基于半夹心Cp*Rh单元的超分子桥环和螺环化合物的构筑策略
高 翔 牟秋水 林悦健 金国新
(复旦大学化学系,分子催化与功能材料上海市重点实验室,聚合物与分子材料国家重点实验室,上海 200433)
Cyclic compound was one kind of the most significant compound in organic chemistry.If some atoms simply connected head to tail, regular cyclic compounds were obtained such as cyclohexane or tetrahydrofuran.However, some complex situations usually happen in common compounds.Two or more atoms were shared by two or more rings in some compounds,which were called bridged compounds such as bicyclo[3,2,0]heptane.Similarly, spirocyclic compounds were defined by the situation in which only one atom was shared by two or more rings, such as sprio[4,5]decane(Fig.1a)[1].With the rapid development of modern chemistry, the amount of research fields was increasing and some scientists in supramolecular chemistry pay attention to cyclic compounds.
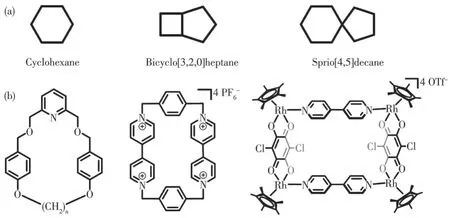
Fig.1 (a)Organic cyclic hydrocarbon compounds(left),bridged hydrocarbon compounds(middle),and spirocyclic hydrocarbon compounds(right);(b)Examples of supramolecular cyclic compounds
In recent decades, scientists have synthesized some supramolecular macrocycles referring to the shape of regular organic cyclic compounds in the supramolecular chemistry field[2].David A.Leigh and his co-workers synthesized an ether macrocycle by organic coupling reaction,which was a pure organic electroneutral molecule (Fig.1b, left)[3].J.Fraser Stoddart and his co-workers obtained a cationic macrocycle by coupling reaction of 1,4-dibromoxylene and 4,4′-bipyridine,which was neutralized by PF6-(Fig.1b, middle)[4].The supramolecular macrocycle mentioned above were organic compounds without metal ions.Coordinationdriven chemical self-assembly developed rapidly in recent decades[5].Therefore, plentiful scientists tried to construct supramolecular macrocycles by inorganic selfassembly method.M.Fujita′s[6]research group and Peter J.Stang′s[7]research group constructed coordinative macrocycles by Pd(Ⅱ)or Pt(Ⅱ)metal corners.In the past 20 years, the Cp*Rh unit attracted scientists′sight, which had three free orthogonal coordinative sites and was convenient for designing macrocycles.JIN Guoxin and his co-workers synthesized a supramolecular macrocycle by coordination-driven self-assembly of 4,4′-bipyridine and Cp*Rh units(Fig.1b,right)[8].In addition, some more complicated derivates of supramolecular macrocycles were synthesized such as catenanes[9-10], knots[11-12], and ravels[13].However, too many sights concentrated the research on macrocycles and their derivates but ignored research on other cyclic supramolecular compounds.
In this article, we chose to research some abnormal rings rather than regular cyclic compounds.We design supramolecular bridged cyclic compounds and spirocyclic cyclic compounds referring to the shape of organic bridged or spirocyclic molecules by coordination-driven self-assembly method featuring half-sandwich Cp*Rh building blocks.Through observing the self-assembly process of regular macrocycles in previous research (Fig.2a), we found that if an extra arm could be inserted into the macrocycle as a‘bridge’,extra coordinative sites were necessary for the linear ligand to immobilize extra metal ions.After the addition of the assistant bidentate ligand with the proper length to connect two extra metal ions, a supramolecular bridged cyclic compound was synthesized successfully as shown in Fig.2b.Besides, a similar strategy could also be applied to construct supramolecular spirocyclic compounds featuring Cp*Rh building blocks.Subsequently, we start designing proper ligands and choosing proper building blocks to construct supramolecular bridged or spirocyclic compounds to demonstrate our strategy.
