Smart epidermal electrophysiological electrodes:Materials,structures,and algorithms
Yuanming Ye, Haochao Wang, Yanqiu Tian, Kunpeng Gao, Minghao Wang, Xuanqi Wang, Zekai Liang, Xiaoli You, Shan Gao, Dian Shao,,a) and Bowen Ji,a)
AFFILIATIONS
1 Unmanned System Research Institute,Northwestern Polytechnical University,Xi’an 710072,China
2Queen Mary University of London Engineering School,Northwestern Polytechnical University,Xi’an 710072,China
3School of Electronics and Information,Northwestern Polytechnical University,Xi’an 710072,China
4School of Life Sciences,Northwestern Polytechnical University,Xi’an 710072,China
5College of Information Science and Technology,Donghua University,Shanghai 201620,China
6College of Electronics and Information,Hangzhou Dianzi University,Hangzhou 310018,China
ABSTRACT Epidermal electrophysiological monitoring has garnered significan attention for its potential in medical diagnosis and healthcare,particularly in continuous signal recording.However,simultaneously satisfying skin compliance,mechanical properties,environmental adaptation,and biocompatibility to avoid signal attenuation and motion artifacts is challenging,and accurate physiological feature extraction necessitates effective signal-processing algorithms.This review presents the latest advancements in smart electrodes for epidermal electrophysiological monitoring,focusing on materials,structures,and algorithms.First,smart materials incorporating self-adhesion,self-healing,and self-sensing functions offer promising solutions for long-term monitoring.Second,smart meso-structures,together with micro/nanostructures endowed the electrodes with self-adaption and multifunctionality.Third,intelligent algorithms give smart electrodes a “soul,” facilitating faster and more-accurate identificatio of required information via automatic processing of collected electrical signals.Finally,the existing challenges and future opportunities for developing smart electrodes are discussed.Recognized as a crucial direction for next-generation epidermal electrodes,intelligence holds the potential for extensive,effective,and transformative applications in the future.
KEYWORDS Epidermal electrodes,Electrophysiological signal monitoring,Smart materials,Smart structures,Intelligent algorithms
I.INTRODUCTION
Epidermal electrophysiological signals reveal the states of bioelectrical activity associated with mainly heartbeats,brain activities,and muscle contractions,which are crucial in medical diagnosis and healthcare monitoring.1–3However,daily recording of bioelectricity is seriously restricted by motion artifacts,low permeability,and risk of skin irritation,which is why recent years have seen attention given to electrodes in stable conformal contact with the skin and providing accurate,long-term,and repeated monitoring.4–6In particular,the emergence of smart electrodes offers a possible self-adaptive solution for compliant interfaces and high-fidelit signal acquisition.Specifically smart electrodes are characterized by smart functions involving self-adaptation,self-sensing,stimuli response,multimodal monitoring,and self-processing,and these novel functions originate mainly from materials,structures,and algorithms(Fig.1).
Smart electrode materials can respond to environmental changes and external stimuli via functions such as self-sensing,self-adaptation,and self-healing,thereby mimicking biological intelligence.7Reversible bonds contribute to adhesion and healing either autonomously or with the aid of stimuli,and piezoelectric materials provide an alternative for signal recording.
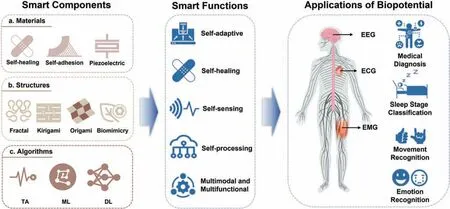
FIG.1.Overview of smart epidermal electrophysiological electrodes from smart components to smart functions to applications.
Smart structures endow electrodes with intelligence via mesoscale and microscale structural designs.These structures enable adaptation to various environments,including complex 3D surfaces,cold and hot temperatures,and moist conditions.8–10Mesostructures facilitate 3D deformation to compensate for plane strain in conformal structures,while biomimetic strategies are used widely for micro/nanostructure array design.
Intelligent algorithms play important roles in signal processing,mainly in the stages of(i)signal acquisition,(ii)preprocessing,(iii)feature extraction,(iv) feature optimization,and (v) feature classification After signal acquisition,preprocessing algorithms are used to eliminate noise.Regarding feature extraction and optimization,deep learning(DL)frameworks have been used widely for accuracy and speed in extracting spatiotemporal features,and then optimizing those features helps in simplifying the feature vector,involving mainly feature selection and dimensionality reduction.Finally,classificatio is performed as required by specifi tasks to better serve practical scenarios such as healthcare11and sentiment recognition classification12
This review highlights recent advances in smart electrodes.First,we discuss examples of smart materials from the perspectives of self-adhesion,self-healing,and self-sensing,then we review meso-structures for 3D conformation and micro/nanostructures targeting self-adhesion and self-drainage.Next,the acquired signals are investigated and discussed in detail in the order of the fiv aforementioned stages of signal processing.Finally,we discuss issues and outlooks for future smart electrodes.With the rapid development of smart electrodes,we expect more-accurate signals,longer monitoring times,and more-convenient applications.
