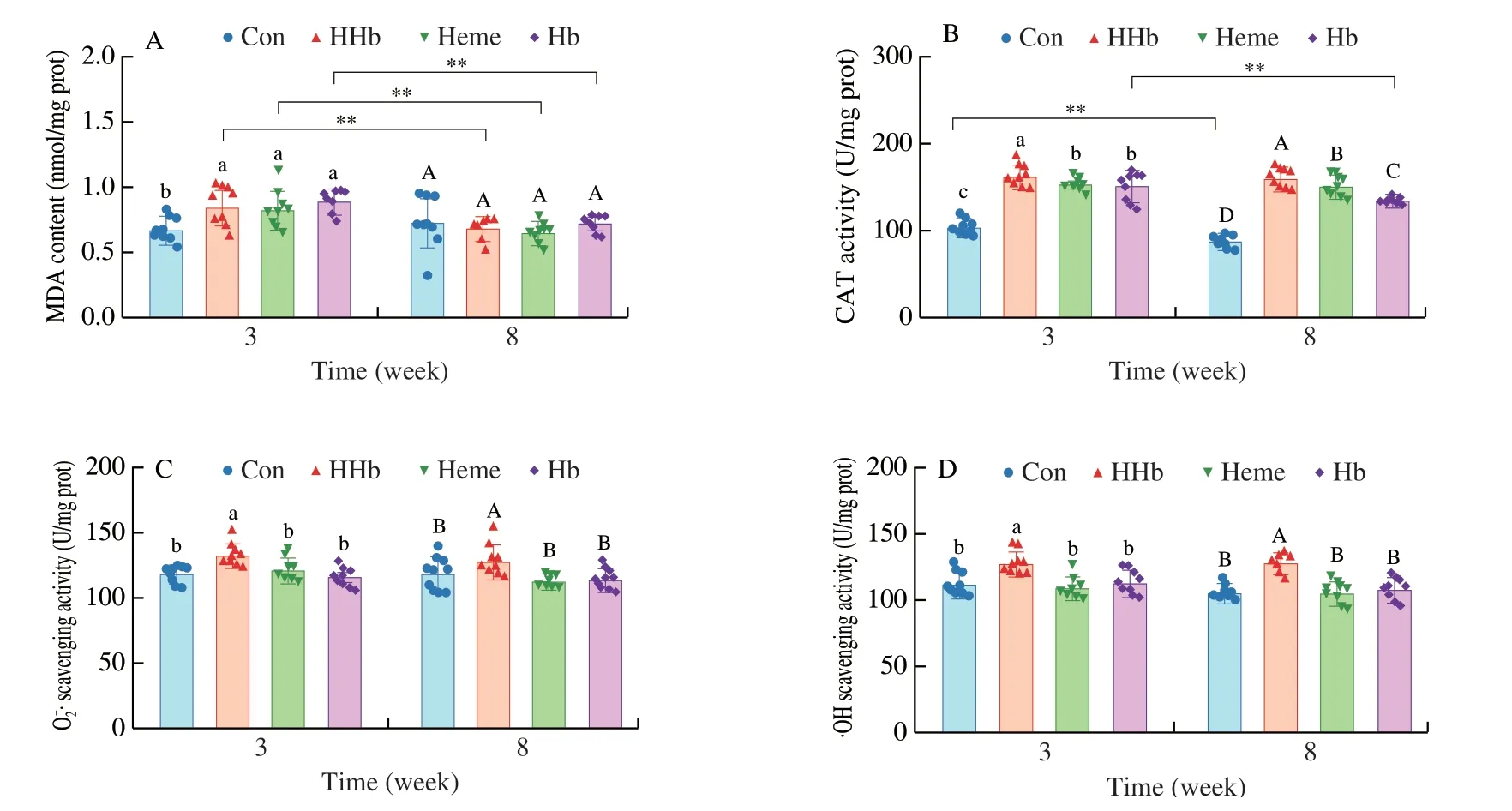
Fig.6 The effect of hemoglobin hydrolysate on liver oxidative stress.(A) The MDA content.(B) The CAT activity.(C) The scavenging activity.(D) The·OH scavenging activity.Con: casein group;HHb: hemoglobin hydrolysate group;Heme: heme group;Hb: intact hemoglobin group.a-c,indicate differences among samples at 3 weeks (P < 0.05);A-D,indicate differences among samples at 8 weeks (P < 0.05);n=10 in each group for panels A-D.**P < 0.01.
