COVID-19 vaccination produces exercise-responsive SARS-CoV-2 specific T-cells regardless of infection history
Kyle A.Smith,Tiffny M.Züñi,Forrest L.Bker,Helen Bttinh,Chrles R.Pedlr,Shne C.Buress,Michel P.Gustfson,Emmnuel Ktsnis,Richrd J.Simpson,h,i,
a School of Nutritional Sciences and Wellness,The University of Arizona,Tucson,AZ 85721,USA
b Department of Pediatrics,The University of Arizona,Tucson,AZ 85724,USA
c Faculty of Sport,Allied Health and Performance Science,St.Mary’s University,Twickenham TW1 4SX,UK
d Institute of Sport Exercise and Health,University College London,London WC1E 7HU,UK
e Department of Animal and Comparative Biomedical Sciences,The University of Arizona,Tucson,AZ 85721,USA
f The University of Arizona Cancer Center,The University of Arizona,Tucson,AZ 85719,USA
g Laboratory Medicine and Pathology,Mayo Clinic Arizona,Phoenix,AZ 85054,USA
h Department of Immunobiology,The University of Arizona,Tucson,AZ 85724,USA
i Department of Medicine,The University of Arizona,Tucson,AZ 85724,USA
j Department of Pathology,The University of Arizona,Tucson,AZ 85724,USA
Abstract Background:The mobilization and redistribution of severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)specific T-cells and neutralizing antibodies(nAbs)during exercise is purported to increase immune surveillance and protect against severe coronavirus disease 2019(COVID-19).We sought to determine if COVID-19 vaccination would elicit exercise-responsive SARS-CoV-2 T-cells and transiently alter nAb titers.Methods:Eighteen healthy participants completed a 20-min bout of graded cycling exercise before and/or after receiving a COVID-19 vaccine.All major leukocyte subtypes were enumerated before,during,and after exercise by flow cytometry,and immune responses to SARS-CoV-2 were determined using whole blood peptide stimulation assays,T-cell receptor(TCR)-b sequencing,and SARS-CoV-2 nAb serology.Results:COVID-19 vaccination had no effect on the mobilization or egress of major leukocyte subsets in response to intensity-controlled graded exercise.However,non-infected participants had a significantly reduced mobilization of CD4+and CD8+naive T-cells,as well as CD4+central memory T-cells,after vaccination (synthetic immunity group);this was not seen after vaccination in those with prior SARS-CoV-2 infection(hybrid immunity group).Acute exercise after vaccination robustly mobilized SARS-CoV-2 specific T-cells to blood in an intensity-dependent manner.Both groups mobilized T-cells that reacted to spike protein;however,only the hybrid immunity group mobilized T-cells that reacted to membrane and nucleocapsid antigens.nAbs increased significantly during exercise only in the hybrid immunity group.Conclusion:These data indicate that acute exercise mobilizes SARS-CoV-2 specific T-cells that recognize spike protein and increases the redistribution of nAbs in individuals with hybrid immunity.
Keywords: Anti-viral;COVID-19;Exercise immunology;SARS-CoV-2;T-Cells;Vaccine
1.Introduction
Enhanced immunity against viral infection has been attributed to healthy lifestyle factors such as engagement in regular physical activity.1,2Participation in moderate-to-vigorous intensity exercise has been shown to elicit several responses that improve anti-viral immune surveillance,including exchange of natural killer (NK) cells and virus-specific T-cells (VSTs)between the tissues and peripheral circulation as well as the augmented redistribution of immunoglobulins.1,3-5Indeed,the protective benefits of exercise against viral disease have been demonstrated in animal models6-8and have become evident in humans during the coronavirus disease 2019 (COVID-19)pandemic,as individuals who regularly exercise have shown improved clinical outcomes and reduced symptoms following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)infection.9-14
Single exercise bouts elicit transient immune responses that may protect against infection.Among these is the extensive,rapid mobilization and redistribution of effector lymphocytes(e.g.,NK cells,CD8+T-cells,and gd T-cells) with high lytic function and migratory capacity.1,4More specifically,exercise preferentially mobilizes VSTs with enhanced proliferative properties that may increase lymphocyte trafficking and inhibit the reactivation of latent viruses (e.g.,cytomegalovirus and Epsteinarr virus).15-17These preferentially mobilized VSTs exhibit increased responsiveness to viral peptide stimulationex vivoand,therefore,may exert potent anti-viral responses upon reintroduction to viral antigens.16,17Consequently,the summation of acute exercise bouts (i.e.,regular exercise) may routinely increase immunosurveillance against viral pathogens,providing beneficial anti-viral health effects over the long term.1,5Existing evidence indicates that regular exercise may not only reduce the risk of acquiring respiratory infections but also decreases the severity of symptoms.18,19
While exercise has been shown to promote anti-viral immunity,the mechanisms by which exercise can ameliorate symptoms and enhance immunity against SARS-CoV-2 are still unknown.Our group was of the first to report on the immune responses to acute exercise following natural SARS-CoV-2 infection and reported that exercise mobilizes functional SARS-CoV-2 specific T-cells and increases transportation of neutralizing antibodies (nAbs).20,21However,it is unknown whether vaccination in the absence of infection elicits similar responses.