II.SMART MATERIALS
Regarded as the fourth generation of materials,smart materials lead a new trend for materials that can intrinsically sense or respond to the environment,such as via mechanical,optical,thermal,electrical,and magnetic stimulation.13By embedding smart materials into electrodes,the resulting smart devices achieve self-adaptive functions such as self-adhesion,self-healing,and self-sensing.
A.Self-adhesive materials
With the aim of long-term monitoring,the mechanical mismatch between tissues and electronics has been studied extensively for the corresponding challenges of unstable signals,high interfacial impedance,motion artifacts,and even inflammator responses.14Currently,self-adhesive materials are regarded as candidates for resolving the above issues because of their strong adhesion and balanced cohesion when mechanical deformations appear.Moreover,much effort has gone into developing an all-in-one electronic system based on self-adhesive materials.15
1. Mechanical design strategies
On one hand,soft materials with lower mechanical modulus are more adaptable.For example,in 2014 Leeet al.16reported a mixture of carbon nanotubes (CNTs) and adhesive polydimethylsiloxane (aPDMS) that had a modulus of 27.5 kPa,comparable to that of skin,thereby enabling it to conform to skin wrinkles with higher mechanical matching [Fig.2(a)].On the other hand,thin film provide higher adhesion compared to bulk systems,a representative example being a sub-300-nm electrode with a similar surface morphology to that of skin.17Its larger contact area and surface roughness were attributed to its adhesiveness,resulting in an average peel strength of 135.09 mN/cm and long-term monitoring for up to 10 h[Fig.2(b)].Therefore,appropriate modulus and low thickness enhance the self-adhesion performance of electrodes mechanically.
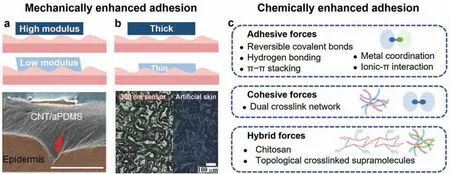
FIG.2.Designs of self-adhesive materials in epidermal electrophysiological electrodes.(a)Upper two images:improving adhesion by reducing mechanical modulus;lower image:penetration of mixture of carbon nanotubes(CNTs)and adhesive polydimethylsiloxane(aPDMS)into wrinkles on skin(scale bar:100 μm).16 (b)Upper two images:improving adhesion by reducing fil thickness;lower image:similar morphologies of 300-nm ultra-flexibl fil (left)and artificia skin(right).17 (c)Overview of chemically enhanced adhesion by adhesive,cohesive,and hybrid forces.Figures reprinted with permissions from (a) Springer Nature,Copyright 2014;(b) John Wiley and Sons,Copyright 2018.
2. Supramolecular chemistry design strategies
Hydrogels and ionic gels are crosslinked polymers with enriched water content and dispersed ions,respectively.Thanks to their abundant hydroxyl groups,adjustable softness,and biocompatibility,gels are possible candidates for self-adhesive materials,18and their weak intermolecular forces,covalent bonds,and mechanical topological crosslinks work either individually or cooperatively to form dynamic multiple networks.
a.Enhanced interfacial adhesive forces.Interfacial chemical interactions are represented by covalent and noncovalent bonds.Noncovalent intermolecular forces including hydrogen bonding,metal coordination,π–π stackings,and ionic–π interactions occur at the electrode–skin interface,chemically strengthening the adhesion.Polydopamine (PDA),19tannic acid (TA),20D-sorbitol,1and polyacrylic acid(PAAc)21are typical adhesives that blend physically with the conductive and network-supporting components.
PDA chains are rich in catechol groups,whose phenol hydroxyl groups bond strongly with active groups on the skin,and other supramolecular interactions such as cation–π and π–π interactions also occur at the interface,enabling a hydrogel with self-adhesive properties.For example,a self-adhesive hydrogel involving PDAdecorated CNTs maintained a tissue adhesion of over 60 kPa for 30 d at room temperature9[Fig.3(a)].Meanwhile,TA is a biocompatible polyphenol similar to dopamine [Fig.3(b)],and the catechol groups afford various covalent and dynamic noncovalent bonds with the skin,especially multiple hydrogen bonds.20For example,a ternary system of a hydrogel reinforced with TA-coated cellulose nanocrystals provided repeatable self-adhesion by bridging the crosslinks of poly(acrylic acid) (PAA) and polyaniline (PANI),20and the self-adhesion could be also realized under water.22Furthermore,D-sorbitol is another inherent bioadhesive component.For example,in 2020 Zhanget al.1fabricated a poly(ethylene dioxythiophene):poly(styrene sulfonate)/waterborne polyurethane/D-sorbitol(PEDOT:PSS/WPU/D-sorbitol) fil that kept conformal contact with human skin with body movements[Fig.3(c)].Besides hydrogels,biocompatible ionogels also show promising applications.For example,a PAAc–DES gel21based on the abundant–COOH groups of PAAc and deep eutectic solvents (DES) had an adhesion of 100 N/m[Fig.3(d)].