1 Experimental
Compound (Cp*Rh(OTf)2)2[14], binuclear building blocks B1[15], B2[16], B3[17], B4[18]and rigid ligand LA[19],LB[20]were synthesized as the literature report, where B1=[(Cp*Rh)2(μ-η2-η2-C2O4)](OTf)2,OTf-=CF3SO3-,B2=[(Cp*Rh)2(dhbq)] (OTf)2, dhbq=2, 5-dihydroxy-1, 4-benzoquinone, B3= [(Cp*Rh)2(tpphz)] (OTf)4), tpphz=tetrapyrido[3,2-a∶2′,3′-c∶3″,2″-h∶2‴,3‴-j]phenazine,B4=[(Cp*Rh)2(bibzim)](OTf)2, bibzim=2,2′-bisbenzimidazole,LA=3,3′-di(pyridin-4-yl)-2,2′-bipyridine,LB=4,4′-di(pyridin-4-yl)-1,1′-biphenyl (Scheme 1).Assistant ligand pyrazine and 1,2-di(pyridin-4-yl)ethane(bpea) were purchased in the common commercial way.Methanol (≥99.7%), diethyl ether (≥99.7%), andN,Ndimethylformamide (DMF, ≥99.5%) were directly used after purchase, and no more purified.Elemental analyses (C, H, N) were carried out on an Elemental Vario EL Ⅲanalyzer.NMR spectra (1H,1H-1H COSY, and1H DOSY) were recorded on Bruker AVANCE ⅢHD 400 MHz spectrometers at room temperature.Chemical shifts (δ) were reported relative to the residual solvent peak(δH=1.94 for CD3CN).X-ray intensities of the complexes were collected on a Bruker D8 Venture diffractometer with CuKα(λ=0.154 178 nm) or GaKα(λ=0.134 138 nm) using the SMART and SAINT programs.The structure was solved by direct methods and refined onF2by full-matrix least-squares methods with SHELXTL-2018.In the structural refinement, except the partly occupied solvent molecules and the disordered parts in the cage, the other non-hydrogen atoms were refined anisotropically and hydrogen atoms within the ligand backbone were fixed geometrically at calculated distances and allowed to ride on the parent nonhydrogen atoms.The highly disordered state of the incorporated molecule solvents meant that lots of them could not be located,and hence in the final refinement,the electron density was treated with the SQUEEZE routine in the PLATON program package.The NMR spectra, ESI-MS data, and crystal data of the complexes can be found in the Supporting information.
1.1 Synthesis of[(Cp*Rh)6(μ-η2-η2-C2O4)2(μ-C2O4)(LA)2](OTf)6(1)
Compound B1 (38.8 mg, 0.045 mmol) was dissolved into 6.0 mL methanol, followed by the addition of ligand LA(9.30 mg, 0.03 mmol).After stirring overnight, much precipitate was observed.Therefore, DMF(1.5 mL) was added into the self-assembly system,which improved solubility obviously after stirring for another 8 h.Orange solution was obtained after centrifugation.The solution was filtered through a filter membrane, and the filtrate was crystallized by diethyl ether diffusion to obtain the bridged cyclic compound 1,which was washed with diethyl ether and dried under a vacuum.Yield: 41.4 mg, 86%.1H NMR (400 MHz,CD3CN):δ8.99(s,α-H in middle pyridyl,4H),8.63(d,J=8.4 Hz,β-H in middle pyridyl, 4H), 8.56 (d,J=8.4 Hz,γ-H in middle pyridyl,4H),8.26(d,J=5.7 Hz,α-H in terminal pyridyl, 8H), 7.80 (d,J=5.7 Hz,β-H in terminal pyridyl, 8H), 1.60-1.63 (90H, H of Cp*).Anal.Calcd.for C112H118O30N8S6F18Rh6(%): C 41.93, H 3.71,N 3.49;Found(%):C 41.77,H 4.06,N 3.63.