In the present study,we compared the exercise-induced immune response to SARS-CoV-2 in fully vaccinated individuals that were either naturally infected (hybrid immunity) or uninfected (synthetic immunity) prior to vaccination.We demonstrate that: (a)recent vaccination in uninfected individuals elicits shifts in T-cell maturation and blunts the mobilization of naive CD4+and CD8+T-cells with exercise;(b)exercise mobilizes T-cells that react to spike (S) protein after vaccination regardless of infection history;and (c) nAb titers in blood are elevated during exercise after vaccination in individuals with hybrid immunity.
2.Methods
2.1.Participants
We recruited 18 participants(8 males,10 females)to participate in this investigation.Participants were young,healthy individuals(21-44 years)free of any cardiovascular,pulmonary,or autoimmune diseases.Eleven virus-naive participants received the Pfizer 2-dose COVID-19 vaccine regimen during this study period and were considered to have “synthetic” immunity.An additional 7 participants who were previously infected and subsequently recovered also received a COVID-19 vaccine (Johnson and Johnson,n=5;Pfizer,n=2) and were considered to have“hybrid” immunity.All post-vaccination trials took place 3-4 weeks after final inoculation.All participants completed a brief medical history questionnaire as well as the American Heart Association/American College of Sports Medicine Pre-participation and Screening Questionnaire22to ensure they were identified as “low risk” for cardiovascular disease and able to perform vigorousintensity exercise.In addition,participants were eligible if they reported an active-physical activity rating>4.23Research participants were non-users of tobacco products,consumed<10 standard alcoholic beverages per week,and were not taking prescription medications that could affect cardiovascular,metabolic,or immune responses to exercise.After screening,all participants provided written and oral informed consent,and all experimental procedures were conducted in accordance with the ethical guidelines provided by the Belmont Report.The University of Arizona Institutional Review Board granted ethical approval,and the trial was registered at www.clinicaltrials.gov(NCT05019456).
2.2.Experimental design
The exercise protocol and metabolic responses to exercise used in these research participants have been previously described.24In brief,participants first completed a submaximal graded exercise test on a cycle ergometer (Velotron,Quarq Technology,San Diego,CA,USA) to determine predicted maximal oxygen consumption (VO2max) (Quark CPET,COSMED,Pabona di Albona Laziale,Italy).Upon successful completion of submaximal testing,individualized power outputs for the experimental trials were determined via simple linear regression between VO2and power (W).Participants then returned to the laboratory twice more for experimental trials,once before and once after COVID-19 vaccination.Trials consisted of 4 incremental 5-min stages with power outputs corresponding to 50%,60%,70%,and 80% of the individual predicted VO2max.Blood was collected at rest,during the 60%VO2maxstage,during the 80%VO2maxstage,and 1 h after exercise cessation.To maintain consistency,the absolute cycling power outputs for each individual were identical during the experimental trials.Heart rate and rating of perceived exertion (Modified Borg Scale) were recorded during the final 15 s of each stage.A schematic of the experimental trial design is displayed in Fig.1(Created with BioRender.com).
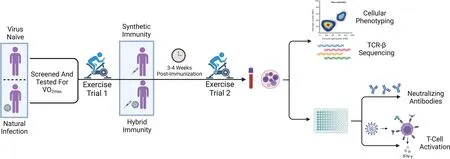
Fig.1.Schematic of experimental design.Virus-naive(n=9)and naturally infected(n=5)participants were screened and performed submaximal graded exercise testing prior to their first main exercise trial(pre-vaccination).Participants performed a second exercise trial 3-4 weeks post-vaccination.Those who were virusnaive and subsequently vaccinated were included in the synthetic immunity group,with the addition of 2 participants who only performed the exercise trial postvaccination(n=11).Those who were naturally infected and subsequently vaccinated were included in the hybrid immunity group,with the addition of 2 participants who only performed the exercise trials post-vaccination(n=7).Blood samples were taken at rest,during the exercise trial,and 1 h post-exercise for further analysis utilizing flow cytometry,TCR-sequencing,whole-blood peptide stimulation,and ELISA.Diagram was created with BioRender.com.ELISA=enzymelinked immunosorbent assay;TCR=T-cell receptor;2max=maximal oxygen consumption.