Additionally,because hydrogen bonds are highly sensitive to temperature,temperature-responsive hydrogels have been developed to realize multiple adhesion and detaching.At elevated temperatures,the breaking of interior hydrogel bonds enables more interfacial interactions with the skin.In 2022,Liuet al.26developed a PVA/PA/Gel hydrogel that controlled adhesion by activating ionic and hydrogen bonds at the machine–skin interface by heating.Another temperature effect is phase transformation.For example,a paintable gelatin biogel with a fluid–ge transition temperature for on-skin application had dynamic compliance with a hairy scalp,27resulting in reversible adhesion of 0.80 ± 0.076 N/cm by spontaneous gelatinization and hot-watertriggered detachment.Therefore,blending traditional substrate materials with adhesive materials is one of the main approaches to self-adhesive electrodes.
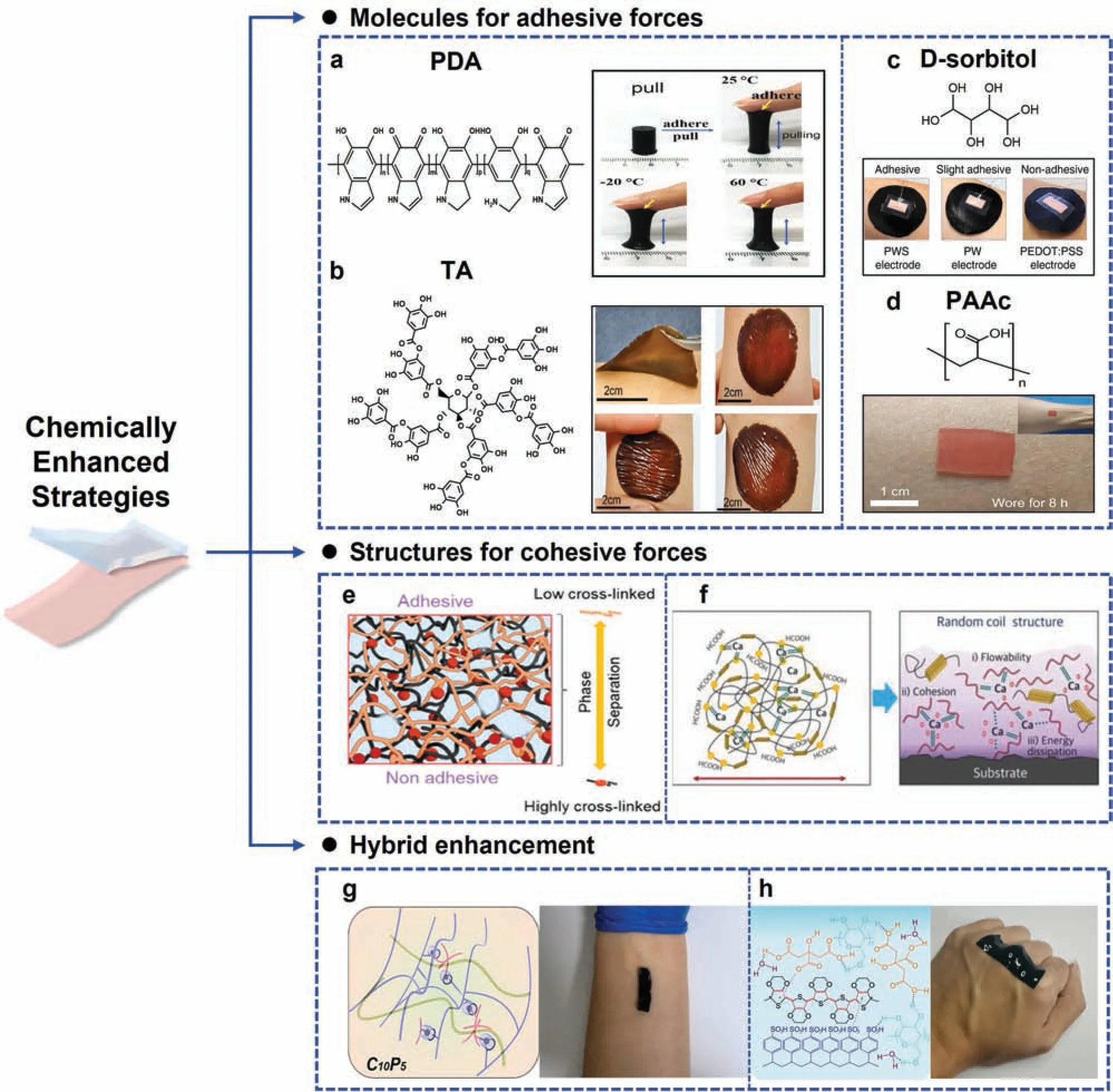
FIG.3. Strategies for chemically enhanced adhesion.(a) Left: chemical structure of polydopamine (PDA);right: adhesion of hydrogel containing PDA-decorated CNTs at various temperatures.9 (b)Left:chemical structure of tannic acid(TA);right:self-adhesion of WDT(water dispersible transition)eutectogels on skin at rest,stretched,compressed,and twisted.22 (c)Upper:chemical structure of D-sorbitol;lower:adhesion of PEDOT:PSS-based electrode.1 (d)Upper:chemical structure of polyacrylic acid(PAAc);lower:skin adhesion of PAAc–DES ionogel.21(e)Crosslink density and adhesion controlled by hyaluronic acid.23(f)Dual crosslink network and Ca2+synergistically enhanced adhesion.24(g)Chitosan-based C10P5 hydrogel and its adhesion on an arm.25(h)Topological crosslinked supramolecular solvent solution and the adaptability of the electrode to the skin.15 Figures reprinted with permissions from(a)John Wiley and Sons,Copyright 2018;(b)American Chemical Society,Copyright 2021;(c)Springer Nature,Copyright 2020;(d)Springer Nature,Copyright 2021;(e)American Chemical Society,Copyright 2022;(f)John Wiley and Sons,Copyright 2018;(g)Elsevier,Copyright 2022;(h)Springer Nature,Copyright 2022.
