Resistance of Microbial Community of Artemisia annua L. to Pathogenic Fungi
Zerong GENG, Tianhua YU, Zhannan YANG, Shiqiong LUO
1. Key Laboratory for Information System of Mountainous Areas and Protection of Ecological Environment of Guizhou Province, Guizhou Normal University, Guiyang 550018, China; 2. School of Life Sciences, Guizhou Normal University, Guiyang 550018, China
Abstract [Objectives] This paper was to figure out whether the dominant bacterial community has the role and effect of bacterial community and its defense mechanism against potential pathogenic fungi in Artemisia annua, and thus establish a systematic model of bacteria-fungus-plant. [Methods] Fifty-eight strains of bacteria and one strain of pathogenic fungi, Globisporangium ultimatum, were used for the experiments. These 58 bacterial strains were assembled into a bacterial community, and the bacteria with abundance in the top 1% were reassembled into a dominant bacterial community as measured by 16S rDNA. [Results] The growth of A. annua seedlings inoculated with bacterial communities and pathogenic fungi or dominant bacterial communities and pathogenic fungi was significantly better than that of A. annua seedlings inoculated with pathogenic fungi during in vitro confrontation, which was evident in both enzymatic and non-enzymatic antioxidant assays. [Conclusions] The results suggest that the dominant bacterial community has a crucial role as a representative core microbial community of synthetic bacterial community, which can protect plants by interfering with the growth of phytopathogenic fungi mediated by chemical signals, and can be used as the main synthetic community of biocides to achieve the effect of biocontrol.
Key words Artemisia annua; Core bacterial communities; Pathogenic fungi; Bacteria versus fungi
1 Introduction
As a novel biological resource, the study of endophyte-host plant interactions and their functions in host plants has received increasing attention[1-2]. Microorganisms are closely related to plants, affecting the growth and health status of host plants, and there are antagonistic interactions between beneficial and pathogenic microorganisms that will affect plant growth and protect plants from pathogens[3]. All types of ecosystems do not exist in isolation in nature but interact with each other, forming specific ecological environments that affect plants. In the process of coexistence between plants and microorganisms, on the one hand, microorganisms influence plants and form the internal law of plant growth and development; on the other hand, plants have different reactions to the changes in microorganisms and change the microorganisms[4-6]. These two aspects constitute a mutualistic relationship between microorganisms and plants. In previous studies, it was reported that microbial communities protect plants by interfering with the growth of plant pathogenic fungi. Among the known reports are the injection of simplified microbial communities on aseptically grown plants to constitute artificially controlled microbial-plant ecosystems[7]and the assembly of simplified synthetic bacterial communities from maize rhizosphere microbes to explore their inhibitory and host-beneficial effects on the pathogenic fungusFusariumverticillioides[8-9]. Simplified core bacterial communities can be colonized on plants to protect them from pathogen invasion.
With the rapid development of society and the popularity of scientific production, there is a growing concern about the effects of endophytes on the plants themselves, and a desire to reduce the reliance on chemicals in crops[10].Artemisiaannua, a representative annual herb of theArtemisiagenus, is the raw material for the extraction of artemisinin, which is used in the treatment of malaria, and is one of the most important Chinese herbal medicines[9]. As mentioned in theMateriaMedicaCompendium[11], the roots, stems, cotyledons, and leaves ofA.annuaare also used for medicinal purposes, and the dried ones are particularly good when roasted for drinking incense. The roots, stems and leaves can be used as medicine. It can cure children’s wind-cold fever, cure labor, down the qi stomach, and stop night sweating and evil ghost poison. It is born in the wilderness, mountain slopes, roadsides, and other places, and its distribution is almost all over China[12]. The present study provides another method to find ways to promote plant growth and development, increase the yield of medicinal plants, and increase secondary metabolites in the future through the relationship between microorganisms and host plant growth. Artemisinin, the anti-malarial drug recommended by the World Health Organization, has the advantages of "high efficiency, quick effect, and low toxicity"[13]. In addition to its anti-malarial function, artemisinin and its derivatives can also be used for the treatment of a series of diseases, such as leukemia, cardiovascular diseases, neurological diseases, and so on[14]. Currently, research onA.annuamainly focuses on improving the yield of artemisinin. Still, there are few reports on the effect of microorganisms onA.annua, which plays a vital role in the growth and health of the plant. There are important interactions betweenA.annuaand microorganisms in previous studies, but it is still not clear how to act against pathogens to make the plant healthy. In the natural, it can be found that there is almost no interference from pests and diseases, so is it possible that the ecosystem ofA.annuaitself has a role in resisting pathogenic fungi? The presence of such a microbiome in the ecosystem ofA.annuacan make it possible to achieve the effect of biocontrol.