2.3.Blood processing and immunophenotyping
Heparinized blood was utilized for a 24 h whole blood peptide stimulation assay and for isolation of peripheral blood mononuclear cells using standard density gradient centrifugation protocols.Ethylenediaminetetra-acetic acid whole blood samples were stained and analyzed via flow cytometry(MACSQuant 10,Miltenyi Biotec,Bergisch Gladbach,Germany) to determine lymphocyte counts and immune cell subsets using the following conjugated antibodies: CD8-VioBlue,CD14-VioGreen,CD3-VioGreen,CD3-FITC,CD4-FITC,CD4-PE,CD62L-PE,CD20-PerCP,PD-1-PerCP,CD45RA-PerCPVio770,CD45-APC,and CD56-APCVio770(Miltenyi Biotec,Bergisch Gladbach,Germany).The percentage of all CD45+lymphocytes expressing the surface marker of interest were multiplied by the total lymphocyte count to enumerate each lymphocyte subset per unit of whole blood.Serum was isolated from each timepoint and frozen for future analysis to determine SARS-CoV-2 nAb titers using commercially available enzyme-linked immunosorbent assay(ELISA)kits(Cayman Chemical,Ann Arbor,MI,USA).
2.4.Whole blood peptide stimulation
Whole blood (1 mL) samples were utilized for whole blood peptide stimulation as previously described.20In brief,whole blood was stimulated with the following:saline(negative control),phytohemagglutinin(10 mg/mL;positive control;Sigma Aldrich,St.Louis,MO,USA),actin pepmix (1 mg/mL;peptide control;JPT Peptide Technologies,Berlin,Germany),or SARS-CoV-2 S,membrane (M),and nucleocapsid (N) pepmixes (10 mg/mL;Miltenyi).After 24 h of incubation at 37˚C and 5% CO2,the samples were centrifuged and plasma was stored at80˚C for future analysis.After thawing,interferon gamma(IFN-g)concentrations within the plasma were determined via a commercially available ELISA kit(R&D Systems,Minneapolis,MN,USA).
2.5.T-cell receptor-b sequencing
Genomic DNA was extracted from peripheral blood mononuclear cells collected at rest,80%VO2max,and 1 h post-exercise in post-natural infection and post-vaccination trials(DNeasy Extraction Kit,Qiagen,Germantown,MD,USA).Genomic DNA was sent to Adaptive Biotechnologies(Seattle,WA,USA),where subsequent T-cell receptor b (TCR-b)sequencing was performed and analysis was carried out utilizing the ImmunoSEQ (Adaptive Biotechnologies) data platform.TCR-b rearrangements specific to SARS-CoV-2 were determined using the ImmunoSEQ T-MAP COVID ImmuneCODE database.The public database VDJdb (https://vdjdb.cdr3.net) was utilized to determine the major histocompatibility complex(MHC)class of each identified SARS-CoV-2 productive rearrangement.This included only those SARSCoV-2 specific T-cell clones with a confidence threshold score of 3(very high confidence)or 2(high confidence).
2.6.Statistical analysis
Differences in participant characteristics were assessed using unpairedttests.For general immune cell phenotype changes across time,2-way repeated measures analysis of variance were used with Bonferonni multiple comparisons.To determine immune cell phenotype changes across time,a mixed model was used with Bonferonni multiple comparisons.Lastly,2-way repeated measures analysis of variance with Bonferonni multiple comparisons was used to assess differences in nAb titers and cytokine secretion across time.All statistical analyses were performed using GraphPad Prism(Version 9.4;GraphPad Software,San Diego,CA,USA).Significance was accepted atp<0.05,and all data are presented as meansstandard error of mean.