1.2 Synthesis of [(Cp*Rh)6(dhbq)2(pyrazine)(LA)2](OTf)8(2)
The (Cp*Rh(OTf)2)2solution (1.5 mL, 0.01 mmol·mL-1) was mixed with ligand LA(9.30 mg, 0.03 mmol)and stirred for 2 h,followed by the addition of B2 solution (27.4 mg in 6.0 mL methanol, 0.03 mmol).After stirring for 5 h, a methanol solution of assistant ligand pyrazine (1.5 mL, 0.01 mmol·mL-1) was added into the turbid liquid and then DMF (1.0 mL) was added to improve solubility.After stirring overnight and centrifugation, the brown solution was filtered through a filter membrane, and the filtrate was crystallized by diethyl ether diffusion to obtain the bridged cyclic compound 2, which was washed with diethyl ether and dried under a vacuum.Yield: 43.7 mg, 81%.NMR (400 MHz, CD3CN):δ8.8-9.5 (α-H in middle pyridyl, 4H),8.73, 7.99(β-H in middle pyridyl, 4H), 8.67, 8.44(α-H in terminal pyridyl,8H),8.49(d,J=4.8Hz,H in pyrazine,8H),8.51,7.97(γ-H in middle pyridyl,4H),8.14,7.88(β-H in terminal pyridyl, 8H), 5.66 (H in B2, 4H), 1.6-1.7(H in Cp*, 90H).Anal.Calcd.for C124H126O32N10S8F24Rh6(%): C 41.39, H 3.53, N 3.89; Found(%): C 41.20,H 3.76,N 4.07.
1.3 Synthesis of [(Cp*Rh)6(tpphz)2(bpea)(LA)2](OTf)12(3)
Bridged cyclic compound 3 was synthesized according to a similar procedure as compound 2 except that B2 was replaced by B3(43.5 mg in 10.0 mL methanol, 0.03 mmol) and pyrazine was replaced by assistant ligand bpea (2.8 mg, 0.015 mmol).Yellow compound 3 was obtained after recrystallization.Yield:55.2 mg, 77%.NMR (400 MHz, CD3CN): δ 10.1-10.4(α-H of B3, 8H), 9.7-9.9 (γ-H of B3, 8H), 9.20, 9.04(α-H in middle pyridyl, 4H), 8.71 (β-H of middle pyridyl,4H),8.69,8.59(α-H of terminal pyridyl,8H),8.61(β-H of B3, 8H), 8.52 (γ-H in middle pyridyl, 4H),8.43 (H in —CH2— of bpea, 4H), 7.96, 7.69 (α-H in pyridyl of bpea, 8H), 7.93, 7.84 (β-H of terminal pyridyl, 8H), 6.61, 6.56 (β-H in pyridyl of bpea, 8H), 1.3-1.8(H in Cp*, 90H).Anal.Calcd.for C172H154O36N22S12F36Rh6(%): C 43.11, H 3.24, N 6.43; Found(%): C 43.29,H 3.58,N 6.26.
1.4 Synthesis of (Cp*Rh)12(bibzim)3Ru(LA)3(LB)3](OTf)10(PF6)4(4)
Pre-4 was a precursor compound of spirocyclic compound 4.It could be described as [Ru(LA)3](PF6)2.It was synthesized as the literature method[21].pre-4(6.61 mg, 0.005 mmol) was dissolved into acetonitrile (3.0 mL), which was poured into a solution of B4 (30.2 mg in 6.0 mL methanol, 0.03 mmol).After stirring for 5 h,assistant ligand LB(4.6 mg, 0.015 mmol) was added into the system before DMF (1.0 mL) was added to improve its solubility.After 8 h stirring and centrifugation, an orange-yellow solution was filtered through a filter membrane, and the filtrate was crystallized by diethyl ether diffusion to obtain the spirocyclic compound 4, which was washed with diethyl ether and dried under a vacuum.Yield: 32.7 mg, 79%.NMR(400 MHz, CD3CN):δ8.54, 8.50, 8.29 (α-H of middle pyridyl, 4H), 8.07, 7.95 (α-H of pyridyl in LB, 8H),8.01 (H of B4, 2H), 7.86 (H of B4, 2H), 7.71, 7.64 (α-H of terminal pyridyl in LA,4H),7.55,7.37(β-H of pyridyl in LB, 4H), 7.51 (H of B4, 2H), 7.44 (H of B4, 2H),7.32 (γ-H of pyridyl in LA, 2H), 7.22, 7.02 (β-H of terminal pyridyl in LA, 4H), 7.20 (outer H of biphenyl in LB, 4H), 6.76, 6.68 (inner H of biphenyl in LB, 4H),5.89 (β-H of pyridyl in LA, 2H), 1.1-2.1(H in Cp*,60H).Anal.Calcd.for C340H318O30N42S10F54P4Rh12Ru(%):C 49.33, H 3.87, N 7.11; Found(%): C 49.09, H 4.20,N 6.94.