This study investigates the inhibitory effect of a simplified synthetic bacterial community with a major role in a potential pathogenic fungus obtained from the microbiota aggregated in the root system ofA.annua. The microbial community, fixed in value on plants, has a very high level of complexity. In this study, it was simplified and assembled into a representative synthetic bacterial community to investigate the kinetics and function of the assembled bacterial community fromA.annua’s rhizosphere on sterileA.annuaseedlings. This synthetic bacterial community interfered with resisting the growth of a potentially pathogenic fungus as a way to validate plant-microbe interactions.
To verify that the simplified dominant bacterial community (Y) can approximately replace the overall bacterial community (H) role, we searched for high-abundance bacteria to synthesize by 16S abundance assay, and compared them with the overall bacterial community, which can be approximated as the dominant bacterial community is the core bacterial community. In addition, we compared the response ofA.annuato pathogenic fungi after Y, H, and no inoculation of bacterial community. This study demonstrates that the assembled dominant bacterial community can inhibit the invasion of pathogenic fungi, promote the healthy growth of the host plant, and can be present as a biocide to control biological plant diseases.
2 Materials and methods
2.1 Medium preparationTheA.annuahistolytica seedlings, bacteria, and fungi used in this study were provided by the Key Laboratory for Information System of Mountainous and Protection Ecological Environmental of Guizhou Province, Guizhou Normal University.
A.annuahistoculture medium: 9.52 g of MS medium, 30.0 g of sucrose, 20.0 g of agar, 2 mL of NAA (0.5 mg/mL), 0.4 mL of IAA (0.1 mg/mL), 2.0 L of distilled water, pH natural, and sterilized at 121 ℃ for 20 min. Potato Dextrose Agar medium (PDA): 200.0 g of potato, 20.0 g of dextrose, 18.0-20.0 g of agar, 1 000.0 mL of distilled water, pH natural, and sterilized at 121 ℃ for 20 min. Nutrient Agar medium (NA): 10.0 g of peptone, 3.0 g of beef paste, 5.0 g of sodium chloride, 15.0-17.0 g of agar, 1 000.0 mL of distilled water, pH natural, and sterilized at 121 ℃ for 20 min.
2.2 Preparation of bacterial and fungal suspensionsThe activated bacterial colonies were picked with an inoculating ring into 50 mL of NA liquid medium. The shaker was shaken at 38 ℃ and 200 r/min for 12 h to make the bacterial colonies fully dispersed into the solution. Then the bacterial concentration was diluted with sterile water to 106CFU/mL, and stored at 4 ℃; The mycelium was picked with an inoculation ring into 50 mL of sterile water, shaken at 28 ℃ and 200 r/min for 12 h to make the spores fully dispersed into the solution, and then the spore concentration was diluted to 106CFU/mL with sterile water and stored at 4 ℃. The bacterial colony solution was a mixture of 58 bacterial strains in equal volume.
2.3 Sterile soil preparationChangbai Mountain humus 150 g was filled with tissue culture flasks, autoclaved at 105 ℃ for 20 min, cooled and continued to be sterilized for 20 min, repeated three times. The cooled sterile soil was diluted to 10-5and 10-6concentrations dispersed on NA and PDA medium, and incubated at 28 ℃ for one week. If there was no microbial growth, it could be proved that the sterile soil had been completely sterilized and could be used in the experiment.
2.4 Bacterial community-fungal standoffinvitroInvitro, a standoff of bacterial community fungi was established on a 9 cm diameter NA medium. A hole was punched at the edge of the fungus with a 7 mm pore size punch and moved to the center of the NA plate. A small number of bacteria were dipped into an inoculating needle to draw a straight line 2 cm from the fungal cake, and the one that only transferred the fungus was the control. Finally, the width of the inhibition zone was measured with vernier calipers after incubation for 72 h at 28 ℃.
2.5A.annuainoculated with bacterial communities and fungiThe seedlings ofA.annuawere cultivated in a sterile medium until small rhizomes grew, and then 1 mL of the bacterial colony and fungal suspension was added simultaneously. The fungal control group only added fungal suspension and the bacterial control group added bacterial colony.