3.Results
3.1.Participant characteristics
The demographics of the 18 participants in this investigation can be viewed in Table 1.Nine individuals who were SARS-CoV-2aive (confirmed negative via SARS-CoV-2 spike IgG titers) were assessed before and after mRNA-based vaccination (Pfizer,New York,NY,USA),with the addition of 2 individuals who were only assessed post-vaccination(Pfizer).Five individuals who were previously infected with SARS-CoV-2 were assessed before and after mRNA-based vaccination (Pfizer),with the addition of 2 individuals who were only assessed post-vaccination (Johnson and Johnson,New Brunswick,NJ,USA).There were no significant differences in demographics between groups;SARS-CoV-2 spike IgG was significantly elevated from baseline after vaccination in the entire cohort(p<0.0001).
Table 1 Participant demographics and serostatus(meanE).

Table 1 Participant demographics and serostatus(meanE).
a Includes 5 participants who have prior natural infection.b Includes 4 participants who did not test prior to vaccination.Abbreviations: CMV=cytomegalovirus;PAR=physical activity rating;SARS-CoV-2=severe acute respiratory syndrome coronavirus 2;VO2max=maximal oxygen consumption.
3.2.Naive CD4+and CD8+T-cell mobilization during exercise is blunted after vaccination in virus-naive but not previously infected individuals
All participants successfully completed the exercise trials as prescribed.The effect of acute cycling exercise on the number of circulating lymphocyte subsets and monocytes is displayed in Fig.2.Considering all participants regardless of infection status,there were no differences in the circulating number of total lymphocytes,monocytes,T-cells,B-cells,or NK cells at any timepoint after vaccination.All immune cell subsets assessed displayed a strong mobilization across time,with cell numbers returning to baseline 1 h post-exercise (p<0.0001).Overall,vaccination status did not significantly affect general immune cell subset numbers at rest,during exercise,or during recovery.
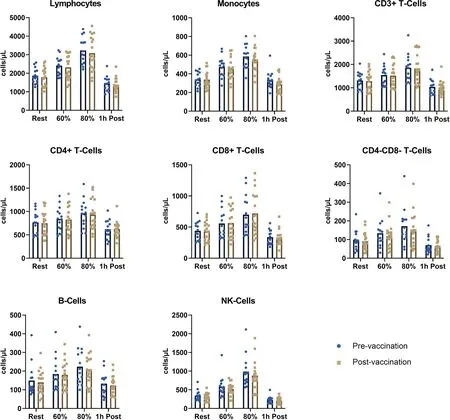
Fig.2.Recent COVID-19 vaccination does not affect major immune subsets during exercise-induced leukocytosis.Major immune cell subsets are displayed across time for all study participants regardless of natural infection.A significant effect of time was found for all subsets analyzed(p<0.0001)with no differences at any specific timepoints between pre-vaccination(n=14)and post-vaccination(n=18).COVID-19=coronavirus disease 2019;NK=natural killer.
Although vaccination did not significantly affect the mobilization and egress of immune cells in the collective cohort,significant differences in T-cell differentiation were observed when the group was split based on a history of past infection.A strong mobilization effect(p<0.0001)was present for major T-cells subsets,but there were no differences between groups pre-and post-vaccination(Fig.3A).In individuals with no history of SARS-CoV-2 infection who were subsequently vaccinated (synthetic immunity),vaccination elicited significant decreases in the exercise-induced mobilization of naive CD4+and CD8+T-cells at 60% and 80% VO2max(p=0.03 andp=0.009 for CD4+T-cells;p=0.002 andp=0.0009 for CD8+T-cells,respectively) (Fig.3B).There were also significant reductions in central memory(CM)CD4+T-cells at 60%and 80% VO2maxpost-vaccination (p=0.004 andp=0.0002,respectively).While there was an increased mobilization of effector memory RA (EMRA) CD4+T-cells across time in the synthetic immunity group after vaccination,it was not significant (p=0.11).The reductions in circulating naive and CM T-cells were not observed in those with a previous history of infection who were subsequently vaccinated (hybrid immunity).Thus,vaccination reduced the mobilization of less-differentiated T-cell subsets in individuals who were virus-naive while causing no changes in those who already possessed immunological memory to SARS-CoV-2.

Fig.3.Recent COVID-19 vaccination alters the T-cell compartment in those acquiring synthetic immunity.Individuals were split into synthetic (n=11) and hybrid(n=7)immunity groups depending on their history of previous infection to assess differences in vaccination responses.(A)Total lymphocytes and major T-cell subsets are displayed across time pre-and post-vaccination;(B)CD4+and CD8+T-cell differentiation status are displayed across time pre-and post-vaccination.A significant effect of time was found for all subsets analyzed(p<0.05).*p<0.05,**p<0.01,***p<0.001.COVID-19=coronavirus disease 2019;CM=central memory;EM=effector memory;EMRA=effector memory RA.