CCDC: 2296510, 1; 2296511, 2; 2296512, 3;2296513,4.
2 Results and discussion
In our construction strategy mentioned above,extra coordinative sites were necessary for rigid ligands.Therefore, a 2,2′-bipyridyl group was inserted into a rigid ligand as a chelate site.After coupling with another two pyridin-4-yl groups, the main ligand LAwas synthesized successfully,which was chosen to selfassemble with building blocks with different lengths including B1, B2, B3, and B4.In addition, another assistant ligand was also necessary to connect two extra metal ions, whose length should match the length of building blocks.By coordination-driven stepwise selfassembly method, supramolecular bridged and spirocyclic compounds were obtained as shown in Scheme 1.
Firstly, building block B1 was chosen to assemble with LA.A bridged cyclic supramolecular compound 1 was obtained as shown in Fig.3a.From its single crystal X-ray diffraction result (Fig.3b), two extra Cp*Rh units,which belong to another B1 originally,are chelated by two 2,2′-bipyridyl in LA.The original tetradentate chelating ligand C2O42-changes to a bidentate coordinative ligand after revolving to connect two Cp*Rh units as a bridge.Therefore, a bridged cyclic compound 1 was synthesized successfully, composed of two B1 units, two main LAligands, two extra Cp*Rh units,and an assistant ligand C2O42-.In ad dition, the location of two extra Cp*Rh was on the same side of ligand LAas shown in the top view of the crystal structure(Fig.3c).Ball-and-stick model reminds us that compound 1 is indeed a bridged cyclic compound(Fig.3d).
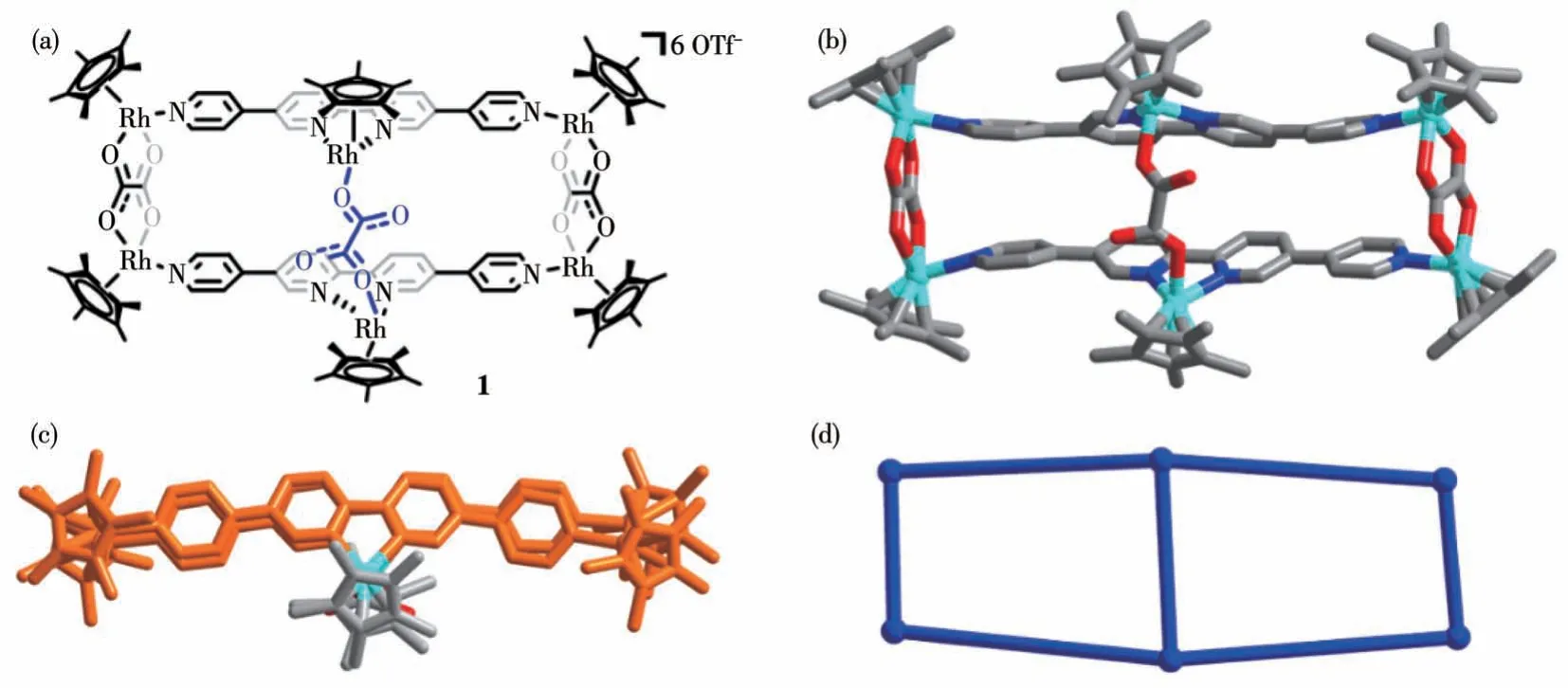
Fig.3 (a)Chemical structure of bridged compound 1;(b)Crystal structure of 1;(c)Top view of 1;(d)Ball-and-stick model of 1
In the above description, compound 1 could be constructed by self-assembly of B1 and LAin a molar ratio of 3∶2.One of C2O42-loses its chelating tetradentate coordinative formation and uses bidentate coordinative formation, which has proper length matching the distance between two extra Cp*Rh units.However, the same situation did not happen when longer - length building block B2 was chosen.If the same ratio(nB2∶nLA=3∶2)was applied,no crystal could be obtained.