2.6 Measurement of growth indexes ofA.annuaAfter opening the culture bottle and pulling outA.annua, the soil and medium attached to the root system were rinsed out with tap water, and the water on the surface of the plant was sucked up by filter paper. The height of the plant and the length of the main root system were determined with a ruler. The fresh weight ofA.annuawas weighed with a 1/10 000 electronic balance.
2.7 Measurement of physiological indicators ofA.annua
2.7.1Measurement of peroxidase (POD) activity. Measurement of peroxidase activity can reflect the changes in metabolism inA.annuaduring the period. The colorimetric method was used. 0.05 g ofA.annualeaves were weighed in a mortar, added with 3 mL of 0.05 mol/L phosphate buffer solution (pH 6.0), and ground into a homogenate. All the homogenate was transferred into a centrifuge tube and then centrifuged at a speed of 2 500 r/min for 15 min. As the supernatant was transferred to a test tube, the residue was extracted again with 3 mL of phosphate buffer solution, and then the supernatant was incorporated into a test tube for spare. The countermix solution was obtained by mixing 100 mL of phosphate buffer solution with pH 6.0, 30 μL of 30% H2O2and 30 μL of guaiacol. 0.5 mL of the supernatant and 3 mL of the countermix solution were mixed and the absorbance at 470 nm was measured on a UV spectrophotometer every 30 s. The supernatant and the countermix solution were mixed and the absorption at 470 nm was measured every 30 s. The supernatant and the countermix solution were mixed with 3 mL of countermix solution.
2.7.2Determination of superoxide dismutase (SOD) activity. The photochemical reduction method was used. 0.1 g ofA.annualeaves was taken in a mortar, added with 3 mL of 0.05 mol/L phosphate buffer solution with pH 7.8 and ground into a homogenate. Afterwards, the residue was rinsed with 3 mL of phosphate buffer solution and centrifuged at 2 500 r/min for 20 min, and then the supernatant was transferred to test tubes for reserve. 0.1 mL of supernatant, 1.5 mL of phosphate buffer solution, 0.3 mL of 130 mmol/L methionine solution, 0.3 mL of 750 μmol/L NBT solution, 0.3 mL of EDTA-Na2solution, 0.3 mL of 100 μmol/L riboflavin solution and 0.5 mL of deionized water were added to a clear glass test tube and reacted under fluorescent light for 25 min. The absorbance at 560 nm was measured on a UV spectrophotometer.
2.7.3Determination of malondialdehyde (MDA) content. The thiobarbituric acid method was used, and the enzyme solution preparation was the same as that described in Section2.7.2. 3 mL of supernatant and 3 mL of 0.6% TBA were added to the test tubes, and the reaction was carried out for 15 min in a boiling water bath after mixing. The reaction was cooled down and then centrifuged at 2 500 r/min for 15 min. The centrifuged supernatant was taken and the absorbance was measured on an ultraviolet spectrophotometer at 532, 600 and 450 nm.
2.8 Determination of medicinal secondary metabolites ofA.annua
2.8.1Determination of flavonoid content. It was done using high-performance liquid chromatography. The leaves ofA.annuawere collected from the culture, cleaned and dried with filter paper. 0.1 g ofA.annuawas weighed, cut into pieces, and put into a 20 mL brown storage bottle. After 1 mL of methanol was added, the mixture was extracted by ultrasonic extraction for 30 min, then filtered through 0.45 μm microporous filtration membrane for determination on the machine. The mixed standard solution was obtained by aspirating 0.5 mL of each of the 0.1 mg/mL stock solution of scopolamine lactone, cathelicidin, and cathelicidin yellow with methanol, and the concentration was 33.33 μg/mL.
2.8.2Determination of artemisinin content. The artemisinin content was determined using a liquid-mass chromatograph. The extraction method was the same as that described in Section2.8.1The solution was diluted 200 times and filtered through 0.45 μm microporous membrane into Agilent injection vials for online determination.