3.3.Acute exercise increases circulating nAb concentrations and promotes the mobilization of T-cells responsive to SARSCoV-2 spike protein
In addition to immunophenotyping,serum and whole blood collected from each timepoint were used for the quantification of SARS-CoV-2 nAb titers and to assess T-cell responsiveness to viral peptidesex vivo,respectively.For insight into B-cell responses to vaccination,nAb titers were determined at all experimental timepoints.The hybrid immunity group demonstrated significantly higher nAb titers at 80% VO2maxrelative to rest(p=0.02).Although mean values were elevated at 80%VO2maxfor individuals with synthetic immunity,they were not significantly elevated from resting values(Fig.4A).
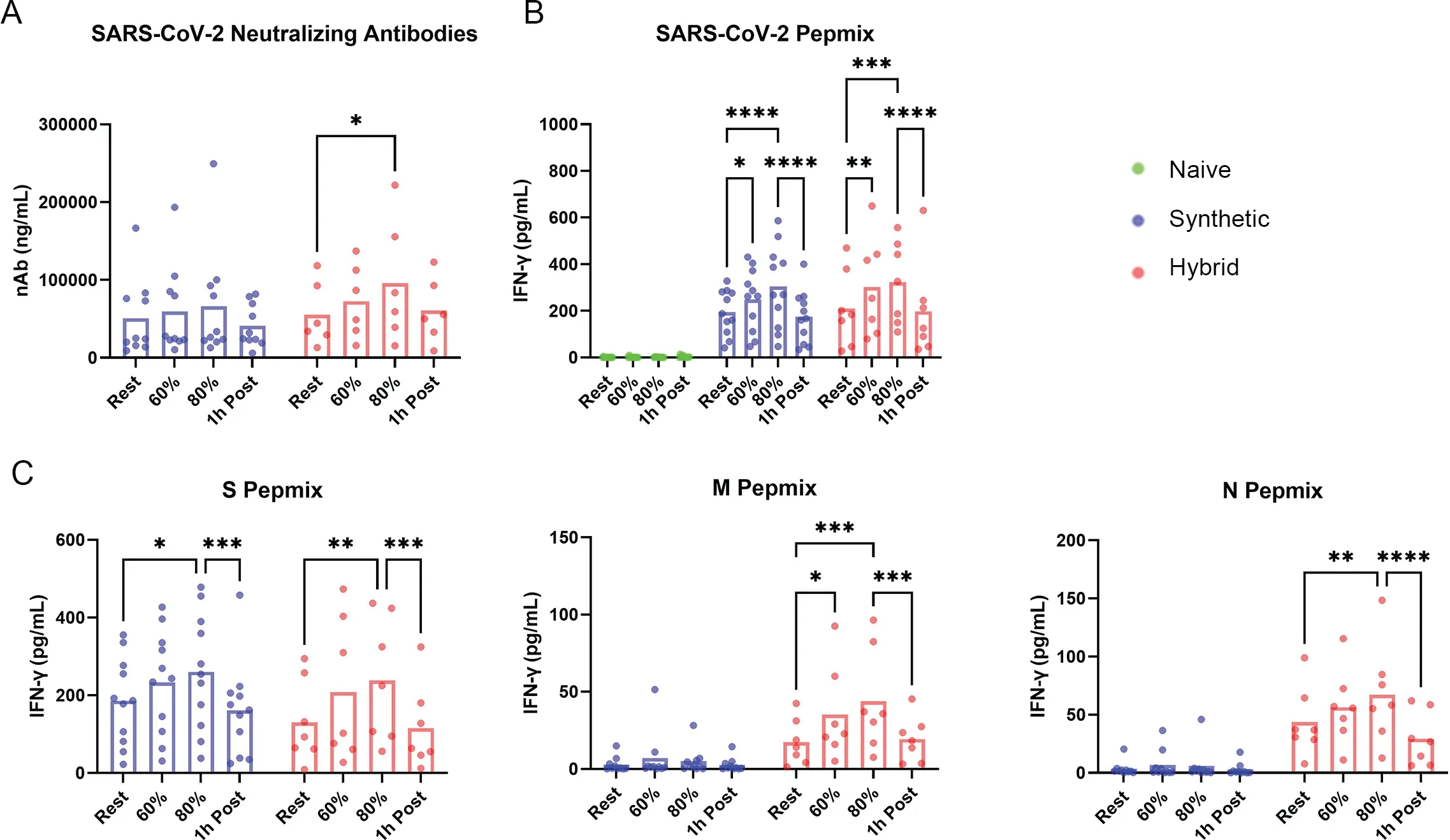
Fig.4.Recent COVID-19 vaccination elicits transient increases in neutralizing antibody titers and an intensity-dependent increase in T-cell function.Humoral and cellular immune measures are displayed across time as neutralizing antibody titers and IFN-g responses in whole blood.(A) nAb concentrations across exercise intensities and through recovery in individuals with synthetic and hybrid immunity;(B) Whole blood IFN-g responses to a SARS-CoV-2 pepmix containing all major structural proteins(S,M,N);(C)Whole blood IFN-g responses to each individual component of the SARS-CoV-2 pepmix,demonstrating the specificity of vaccination.*p<0.05,**p<0.01,***p<0.001,****p<0.0001.COVID-19=coronavirus disease 2019;IFN-g=interferon gamma;SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
In response to a pepmix containing S,M,and N peptides,both groups exhibited enhanced IFN-g secretion during exercise,with values returning to normal 1 h post-exercise.For synthetic immunity responses,the total output of IFN-g significantly increased from rest to both 60% and 80% VO2max(p=0.04 andp<0.0001,respectively) (Fig.4B).Hybrid immunity responses presented enhanced IFN-g secretion at both 60% and 80% VO2maxrelative to resting values(p=0.002 andp=0.001,respectively) (Fig.4B).When T-cell responsiveness to peptide stimulation was assessed for individual SARS-CoV-2 components,hybrid immunity led to robust IFN-g secretion in response to S,M,and N peptides and was significantly higher at 80% VO2maxwhen compared to resting values for all peptides (p=0.002,p=0.0003,p=0.002,respectively) (Fig.4C).Synthetic immunity displayed a predictably robust IFN-g response to S peptides from rest to 80% VO2max(p=0.01),with little to no response to M and N peptides(Fig.4C).
Overall,these results indicate that acute exercise mobilizes SARS-CoV-2 specific T-cells in vaccinated individuals that are highly responsive to antigen stimulation,and that responses to peptides not included in current mRNA vaccines(M and N)are only elicited in those with hybrid immunity.In addition,acute exercise transiently increases the concentration of nAbs in individuals with hybrid immunity,but this response was not observed in those with synthetic immunity.