b.Enhanced cohesive forces.As another important factor contributing to adhesion,increasing the cohesive force is also essential for self-adhesion,and introducing dual networks is a widely developed strategy for doing so.Interactions between crosslinks strengthen the elastic modulus and toughness,offering sufficien cohesion,28but it is also important to consider stretchability when aiming for conformal attachment.29By adjusting the crosslink density within the dual network,it becomes possible to achieve asymmetrical adhesions.For example,the adhesion of a PDMS network was highly dependent on the density of crosslinks,which was manipulated by blending hyaluronic acid23[Fig.3(e)].Meanwhile,metallic ions such as Ca2+provide metal chelation for higher cohesion.For example,Ca-modifie silk fibroi exhibited high random coil content and viscoelasticity for strong cohesion24[Fig.3(f)],with the peel strength reaching 429±18 N/m at 20%humidity.However,findin a balance between adhesive and cohesive forces is crucial for achieving effective overall adhesion,particularly when both forces operate through similar mechanisms.
c.Comprehensively enhanced.In the latest designs,both cohesion and adhesion enhancement mechanisms have been realized.In particular,chitosan (CS)—a cationic biopolymer that is biocompatible,antibacterial,and biodegradable—has been used and modifie for bioadhesives.25Notably,in 2022 Wanget al.25reported a series of conductive hydrogels based on CS,water-soluble polypyrrole,and cucurbit[7]uril,with the hydrogen bonds of CS ensuring strong adhesion and cohesion separately [Fig.3(g)].Another strategy is introducing topological crosslinked supramolecules: the inherent mechanically interlocking network contributes to cohesion,30while thein situtriggered topologically entangled network greatly enhances the interfacial adhesion without specifi functional groups.31Moreover,the interactions between active groups are also beneficial For example,a supramolecular solvent(SMS)-based electrode displayed conformal contact and synchronous deformation with finge joints or arms with body movement;15the SMS was composed of citric acid and cyclodextrin,which offered many carboxyl and hydroxyl groups for synergistic interactions at the interface[Fig.3(h)].The hybrid strategy avoids the competition between adhesive and cohesive forces,thereby providing the material with better self-adhesive intelligence.
3. Multi-function maintenance
Besides self-adhesion,other functions are maintained in bioadhesive hydrogels.Because of their dynamic hydrogen bond network,many self-adhesive hydrogels are characterized by self-healing;32see Sec.II B for further details.Also,introducing antibacterial materials such as silver nanorods,33phytic acid,26and branched polyethyleneimine34avoids the potential risk of bacterial infection.Furthermore,additional functions offer more adaptability for a wide range of environments.For example,conformal contact with human skin was made waterproof without external encapsulation,16and a glycerol–water hydrogel fille with PDA-decorated CNTs could resist both freezing and heating to maintain a large working temperature range of-20○C to 60○C.9
Besides dry electrodes,self-adhesive superporous hydrogel has wide applications in semi-dry electrodes,which combine features of dry and wet electrodes and have a promising future in electroencephalography(EEG)signal acquisition.35They locally release a trace amount of electrolyte to achieve conformal contact between electrode and scalp,reduce the interfacial impedance,and enhance the quality of the acquired EEG signal.36,37More importantly,the self-charging and discharging of electrolytes can be controlled by hydrogel materials and package structures.38For example,selfadhesive polyvinyl alcohol/polyacrylamide double-network hydrogel (PVA/PAM DNH)39can automatically load and unload saline electrolyte,thereby balancing desirable mechanical properties and controllable release of electrolyte.