2.9 16S rDNA enrichment assayA.annuaseedlings in sterile medium and sterile soil were inoculated with H assembled by phylum classification and collected after 7 d of incubation, which we submitted to Sangyo Bioengineering (Shanghai) Co. for microbial taxonomic sequencing. The collectedA.annuawas transported to the laboratory of Sangyo Bioengineering (Shanghai) Co. in an expanded polystyrene foam box filled with dry ice. According to the protocol of the laboratory, the samples were ground into powder form by liquid nitrogen grinding, put into 2 mL centrifuge tubes, added with appropriate amount of steel beads and lysis solution, and extracted after crushing on a crusher. Qubit dsDNA HS analysis kit was used to extract DNA, and the amplification region was selected as 16S V3-V4, then the target sequence was enriched by highly specific primers, and finally, the data were obtained by sequencing for bioinformatics analysis. The relative abundance of the colony was analyzed by OUT to get the bacteria with an abundance greater than 1% and then reassembled to become the Y.
2.10 Statistical analysisMicrosoft Excel 97-2003 worksheets were used to calculate theinvitroinhibition of the standoff experiments, and IBM SPSS Statistics 20 software was used to statistically analyze the means of the treatments in the growth physiology and metabolism experiments and to determine significant differences in the data atP<0.05.
3 Results and analysis
3.1Invitrointeractions between bacterial communities and fungiFirstly, in theinvitroplate confrontation, the fungi grew very fast, and the bacterial demarcation part could be reached by 48 h of incubation. One more day of incubation was used to observe the subsequent growth. In Fig.1, the inhibition rate of bacterial flora and dominant bacterial flora on pathogenic fungi reached 10.7% and 13.3%, and it could be observed that the combined bacterial community did have an inhibitory effect on the growth of pathogenic fungi.
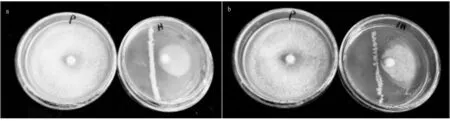
Note: a. Confrontation between bacterial flora and fungi; b. Confrontation between dominant bacterial flora and fungi.
3.2 Effect on the growth ofA.annuaTo understand the effect of inoculated bacterial flora and fungi on the morphological characteristics ofA.annua, the growth indices ofA.annuawere measured and counted (Fig.2). In sterile soil substrate, the plant height, root length, and fresh weight ofA.annuadecreased by 12.1%, 27.3%, and 47.4% in the fungus-only Z group compared with the control group CK, and increased by 15.4%, 22.0%, and 4.3% in the bacterial colony-only H group, while the amount ofA.annuaincreased by 15.4%, 22.0%, and 4.3% in the mixed bacterial colony-fungus co-culture HZ group compared with the Z group, and the height, root length, and fresh weight increased by 29.4%, 24.8%, and 47.5%, respectively. In terms of plant height, root length, and fresh weight ofA.annuagrown in sterile soil, the addition of bacterial community promoted the growth ofA.annua. On the contrary, the addition of fungi inhibited the growth ofA.annua, while the addition of bacterial community and fungi co-cultivation inhibited the growth of fungi.
Also, in this study, the growth index ofA.annuainoculated with bacterial colonies in the MS medium was determined. Consistent with the experimental results in sterile soil, the plant height, root length, and fresh weight ofA.annuain the fungus-inoculated group decreased to different degrees compared to the CK group, while those in the H group increased by 1.5%, 30.0%, and 30.8%, respectively, compared to the CK group, and the co-cultivation of bacterial flora and fungi in the HZ group decreased the plant height, root length and fresh weight ofA.annuaby 7.4%, 0.7%, and 17.7%, respectively, compared to the Z group.
After inoculation ofA.annuagrown in sterile soil with dominant bacterial flora, it could be seen that the plant height, root length, and fresh weight ofA.annuainoculated with dominant bacterial flora were higher than those of the CK group by 44.7%, 1.1% and 151.2%, while those inoculated with fungi were significantly lower than those of the CK group by 49.1%, 6.3% and 15.1%. The plant height, root length and fresh weight ofA.annuainoculated with dominant bacterial flora and fungi simultaneously increased by 69.0%, 10.7%, and 10.4%, respectively, as compared with that inoculated with fungi group.