3.4.TCR-b sequencing of exercise-responsive SARS-CoV-2 specifci T-cells reveals preferential mobilization of MHC-Irestricted clones
Next-generation TCR-b deep sequencing (Adaptive Biotechnologies;ImmunoSEQ T-MAP COVID Immune-CODE database) was utilized to determine the specificity of SARS-CoV-2 specific T-cell clones mobilized during exercise.Two individuals from the vaccine-only cohort (synthetic immunity) and 2 individuals with natural immunity from our previous study(prior to vaccination)21were used for comparison in this descriptive analysis.Complementarity-determining region 3 length motif analysis allowed for the determination of antigen specificity for TCR sequences obtained.Determination of MHC class I or II,utilizing the VDJdb,demonstrated that a percentage increase in MHC-Iestricted T-cells occurred from rest to 80% VO2max,with a percentage decrease in MHC-IIestricted T-cells seen in both individuals within the synthetic group (Fig.5A);however,the total number of MHC-Ind MHC-IIestricted clones increased in response to exercise.This is demonstrative of the larger mobilization effect observed in CD8+T-cells during exercise relative to CD4+T-cells (Fig.2).Accompanying the mobilization of T-cells during exercise,there was an increase in the number of hits for clones specific to SARS-CoV-2 epitopes from rest to 80%VO2maxin 2 individuals with synthetic immunity (Fig.5B).Furthermore,specific clones that were heavily mobilized during exercise tended to egress the blood compartment by 1 h postexercise (Fig.5C).Finally,comparison of epitope specificity of SARS-CoV-2 specific T-cell clones is displayed between individuals with synthetic immunity and those with natural immunity(Fig.5D).Overall,acute exercise did not alter the proportion of SARS-CoV-2 clones being mobilized to circulation,therefore indicating the diversity is maintained even within vaccinated individuals,which was previously observed in a naturally infected cohort.21

Fig.5.TCR-b specificity in vaccinated and naturally infected individuals during exercise and recovery Deep TCR-b sequencing.Performed on T-cells present in the blood at rest,during exercise,and 1 h post-exercise in vaccinated (n=2) and naturally infected (n=2) individuals.(A) Percentage of MHC-I and MHC-II restricted T-cells across time in 2 individuals with synthetic immunity;(B) Number of SARS-CoV-2 specific clones across time in 2 individuals with synthetic immunity;(C) Changes in the number of hits for SARS-CoV-2 specific clones during mobilization (rest to exercise) and egress (exercise to 1 h post) alongside epitope specificity in 2 individuals with synthetic immunity;(D) Percentage of epitope dominance across time between vaccinated (n=2)and naturally infected(n=2) individuals.MHC=major histocompatibility complex;ORF=open reading frame;SARS-CoV-2=severe acute respiratory syndrome coronavirus 2;TCR=T-cell receptor.