In short,designing reversible interactions in hydrogels offers a crucial path to self-adhesive materials.However,note that to lower the risk of skin allergies and reduce motion artifacts10caused by extensive sweat buildup,the air and sweat permeability of hydrogels must be considered fully when enhancing their adhesion,and the aforementioned superporous hydrogel may be a possible solution in that regard.
B.Self-healing materials
Unexpected damage such as scratches and cuts seriously affects the performance of epidermal electrodes,so smart materials with self-healing capabilities are ideal for extending electrode life.To date,the development of self-healing materials has centered on high recovery efficiency healing response,and biocompatibility,and the two main materials currently used in biopotential measuring are elastomers and hydrogels.
1. Self-healing hydrogel-based electrodes
Designs of self-healing hydrogels are based on reversible covalent bonds and noncovalent interactions,including boron ester bonds,hydrogen bonds,coordination bonds,π–π stackings,and electrostatic interactions40–42[Figs.4(a) and 4(b)].For example,in 2022 Zhouet al.40proposed a self-healing SV3/PVA electrode for electrocardiography(ECG)monitoring;the hydroxyl groups of PVA and sodium tetraborate contributed reversible ester bonds,and enhancement came from PEDOT:PSS,propylene glycol (PG),and diethylene glycol (DEG),which offered more hydrogen bonds to facilitate recovery.The recombination process finishe within 2 s with a calculated efficienc of 100%for conductivity.
Because of the dynamic nature of functional bonds,the selfhealing capability responds to external stimuli such as near-infrared(NIR) light43and temperature.26For example,silver nanowires(AgNWs)in a hydrogel sensor adsorbed 808-nm NIR for the formation of Ag–S bonds,44and NIR light also accelerated the self-healing process of an Sb2S3@PPy-DA incorporated gel,43with tensile stress and strain recovering completely within 90 s[Fig.5(a)].
Besides hydrogels,ionogel electrodes with self-healing capability have attracted much attention recently because of their excellent stability underwater,but their application to biopotential monitoring is still in its infancy.Notably,in 2021 Yuet al.45reported a series of IG-x ionogels whose C-F groups from polymeric acryloyloxyethyltrimethylammonium bis(trifluoromethan sulfonyl) imide (poly[DMAEA-Q][TFSI]) enabled self-healing under water [Fig.5(b)],with both the mechanical and conductive properties self-healed with a drift of less than 1%.Designing reversible bonds provides smart self-healing for longer-lasting and more-stable applications.
2. Self-healing elastomer-based electrodes
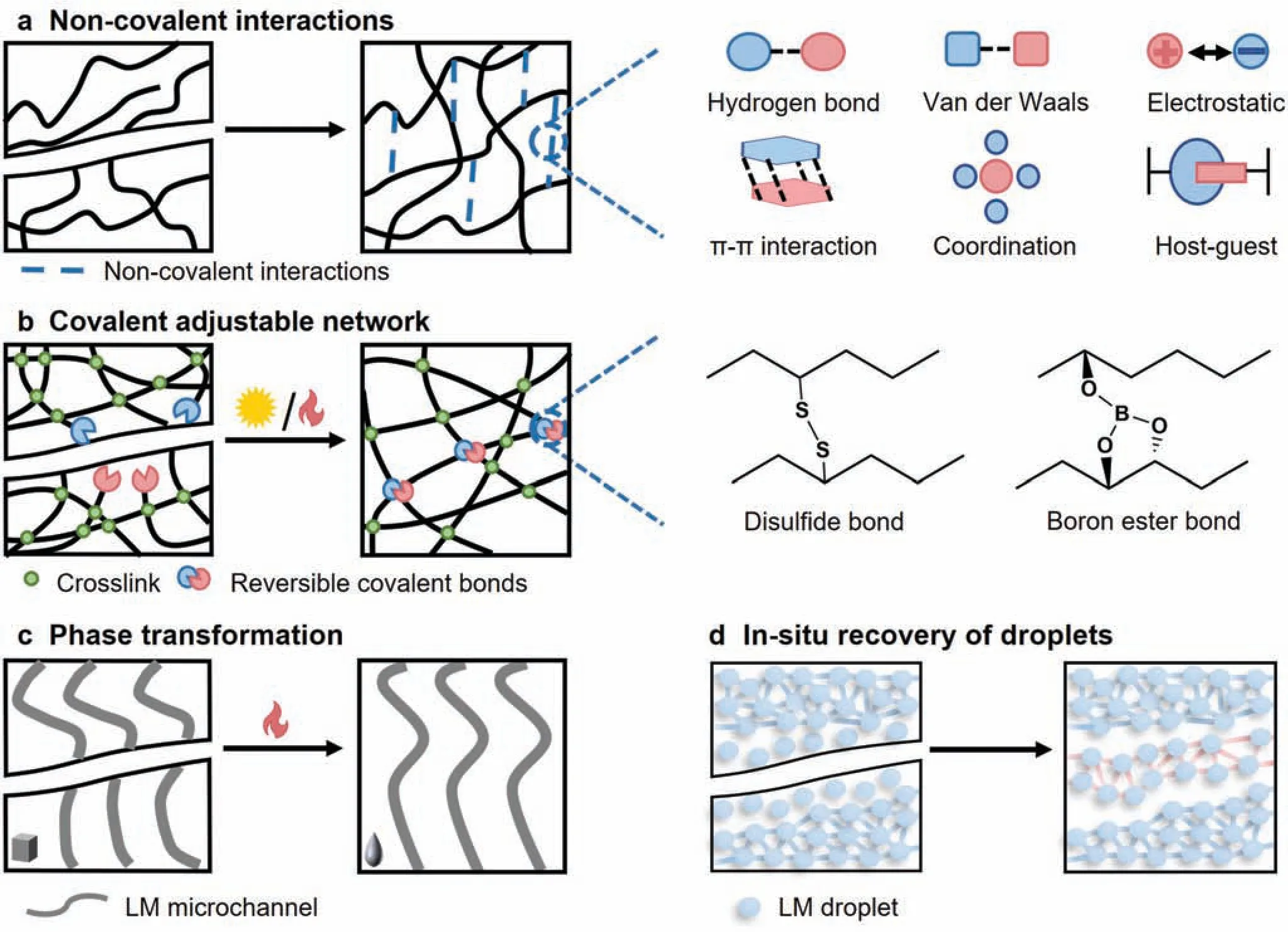
FIG.4.Mechanisms of self-healing:(a)self-healing of substrate based on intermolecular interactions;(b)self-healing of substrate based on reversible covalent forces;(c)self-healing of conductive liquid metal(LM)microchannel;(d)in situ self-healing of LM droplets.
Elastomers are characterized by higher mechanical modulus compared to hydrogels,but they are intrinsic insulators,requiring additional conductive filler for biopotential measurement.Conductive filler include 1D nanowires such as CNTs46and liquid metals (LMs).47For the firs case,strategies are focused mainly on the self-healing ability of the elastomer itself,while the conductive metals heal by following the substrate.46In contrast,LMs have a self-healable conductive network.47