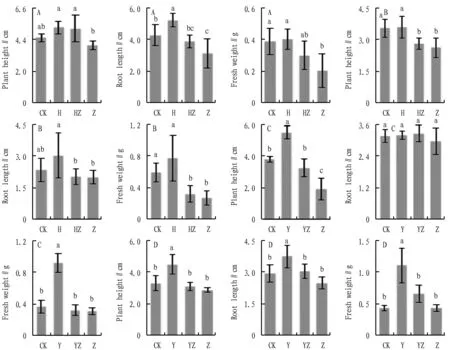
Note: A, B, C, and D are the plant height, root length, and fresh weight of A. annua under four conditions; A. Inoculation of bacterial community in sterile soil; B. Inoculation of bacterial community in sterile medium; C. Inoculation of dominant bacterial community in sterile soil; D. Inoculation of dominant bacterial community in sterile medium; CK. No inoculation of any microorganisms; H. Inoculation of only bacterial community; Y. Inoculation of dominant bacterial community only; HZ. Simultaneous inoculation of bacterial community and fungi; YZ. Simultaneous inoculation of dominant bacterial community and fungi; Z. Inoculation of fungi only; different lowercase letters indicate significant differences between treatments (P<0.05).
Inoculation ofA.annuagrown in MS medium with dominant bacterial flora increased the plant height, root length, and fresh weight ofA.annuaby 36.0%, 27.0%, and 154.7%, respectively, compared with the CK group, and decreased by 13.0%, 15.7%, and 0.8% in the Z group compared with the CK group. In comparison, co-cultivation of dominant bacterial flora and fungi increased the plant height, root length, and fresh weight by 8.0%, 22.7%, and 52.8% in the YZ group compared with the Z group.
In terms ofA.annuaphenotypes (plant height, root length, and fresh weight), inoculation with dominant bacterial flora and inoculation with bacterial flora had the same trend in both substrates, which can be approximated as the dominant bacterial flora being the core flora of the bacterial flora.
3.3 Effects on the physiology ofA.annuaPeroxidase (POD) is an oxidoreductase enzyme that participates in the photorespiratory process of plants and hydrolyses hydrogen peroxide to protect plant cells. Increased POD activity enhances the metabolism ofA.annuafor self-regulation[15]. As shown in Fig.3, inoculation ofA.annuagrown in bacterial colonies in both sterile soil and sterile medium showed a slight decrease in POD activity compared to CK, 4.1% and 9.5%, respectively, while inoculation with fungi showed a significant increase in POD activity by 177.1% and 750.2%, and simultaneous inoculation with both bacterial colonies and fungi showed a lower POD activity than that of inoculation with fungi, with a decrease of 45.9% and 17.0%, indicating that the inoculation of fungi would makeA.annuastressed to maintain the metabolic balance by increasing the POD activity in the body. The additional inoculation of bacterial flora had a significant effect on the maintenance of metabolic balance and stress resistance inA.annuaplants, indicating that the bacterial flora would inhibit the pathogenic fungi and maintain the metabolic balance in the body ofA.annua.

Note: A and B are in sterile soil substrate; C and D are in sterile medium substrate; CK. No inoculation of any microorganisms; H. Inoculation of bacterial community only; Y. Inoculation of dominant bacterial community only; HZ. Simultaneous inoculation of bacterial community and fungi ; YZ. Simultaneous inoculation of dominant bacterial community and fungi ; Z. Inoculation of fungi only; different lower case letters denote significant differences between the treatments (P<0.05); the same below.
Inoculation ofA.annuagrown with dominant bacterial flora in two substrates, sterile soil, and sterile medium, showed a significant increase in POD activity in the sterile soil substrate and a 10.9% increase in the sterile medium substrate compared to CK, and a significant increase in POD activity ofA.annuainoculated with fungi by 2 026.4% and 484.5%. The POD activity of both dominant bacterial flora and fungi inoculation was significantly lower than that of fungi inoculation by 74.4% and 25.0%, respectively, indicating that additional inoculation of dominant bacterial flora is important for the maintenance of metabolic homeostasis inA.annuaplants and that inA.annua, dominant bacterial flora inhibits the pathogenic fungi.
The POD activity profiles ofA.annuainoculated with dominant bacterial flora and bacterial flora were the same in both substrates, sterile soil and sterile medium, with the same trend, so that the dominant bacterial flora can be approximated to be the core flora of the bacterial flora in terms of exploring the POD activity.