3.7 Hemoglobin hydrolysate affected hepatic lipid synthesis
The diet type significantly affected the lipid synthesis in liver(P< 0.05,Table 2).The TG,T-CHO,LDL and CE contents of HHb,Heme and Hb groups were higher than the Con group at 3 weeks(P< 0.05),and no significant difference was found among the HHb,Heme and Hb groups (P> 0.05,Figs.8A-D).Feeding time also had a significant impact on lipid synthesis in liver,with a time-dependent reduction in the TG and CE contents (P< 0.05,Table 2).There was no significant difference in TG,T-CHO and CE contents among the Con,HHb,Heme and Hb groups at 8 weeks (P> 0.05,Figs.8A-B,D).In addition,the Heme group showed the higher LDL level than other groups at 8 weeks (P> 0.05,Fig.8C).These results indicated that the HHb,Heme and Hb diets increased lipid synthesis in liver after feeding for 3 weeks,and the up-regulation of lipid synthesis disappeared during subsequent 5-week feeding.Oil red O staining observations showed the similar results (Fig.8E).

Fig.8 The effect of hemoglobin hydrolysate on liver lipid synthesis.(A) The TG content.(B) The T-CHO content.(C) The LDL content.(D) The CE content.(E) The oil red O staining image of liver tissue (magnifications ×100).Con: casein group;HHb: hemoglobin hydrolysate group;Heme: heme group;Hb: intact hemoglobin group.a-b,indicate differences among samples at 3 weeks (P < 0.05);A-B,indicate differences among samples at 8 weeks (P < 0.05);n=10 in each group for panels A-D.*P < 0.05,**P < 0.01.
4.Discussion
Iron fortifier,as an important method of treating iron deficiency,has good commercial value[10-11].Many proteolytic peptides have been developed as non-heme iron fortifiers because of their ability to promote the non-heme iron absorption through strong binding capacity of non-heme iron[17-18].At present,the utilization ratio and commercial value of hemoglobin are low[5-6].Hemoglobin hydrolysate is an important product of hemoglobin commercial processing.If hemoglobin hydrolysate can be developed into a non-heme iron fortifier,the commercial value of hemoglobin may be enhanced.However,it is still unknown whether hemoglobin hydrolysate promote the absorption of non-heme ironinvivo.
Dietary iron is absorbed in the duodenum and transferred to the liver through the portal vein for storage,and the hepatic iron will be utilized when the iron supply is not sufficient[33-34].Thus,the duodenum and liver play a key role in iron circulation[34].In this study,the iron content in the duodenum and liver of HHb group were significantly higher than the Con group at 3 weeks,and hepatic iron content in HHb group was higher than Heme and Hb groups.In previous studies,heme and hemoglobin,as the main sources of biological iron in diet,were often considered to have higher bioavailability than inorganic iron[35].Therefore,hemoglobin hydrolysate is able to enhance the absorption of non-heme iron in diet with a high efficiency.
The ability of hemoglobin hydrolysate to promote absorption of non-heme iron may be attributed to the special amino acids and peptides produced from hemoglobin hydrolysis.The poor absorption efficiency of non-heme ironinvivois due to its low solubility[36].The carboxyl,hydroxyl and amino groups of amino acids and peptides increase the solubility of non-heme iron in combination with it[18,37].For example,a heptapeptide (SVNVPLY) derived from barley spontaneously formed complexes with non-heme iron,and then significantly increased iron absorption in Caco-2 cells compared to ferric sulfate[38].Meanwhile,meat protein hydrolysate also promoted non-heme iron absorption in a rat intestinal epithelial tissue model through binding non-heme iron to improve its solubility[17].In our study,hemoglobin hydrolysate spontaneously bound non-heme iron during simulated digestion.Such spontaneous binding may enhance the solubility of non-heme iron in the intestinal cavity,thereby improved the absorption of non-heme iron in mice.