According to the states of conductive filler in the elastomer composite,the electrodes can be classifie into three categories.First,the conductive fil is deposited directly on the substrate.The representative covalent adaptable networks (CANs) have attracted extensive attention because of being dynamic crosslinked networks,which are responsive to stimuli including heat and light48[Fig.4(b)].When heating over the topology freezing transition temperature,the topological arrangement of crosslinks is induced for self-healing.48For example,in 2022 Konget al.49reported a dynamic crosslinked polyurethane(PU)based on disulfid CANs,reaching 73%recovery of stretchability at 130○C for 1 h.
Second,the conductive network is embedded with the elastomer matrix.The healing of the nanowire conductive network can be promoted by the surrounding self-healing matrix because of the chain mobility of dynamic crosslinks.46The firs observation of the self-reconstruction of a conductive CNT network was achieved by embedding self-healing PDMS–MPU0.4–IU0.6;46the line-shaped damage self-healed within 12 h at room temperature,exhibiting 200% strain.Modifying conductive components with reversible bonds provides another path to self-healing of the conductive network.For example,disulfide-modifi AgNWs offered reversible Ag–S coordination and disulfid bonds,showing recovery when stimulated by NIR light44[Fig.5(c)].Consequently,the conductivity approached stability after 200 s of illumination.

FIG.5.Self-healable materials for epidermal electrodes.(a)Infrared thermal images of near-infrared(NIR)-responsive self-healing of Sb2S3@PPy-DA.43 (b)Left:optical microscopy images of self-healing process underwater;right:mechanical recovery of different ionogels under different conditions.45 (c)Left:scanning electron microscopy images of Ag and S element mapping;right: self-healing of conductivity over time.44 (d) Left: adhesion on finge joints;right: mechanical recovery over time.47 Figures reprinted with permissions from(a)Elsevier,Copyright 2023;(b)John Wiley and Sons,Copyright 2021;(c)Elsevier,Copyright 2020;(d)John Wiley and Sons,Copyright 2022.
The third category involves applying self-healable LMs.Generally,external stimuli are required for either the construction of a conductive pathway or its restoration.Constructing microfluidi circuits for LMs has been demonstrated to restore functionality50[Fig.4(c)];because of its low melting temperature,broken solid gallium can be reconnected by the body temperature.Meanwhile,Ga fiber also have shape memory properties in phase transformation,imparting shape recovery to the electrode.However,this pathway has limited applications.Dispersing LM droplets in the elastomer matrix is another promising method.41Once destroyed,the broken LM droplets self-reconnectin situto form a conductive pathway instantaneously,keeping a constant conductivity [Fig.4(d)].In 2022,Peiet al.47developed a composite elastomer with dispersed EGaIn droplets.The elastomer realized self-healing of both substrate and conductive pathway with light,mechanical stress,or elevated temperature,almost recovering its stress–strain response in 4 h[Fig.5(d)].