SOD is also a common antioxidant enzyme, which plays an important role in scavenging harmful free radicals during plant growth, maintaining the balance of reactive oxygen species metabolism in plants, and protecting membrane structure, contributing to plant stress tolerance[16]. The increase in SOD activity is also known to enhance the metabolism ofA.annuafor self-regulation. As shown in Fig.4, in sterile soil and sterile medium, the SOD activity ofA.annuainoculated with bacterial flora increased by 385.1% and 28.8% compared to CK, and that inoculated with fungi also increased significantly by 333.2% and 42.9%. Compared with the Z group, the SOD activity ofA.annuainoculated with bacterial flora and fungi simultaneously was significantly reduced by 20.1% and 11.0%, indicating that the inoculation of fungi will makeA.annuastressed and thus increase the SOD activity to enhance the resilience ofA.annua. The additional inoculation of bacterial flora has a significant role in maintaining the metabolic homeostasis and resilience ofA.annuaplants, and the bacterial flora inA.annuaand the pathogenic fungi fight against each other. The bacterial flora inA.annuacounteracts the pathogenic fungi and contributes to the metabolic balance.
In both sterile soil and sterile medium substrates, the SOD activity ofA.annuainoculated with dominant bacterial flora increased by 53.0% and 11.6% compared with CK, while in sterile soil substrate, the SOD activity ofA.annuainoculated with fungi was on the contrary lower. The SOD activity ofA.annuainoculated with fungi increased by 40.6% in the sterile medium substrate. The SOD activity ofA.annuainoculated with dominant bacterial flora and fungi simultaneously decreased by 29.6% compared with that in the Z group. Inoculation of dominant bacterial flora in both substrates had the same trend as previous inoculation of bacterial flora. In terms of probing SOD activity, it can be approximated that the dominant bacterial flora is the core flora of the bacterial flora.

Fig.4 Effect on SOD, a physiological indicator of Artemisia annua
MDA is a decomposition product when membrane lipid peroxidation occurs in plants, which can reflect the degree of plant injury under adverse conditions. Its accumulation will damage plant membranes and cells, and the oxidation of membrane lipids is also an important indicator of the impact on plants[17]. In sterile soil and sterile medium substrate, compared to CK, the MDA content after inoculation of bacterial flora increased by 56.7% and 21.5%, and inoculation of fungi resulted in a significant increase of MDA content by 70.3% and 502.5%, while co-inoculation of bacterial flora and fungi resulted in a reduction of MDA content by 9.0% and 56.7% compared to inoculation of fungi (Fig.5). In sterile soil, the MDA content ofA.annuainoculated with dominant bacterial flora was decreased compared to CK, and it was increased in the sterile medium. In both substrates, inoculation of fungi significantly increased the MDA content by 50.4% and 57.8% compared to the CK group, whereas the MDA content ofA.annuaafter co-cultivation of dominant bacterial flora and fungi was significantly reduced by 55.2% and 43.5% compared to the Z group. It indicates that in both substrates, inoculation with fungi stressesA.annuaand thus produces more MDA, and additional inoculation with bacterial flora and dominant bacterial flora has a significant effect on maintaining theinvivostress tolerance ofA.annuaplants and reduces the extent of the deleterious effect of inoculation with fungi. It indicates that bacterial flora and dominant bacterial flora will increase the resistance ofA.annuaby inhibiting pathogenic fungi. The MDA content ofA.annuainoculated with dominant bacterial flora and bacterial flora also had the same trend in both substrates, so the dominant bacterial flora can be approximated as the core flora of the bacterial flora in terms of MDA content.
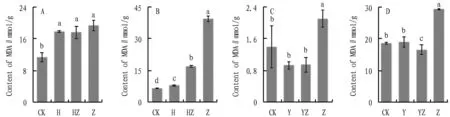
Fig.5 Effect on MDA, a physiological indicator of Artemisia annua
3.4 Effects on medicinal secondary metabolites ofA.annua
Artemisinin is extracted fromA.annuaand has a typical representative secondary metabolite, which is still the most effective anti-malarial drug, playing an important role in the treatment of tumors and the inhibition of cancer cells[18-19]. For this reason, the demand for artemisinin production is high. In Fig.6, the artemisinin content ofA.annuainoculated with bacterial communities decreased by 4.5% and 41.2%, and that inoculated with fungi significantly decreased by 98.4% and 23.8% compared to CK, but the artemisinin content ofA.annuainoculated with bacterial communities and fungi simultaneously in both matrices increased significantly compared to CK and Z groups. The artemisinin content ofA.annuainoculated with bacterial communities and fungi simultaneously in CK and Z group increased significantly. The artemisinin content ofA.annuainoculated with dominant bacterial communities and fungi simultaneously in both substrates was also significantly higher than that in CK and Z groups. This indicates that the inoculation of fungi in sterile soil and sterile medium will inhibit the metabolism ofA.annuainvivo, and the additional inoculation of bacterial flora and dominant bacterial flora can maintain the metabolic balance ofA.annuainvivo, andinvivobacterial flora and dominant bacterial flora will fight with the pathogenic fungi, thus balancing the metabolism and producing more artemisinin. The artemisinin content ofA.annuashowed the same trend when inoculated with dominant bacterial flora and bacterial flora, and it can be approximated that the dominant bacterial flora is the core flora of the bacterial flora in terms of artemisinin content.