To synthesize a bridged cyclic compound by B2 and LA,a stepwise assembly strategy was applied.Firstly, [Cp*Rh(OTf)2]2was mixed with LAat 1∶2 ratio to occupy chelating sites, followed by the addition of B2(nB2∶nLA=2∶2).Eventually, pyrazine was added into the mixture as assistant ligand to connect two Cp*Rh unit(npyrazine∶nLA=1∶2), which constructed bridged cyclic compounds 2 successfully as shown in Fig.4a.
The single crystal X-ray diffraction result reminds us that 2 is indeed a bridged cyclic compound (Fig.4b).The length of pyrazine matches the distance between two extra Rh ions properly.Besides, we can observe that the location of two extra Cp*Rh is also at the same side of ligand LAfrom its crystal structure, which was similar to compound 1.
After compound 2 was synthesized, we continued extending the length of the building blocks.Therefore,building block B3 was chosen with a 1.286 nm length.Compound 3 was synthesized in the same procedure as that of compound 2 except different assistant ligand.In the case of B3 (1.286 nm) is much longer than B2(0.799 nm), the assistant ligand should also be extended longer.Therefore, a flexible bidentate ligand bpea was chosen to connect two extra Rh ions as the assistant ligand.
A bridged cyclic compound 3 was obtained by the stepwise assemble method (Fig.5a).Single crystal Xray diffraction result reminds us compound 3 is also a bridged cyclic compound as shown in Fig.5b.Different phenomenon is observed in its crystal structure that two extra Cp*Rh units locate at both side of LAwhen they are at same side in compounds 1 and 2 (compared Fig.5c with Fig.3c).Enough length and its flexibility might be reasons of this phenomenon.Ball-and-stick model of 3 also exhibited different sides of two extra Cp·Rh units(Fig.5d).