The three categories of materials discussed above show increasing intelligence because of the better healing capability of conductive materials,which is essential to the practical use of electrodes.To achieve more practical applications,self-healing materials should possess faster healing rates and better healing effects.Establishing a more comprehensive evaluation system for the material’s performance is equally important,encompassing aspects such as mechanical properties,electrical conductivity,stability,and reproducibility of stable self-healing performance after repeated breaking–healing cycles.
C.Self-sensing piezoelectric materials
In contrast to measuring biopotential signals directly,indirect monitoring and signal transformation offer another pathway for obtaining ECG signals.Cardiac activities generate both electrical potentials and periodic vibrations at the surface of the chest.Based on the research of Ahmad,51Fourier transformation can be used to construct a direct connection between piezoelectric voltage output and ECG signals.Therefore,self-sensing piezoelectric materials can be attached to the chest to extract ECG signals.A series of stretchable piezoelectric materials have been developed to date,including lead zirconate titanate (PZT),52zinc oxide (ZnO),52aluminum nitride(AlN),53and polyvinylidene fluorid (PVDF).54Theoretically,PZT,ZnO,and AlN are brittle materials and not biocompatible;by contrast,PVDF combines biocompatibility and mechanical stiffness,making it more suitable for being patterned to be stretchable and drawing more attention to its stretchability.52,55
Moreover,mechano-acoustic signals including those from phonocardiography,seismocardiography (SCG),balistocardiography,and forcecardiography are complementary means of monitoring cardiovascular activities,56where piezoelectric materials can record the contraction of the heart.For example,in 2021 Hesaret al.52presented a 28-μm-thick foil based on PVDF [Fig.6(a)];serpentine designs were used to optimize the stretchability and sensitivity,and the contactless epidermal sensor reduced the impact of body motion and obtained continuous SCG signals that were highly synchronized with the referenced SCG and ECG signals measured by commercial sensors.
However,acquiring single signals is not enough for reliable monitoring of cardiovascular activities,and the trend toward multimodal functionality is realized by integrating multiple sensors.For example,in 2016 Liuet al.57reported a mechano-acoustic electromyography(EMG)sensing patch that was coupled with a triaxial accelerometer and dry electrodes.Moreover,in 2019 Haet al.54proposed a dual recording system based on serpentine-patterned PVDF sheets;the sensitivity of the dual-mode sensor was identical to that of a commercial accelerometer,and the former recorded ECG and SCG simultaneously with high fidelit [Fig.6(b)].As an aid in recording ECG signals,the intelligence of the sensing material for mechanoacoustic signals further improves the performance of electrodes in terms of accuracy.

FIG.6.Cardiovascular monitoring electrodes based on piezoelectric material:(a)structure of seismocardiography(SCG)sensor based on polyvinylidene fluorid (PVDF),and comparison between measured SCG and Ref.52;(b)structure of dual PVDF sensor system for electrocardiography(ECG)and SCG recording,showing the same sensitivity as that of a commercial accelerometer.54 Figures reprinted with permissions from(a)American Chemical Society,Copyright 2021;(b)John Wiley and Sons,Copyright 2019.
III.SMART STRUCTURES
Besides smart materials,smart structures provide another strategy for achieving self-adaptive designs.Humidity,extreme temperature,human motion,and the presence of hair cause unstable attachment,thereby impeding accurate health monitoring,but here we review a series of meso/micro/nanostructure designs that enable either long-term monitoring in various conditions or integrated multifunctions for multimodal recording.
A.Smart meso-structures
The sophisticated 3D morphology of human skin challenges the functionalities of wearable electrodes at the dynamic and curvilinear surface.Notably,meso-structure design is an emerging strategy for morphable and adaptive structures even though their materials do not intrinsically match the modulus of human skin.With dimensions ranging from nanometers to micrometers,mesostructures including fractal,kirigami,and origami structures selfadapt themselves via pre-programming to manipulate macroscopic deformations for creating conformal contact.
1. Fractal structures
Fractal structures are self-similar and include typical geometries such as Peano lines,Moore loops,Greek crosses,etc.,58and their spontaneous out-of-plane bending and twisting accommodate the strain associated with large deformation.Typically,various reconfigurabl patterns and shapes can be controlled by cuts in a hierarchy with different motifs,59presenting huge potential variations.The application in large-area physiological monitoring was led by the design of Tianet al.2in 2019,who integrated 17 Greek cross-like fractal electrodes into an area larger than 200 cm2.The conformal contact significantl reduced the effects of impedance and motion artifacts for long-term EMG and EEG.Moreover,the fractal design was also applied in an all-in-one patch for ECG monitoring,enduring 30%stretching and 180○bending60[Fig.7(a)].