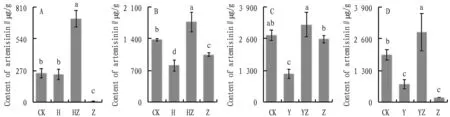
Fig.6 Effect on artemisinin, a secondary metabolite of Artemisia annua
In addition to artemisinin, scopoletin, cathelicidin phenol, and cathelicidin flavin all have really good antioxidant activity and also have a certain potentiation for artemisinin, which can synergistically improve the antimalarial ability of artemisinin[20]. In Fig.7, the scopoletin content ofA.annuainoculated with fungi was significantly lower than that in the CK group, and the content inoculated with bacterial flora and dominant bacterial flora were significantly higher than that in the CK group, while that in groups HZ and YZ in both substrates was significantly increased in comparison to Z group. This indicates that inoculation of fungi in sterile soil and sterile medium will inhibit the metabolism ofA.annuainvivo, and additional inoculation of bacterial flora and dominant bacterial flora can induce the relevant chemicals produced by the metabolism ofA.annua, thereby directly resisting the pathogenic fungi. In terms of scopoletin content, inoculation of dominant bacterial flora was consistent with inoculation of bacterial flora and could be approximated as the dominant bacterial flora being the core flora of the bacterial flora.

Fig.7 Effect on scopoletin, a secondary metabolite of Artemisia annua
In the sterile soil and sterile medium, the cathelicidin content ofA.annuain groups HZ and YZ was significantly higher than that in groups CK and Z (Fig.8). There was a consistency in the trend, additional inoculation of bacterial flora and dominant bacterial flora improved secondary metabolic resistance ofA.annuaagainst pathogenic fungi, and it can be approximated that the dominant bacterial flora is the core flora of the bacterial flora concerning cathelicidin content.
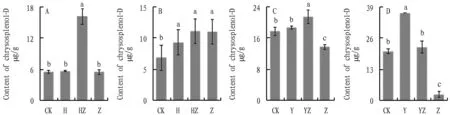
Fig.8 Effect on chrysosplenol-D, a secondary metabolite of Artemisia annua
In both substrates, the cathelicidin content ofA.annuain groups HZ and YZ was significantly higher than that in group Z and was significantly higher than that in group CK, and there was a consistency in the trend (Fig.9). Additional inoculation of bacterial flora and dominant bacterial flora improved the secondary metabolism ofA.annuato resist the pathogenic fungi, and it can be approximated that the dominant bacterial flora is a core group of the bacterial flora about cathelicidin content.
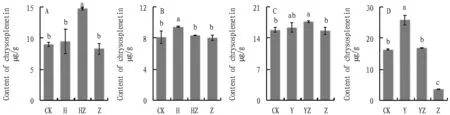
Fig.9 Effect on chrysosplenetin, a secondary metabolite of Artemisia annua
4 Discussion
4.1 Combinatorial bacterial communities and dominant bacterial flora against pathogenic fungiIn nature, all plants are subjected to stress from different levels, andA.annua, as the optimal source of artemisinin[21], is rarely attacked by pests and diseases, so there may be a link between the plant and bacterial community for pathogen suppression. The level of adaptation of the plant to its environment may stem from the influence of some of the bacterial communities[22], but it is unclear howA.annuaand bacterial community go about adapting to their environment. After conductinginvitrointeractions between bacterial communities and fungi and determining that bacterial communities inhibit fungi, the role of H and Y against pathogenic fungi inA.annuaplants was explored.