Fig.5 (a)Chemical structure of bridged compound 3;(b)Crystal structure of 3;(c)Top view of 3;(d)Ball-and-stick model of 3
In bridged cyclic compounds 1,2,and 3,[(Cp*Rh)2(assistant ligand)] is the bridge to connect two rings.If we expect to obtain spirocyclic compounds,only one atom can be shared by different rings, which are metal ions in general.However,the same Cp*Rh unit can not be the shared center as compounds 1, 2, and 3 do.The reason is that Cp*Rh has only three free coordination sites,which does not allow the existence of two or more chelating groups.Therefore, another connection center should be considered.We observed free Rh (Ⅲ), Ir(Ⅲ),and Ru(Ⅱ)ions without Cp* have six free coordination sites, which can be chelated exactly by three 2,2′-bipyridyl groups.Considering obstacles of reactivity between RhCl3/IrCl3and 2,2′-bipyridyl groups,Ru(Ⅱ)ions were selected as the shared center.
Although chelating reactivity between RuCl3and LAis better than RhCl3/IrCl3, a high reaction temperature (180 ℃) is still acquired in the chelating procedure, which is much different from the following selfassembly process.Therefore, stepwise assembly is still necessary in the construction of this spirocyclic compound.The first step was the synthesis of pre-4 by the reaction of LAand RuCl3·3H2O in glycol under 180 ℃,in which Ru(Ⅲ)was reduced to Ru(Ⅱ)to obtain six free coordination sites.After the successful construction of pre-4, it was selected to assemble with building block B4 (6.0 Equiv.to pre-4).Followed by the addition of assistant ligand LBto occupy residual coordination sites, the spirocyclic compound 4 was obtained(Scheme 2).A situation should be explained that LAcannot become the assistant ligand because of its extra chelating sites which will continue coordinating with other ions and make the situation too complicated and unpredictable.Therefore, ligand LB(Scheme 2) was selected to be the assistant ligand due to its almost the same length as main ligand LAbut no extra coordination sites.

Scheme 2 Stepwise assemble synthesis of spirocyclic compound 4
Single crystal X-ray diffraction result reminds us of the successful construction of spirocyclic compound 4 (Fig.6b), demonstrating the validity of our strategy shown in Scheme 2.From Fig.6b, we can observe that each ring is made up of two different ligands (an LAand an LB), which profit from the stepwise assembly method.The LAarm links a Ru(Ⅱ)ion by its chelating sites with another two LAarms in different rings.The LBarm only plays a connection role to complete the formation of a rectangle ring.The Ru(Ⅱ)ion is the only connection between the three rings and the supramolecular spirocyclic compound 4 is synthesized successfully as we predicted.Due to the narrow space in each ring which is caused by the short length of the building block B4 (0.560 nm from Rh to Rh), three rings of 4 can not cross with each other (Fig.6c).The simplified structure of 4 is more obvious (Fig.6d), which exhibits topology of 4 is a supramolecular spirocyclic compound exactly.

Fig.6 (a)Chemical structure of spirocyclic compound 4;(b)Composition of each ring in compound 4;(c)Single-crystal structure 4;(d)Simplified structure of 4
3 Conclusions
Supramolecular macrocycles were synthesized by modeling organic cyclic compounds in the past few decades.In this work,we extended the research to synthesize supramolecular bridged compounds and spirocyclic compounds by coordination - driven chemical assembly method featuring half-sandwich Cp*Rh units.Through detailed observation of previous regular macrocycles, we guess the addition of extra chelating sites in rigid ligands contributed to these constructions.Therefore, a rigid ligand (LA) with an extra 2,2′-bipyridyl group was designed and chosen to assemble with different building blocks as the main ligand.Through stepwise assembly with building blocks and assistant ligand, three coordinative supramolecular bridged cyclic compounds (compounds 1-3) and one supramolecular spirocyclic (compound 4) were constructed successfully as we designed and predicted.This coordination-driven stepwise assemble strategy is particularly effective in the construction of these various supramolecular compounds, which is also significant in providing reference to synthesize more complicated compounds in the near future.
Supporting information is available at http://www.wjhxxb.cn