Diet-based iron exists in blood in the form of transferrin-bound iron (TBI) or nontransferrin-bound iron (NTBI)[39].As a basic index reflecting the potential iron-binding capacity of transferrin in serum[40],the reduction of UIBC indicated that hemoglobin hydrolysate increased the content of NTBI in the serum.Free Fe2+in NTBI mainly enters the liver through DMT1 and ZIP14[34].The increase of DMT1 content indicated that hemoglobin hydrolysate promoted more free Fe2+to get into the liver.In general,free Fe2+produces hydroxyl free radicals by Fenton reaction,and induces body impairment through oxidative stress[41].When free Fe2+is highly accumulated in liver,CP that catalyzes the transformation of Fe2+into Fe3+,and PCBP1 and ferritin that transport and store free iron will be released to protect liver from oxidative stress[42-43].In this study,the mRNA levels of hepaticCPandPCBP1of the HHb group were increased at 3 weeks,but the high expression of these proteins did not prevent oxidative stress induced by free Fe2+,which was shown by an increase in MDA content.This may be because HHb promotes too much nonheme iron to enter the liver,which exceeding the binding capacity of ferritin.However,the hepatic antioxidant system such as free radical scavenging ability and CAT activity was significantly activated in mice fed with hemoglobin hydrolysate.This may be related to the strong scavenging ability of DPPH radical,and ·OH in hemoglobin hydrolysate,which can be proven byinvitroexperiments.
Enzymatic hydrolysis is an effective way to expose active peptides in proteins,which has been proven in many proteins[44].For example,the porcine hemoglobin hydrolysate produced by papain hydrolysis showed higher oxygen radical scavenging capability[7].The antioxidant activity of peptides is mainly associated with the type and sequence of amino acids,relative molecular weights and protein hydrolysis degree[45-46].The peptides with low molecular weight(< 2 kDa) often show stronger antioxidant activity than macromolecular proteins and peptides[45].Invivo,low molecular weight peptides are more likely to expose antioxidant sites during digestion,and the digested peptides are more likely to pass through the intestinal barrier in a complete form and enter the blood circulation system,then affecting the activities in different organs[46].In the previous study,hemoglobin produced 80% of small peptide fractions(< 2 kDa) after protease A hydrolysis[21],which may account for the high antioxidant function of hemoglobin hydrolysate.Furthermore,the combination of non-heme iron to the peptide also helps to enhance the antioxidant activity of peptides[47].Therefore,antioxidant peptides in hemoglobin hydrolysate may be the material basis for inducing the increased antioxidant capacity in liver.
When oxidative stress occurs,cell homeostasis is often unbalanced,accompanying with the increase of cell membrane permeability,extracellular substance extravasation,and abnormality of cell morphology[48-49].PC is a vital lipid component of cell membrane,and the decrease of its content often results in abnormal increase of cell membrane permeability[50].Lipid peroxidation induced by oxidative stress could reduce the content of PC on cell membrane.In response to the reduction of PC content caused by oxidative stress,cells spontaneously increase the synthesis of lipid droplets[51].Lipid droplets are composed of TG and T-CHO,which play key roles in regulating membrane lipid components,preventing lipid peroxidation and removing damaged proteins and lipids to maintain the dynamic balance of cell membrane and organelles[52].Thus,the increase of hepatic lipid content in mice fed with hemoglobin hydrolysate may be a self-regulation of liver on oxidative stress.
Hepcidin that is secreted by the liver is a key substance in the regulation of body’s iron circulation[53].It regulates the body’s iron circulation by internalizing and degrading the FPN that is only iron output channel in duodenal cell[54].When the iron content exceeds the upper limit of usage and storage,hepcidin is released from liver and acts on the FPN on duodenal surface to regulate iron content[55].Therefore,the high expression of hepcidin was responsible for the changes of hepatic iron content.With the prolongation of dietary intervention,the iron that beyond the body’s need was limited to enter the blood circulation under the regulation of hepcidin.Furthermore,the decrease of lipid droplets content may be due to the normalization of hepatic iron levels.After dietary feeding for 8 weeks,hepatocyte homeostasis including the PC content returns to normal due to the regulation of hepcidin,so the up-regulated expression of lipid droplets became unnecessary.
In summary,hemoglobin hydrolysate promoted the absorption of non-heme iron with high efficiency in mice by binding non-heme iron during digestion.In the short term,the duodenal iron directly entered the blood without restriction,which affected the liver homeostasis through oxidative stress.With feeding time prolonged,the release of duodenal iron was controlled under the regulation of hepcidin and then liver homeostasis restored,but the absorption of non-heme iron in duodenum was not affected by hepcidin.Therefore,hemoglobin hydrolysate has a potential to be a non-heme iron fortifier,and has a certain safety because of the regulation of hepcidin.The results are helpful to provide a guidance for the high-value application of hemoglobin product.
Conflict of interests
None of the authors declare a conflict of interest.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (32072211) and Jiangsu Province Department of Education (Innovation Group of Meat Nutrition and Biotechnology).
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