In the previous study, it was found that combined microbiota on leaf surface produces antibiotics and other substances against pathogenic fungi as a means of achieving normal growth of Arabidopsis plants, which is highly instructive in determining the importance of the microbiota of a plant species as a protective layer[23]. In the current study, H and Y may likewise be maintained and regulated inA.annuaplants through chemical signaling mediation. Artemisinin has been reported to have good antimicrobial activity in addition to its most typical antimalarial effects[18-19]. For example, scopoletin, cathelicidin, cathelicidin flavin,etc.have good antioxidant effects[19,24], which are potentiating for the antimicrobial activity of artemisinin, implying that they may be helpful in the inhibition of pathogenic fungi.
In physiological indicators, i.e. enzymatic antioxidant function, the results of this study also demonstrated that inoculation ofA.annuawith H and Y increases resistance to pathogenic fungi, which can reflect the response ofA.annuato the increased oxidative stress associated with pathogenic fungi[25].
Finally, in terms of the phenotype ofA.annuagrowth, it was possible to visualize the significant effect of plants inoculated with H and Y against pathogenic fungi.
4.2 Relationship between H and YAs reported in the previous study, a simplified synthetic bacterial community was obtained from a unique microbiota aggregated in the maize root system. The assembly of this was very simplified, but representative synthetic bacterial model community enabled the study of the kinetics and function of community assembly on sterile maize seedlings[8]. It interfered with the growth of a phytopathogenic fungus, thus protecting the plant. This model system will prove to be a useful system for future plant-microbe interaction studies[8,26]. In Fig.10, the H was reassembled into a Y by OUT analysis of 16S rDNA to obtain the bacteria with relative abundance in the top 1%. Then the phenotypes, physiological indices, and secondary metabolites were probed to obtain the Y equivalent to the H, proving that the Y is the core group of the H. The assembled bacterial community can be replaced by a more simplified equivalent of the dominant bacterial community, and the function of this simplified representative bacterial community on sterileA.annuaseedlings can be directly investigated[23], which can protect the plant and promote the plant growth by inhibiting the pathogenic fungi.
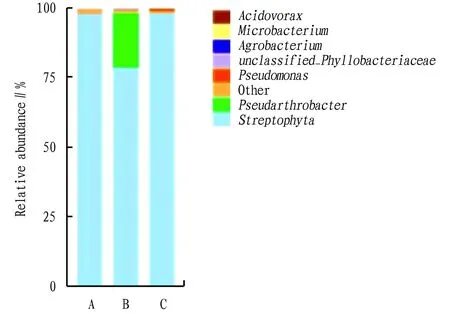
Note: A. A. annua with Proteobacteria assemblage in medium; B. A. annua with actinomycetes assemblage in medium; C. A. annua with thick-walled phyla assemblage in medium.
4.3 Mechanistic conception of simplified bacterial community against pathogenic fungiA blank control group, a control group of the Y, a fungal control group, and a HZ experimental group are often designed. During the validation process, the enzymatic and non-enzymatic antioxidant function can be obtained. Inoculation of fungi will cause harm toA.annua, inoculation of Y to a certain extent will makeA.annuagrow more robustly, and HZ can mitigate and reduce fungal damage to plants. In this study, we found that the Y inhibited the growth of pathogenic fungi in the plant through a signal-mediated manner, thus indicating that the Y can be used as a biocide to help the plant grow normally or to promote the growth of the plant[27]. In conclusion, this Y modeling system with more subtle and detailed mechanisms of interaction with plants deserves more research and exploration in the future and is a mechanistic model with great potential for development[28].
5 Conclusions
Under sterile soil and sterile media substrates, microorganisms with various ecological functions can be assembled into communities and simplified into synthetic bacterial communities with major roles that may be mediated by chemical signals in plants to defend against pathogen invasion. Such simplified bacterial communities can be used as biocides for biological disease control in plants.
- 植物病虫害研究(英文版)的其它文章
- Comprehensive Prevention and Control Technology of Vegetable Diseases and Pests in Shandong Province
- Technical Points of Green Prevention and Control Technology of Major Diseases and Pests in Lixian Rhubarb (Rheum palmatum L.)
- Risk Analysis of Passalora sequoiae Invasion to China with Imported Coniferous Wood
- Determination of Ten Kinds of Alpha-2 Agonists Residues in Animal Derived Food by UHPLC-Triple Quadrupole/Composite Linear Ion Trap Mass Spectrometry
- Investigation and Analysis of Main Diseases of Landscape Plants in the Main Urban Area of Lu’an City
- Effects of Low Temperature Stress on Germination and Physiological Characteristics of Different Sweet Maize Varieties