4.Discussion
Increased levels of physical activity have been associated with a lower risk of hospitalization,disease severity,and mortality in individuals with COVID-19,11,13,25although the mechanisms associated with the protective anti-viral effects of exercise in the context of COVID-19 are still elusive.Building upon our previous findings in individuals with natural immunity,20we sought to determine whether acute exercise enhances cellular and humoral immune responses against SARS-CoV-2 after COVID-19 vaccination.We report here that acute exercise promotes the mobilization and egress of functional SARS-CoV-2 VSTs after vaccination regardless of infection history and transiently increases nAb titers after vaccination in individuals who possess hybrid immunity.
Seropositivity for neutralizing IgG has been demonstrated to be 90% or higher 6-8 months after infection,26,27while vaccination with 2-dose mRNA vaccines elicits strong nAb responses that may surpass concentrations seen after infection.28However,nAb concentrations and memory B-cell frequencies may wane more quickly in individuals with synthetic immunity.28While additional instances of antigen exposure elicit accretion in nAb titers,20,28,29acute exercise has been shown to transiently enhance IgG titers to SARSCoV-2.20We replicate these findings here,as there was an intensity-dependent increase in nAb titers during a 20-min cycling bout.In addition,this transient increase in circulating nAbs was greatest in those possessing hybrid immunity,which has been recently found to maintain significantly higher nAb titers against ancestral variants.28,30,31It is possible,therefore,that each exercise bout will facilitate nAb transportation to increase the likelihood of antibodyirus contact.20While high circulating nAb titers provide significant protective immunity,28it should be noted that they are not present in many cases.28,32The importance of humoral immunity cannot be understated,however epidemiological and animal data indicate that the T-cell-mediated branch of adaptive immunity is paramount for efficient clearance of infection.28
Memory CD8+T-cells elicited by mRNA vaccines recognize diverse S epitopes,33-35typically possess effector memory phenotypes,express IFN-g,and are capable of proliferation.36,37There is less research on adaptive responses with hybrid immunity,but it has been established that T-cell repertoire diversity is maintained,38and some studies have shown improved responses compared to infection or vaccination alone.35Regardless,each subsequent exposure or vaccination further matures the T-cell compartment towards effector phenotypes.28,35Acute bouts of dynamic exercise demonstrate enhanced CD8+T-cell responses against various viral pathogens (e.g.,cytomegalovirus,Epsteinarr virus,adenovirus).16,39,40Although T-cell responses to SARS-CoV-2 are predominated by CD4+VSTs,35we present here that exercise preferentially mobilizes SARS-CoV-2 specific CD8+VSTs.Moreover,we observed decreases in the exerciseinduced mobilization of naive and CM T-cells shortly after vaccination,suggesting the maturation and expansion of an antigenspecific SARS-CoV-2 T-cell repertoire.This effect was observed in those who were virus-naive relative to those who were previously infected;we speculate that vaccination in virus-naive individuals elicited the differentiation of naive T-cells,while vaccination expanded the memory compartment in those who were previously infected.Expectedly,individuals with synthetic immunity exhibited robust IFN-y secretion in response to S pepmixes during exercise,while those with hybrid immunity had robust responses to S,M,and N pepmixes.
Overall,there have been more unique SARS-CoV-2 T-cell epitopes identified for CD8+T-cells than for CD4+T-cells.41Within the main structural antigens (S,M,and N),CD4+T-cells have more pronounced immunodominant regions while CD8+T-cell responses are more evenly distributed.41Indeed,details on TCR specificity and responses to specific epitopes are well-documented;41however,we present here the exercise-mobilization response of SARS-CoV-2 clones between synthetic and natural immunity in a small number of individuals.Although clones specific to surface and ORF1ab displayed the greatest mobilization,there was consistency in epitope recognition across all 3 timepoints regardless of synthetic or natural immunity.Clones that were mobilized to the greatest extent displayed the greatest egress,suggesting these clones may possess higher migratory potential and are more readily redistributed to local sites of need.Moreover,there was a large increase in the percentage and total number of MHC-I (e.g.,CD8+T-cells) restricted clones during exercise regardless of how SARS-CoV-2 immunity was acquired.It is plausible,therefore,that the exercise-induced mobilization of diverse antigen-specific clones enhances the potential for viral resolution and general immunosurveillance.35
The evolution and emergence of SARS-CoV-2 variants is a critical area of investigation and one that is not specifically addressed in this study.However,Tarke et al.33have demonstrated that vaccination elicits memory and reactivity against all currently existing variants.In addition,our previous work in infected individuals demonstrated that exercise mobilized VSTs collected during the ancestral strain outbreak respond to both alpha and delta variants.21The impact of SARS-CoV-2 mutations on memory CD4+and CD8+T-cells is limited;however,predictive models demonstrate that,while newer variants (e.g.,omicron) have fewer conserved epitopes in multiple proteins,the majority of both CD4+and CD8+T-cell epitopes are unaffected by mutations.41It is possible that the regular mobilization and exchange of SARS-CoV-2 VSTs to peripheral tissues with every bout of exercise may be beneficial in this regard;the broad recognition of thousands of epitopes suggests T-cells may be well-poised to recognize developing variants of concern as exercise enhances the immunosurveillance of T-cells.1
We acknowledge that the small sample size within our hybrid immunity group may limit the scope of our findings.However,the recruitment of virus-naive individuals is currently impractical with the global spread of SARS-CoV-2 and large-scale administration of vaccines.While our investigation did not specifically address evolving variants,our work still details the mobilization of diverse antigen-specific T-cells with effector phenotype and function.Subsequent studies are necessary to understand whether exercise training influences the durability of T-cell and humoral immune responses to SARS-CoV-2.The results demonstrated within the TCR-b sequencing analyses are only representative of 2 individuals who were naturally infected and 2 who obtained synthetic immunity.Moreover,we were not able to compare TCR-sequencing for individuals in the hybrid group,although our data corroborates previous findings,35further detailing the mobilization of MHC I (i.e.,CD8+T-cells) restricted SARS-CoV-2 clones.Future work should investigate,in more detail,the specific clones mobilized and egressed with exercise in those with synthetic,natural,and hybrid immunity.Finally,there is a need to determine whether repeated bouts of physical activity resolve symptoms of long COVID,which may be influenced by T-cell and nAb exercise responses reported here.
5.Conclusion
This is the first study to show that a single bout of exercise preferentially mobilizes MHC-Iestricted SARS-CoV-2 VSTs in vaccinated individuals and elevates circulating nAbs after COVID-19 vaccination in previously infected individuals.We have also demonstrated that exercise-mobilized VSTs are highly responsive to SARS-CoV-2 peptidesex vivo.We contend that the elevations in VST responsiveness and nAb titers could be compounded over time in those regularly engaging in physical activity and could putatively provide enhanced immunosurveillance and protection against SARS-CoV-2.Future studies should investigate the potential for chronic exercise to stimulate the persistence of SARS-CoV-2 VSTs and nAbs,further contributing to long-lasting immunity and protection from re-exposure or variant mutations.
Authors’contributions
KAS and TMZ collected and analyzed the data,performed the statistical analysis,and drafted the manuscript;FLB and HB collected the data;CRP,SCB,MPG,and EK contributed to the design of the study;RJS designed the study and supervised all aspects of manuscript preparation.All authors aided in revision of the manuscript.All authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
 Journal of Sport and Health Science2024年1期
Journal of Sport and Health Science2024年1期
- Journal of Sport and Health Science的其它文章
- Commentary on“Speed and surface steepness affect internal tibial loading during running”
- Doseesponse associations,physical activity intensity and mortality risk:A narrative review
- A brief history of the Compendium of Physical Activities
- The 2024 Compendium of Physical Activities and its expansion
- Speed and surface steepness affect internal tibial loading during running
- Acute effect of foot strike patterns on in vivo tibiotalar and subtalar joint kinematics during barefoot running