2. Kirigami structures
Kirigami structures originate from arrays of cuts in 2D thin sheets,59which allow elaborate shapes and tunable configurations By introducing different cuts,the structure can be highly programmable and can respond reversibly to mechanical stimuli by mesoscale bending and twisting.63Compression at selected points63or a combination of buckling and rotational twist64are typical mechanical driven forces,which can be obtained by pre-stretched substrates.For example,in 2022 Liuet al.61presented a wearable kirigami electronic system whose high stretchability of the network benefite from its self-assembled hyper-connective fib rilla.Because of the pre-structure of the uniaxial,biaxial,and square spiral,the kirigami electrode maintained its resistance at 105% uniaxial strain,360○twisting,and 90○bending,separately65[Fig.7(b)].It could tell mental states by recording differences in frequencies and intensities,providing opportunities for smart health monitoring.
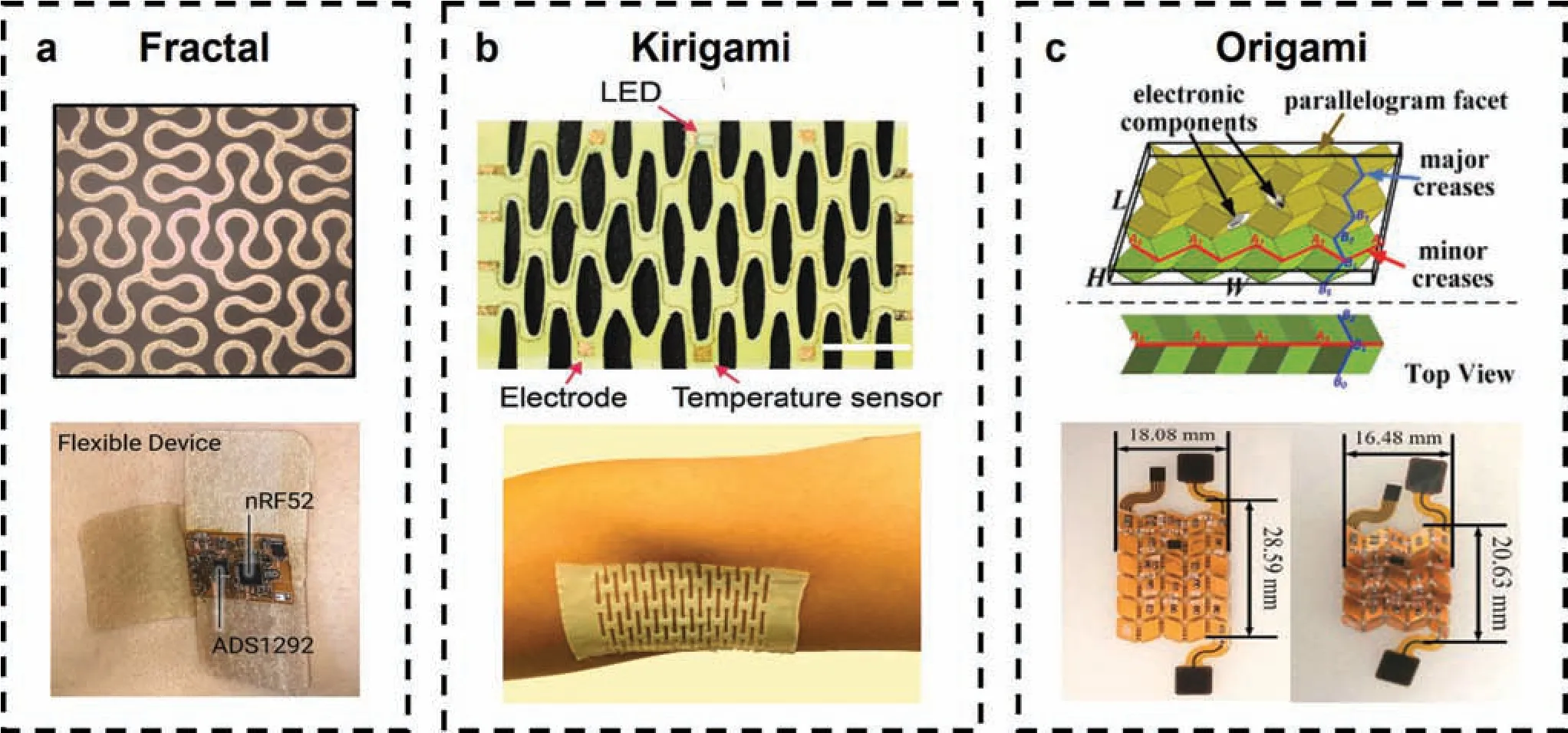
FIG.7.Highly adaptive meso-structures for epidermal electrophysiological electrodes:(a)fractal serpentine structure and conformal contact with the chest;60 (b)Kirigami structure,which bends and twists into 3D shapes to provide stretchability;61 (c) origami structure and corresponding stretchable circuit board.62 Figures reprinted with permissions from(a)American Chemical Society,Copyright 2023;(b)John Wiley and Sons,Copyright 2022;(c)Springer Nature,Copyright 2021.
3. Origami structures
Origami is a 3D folding structure that is compact and deployable.As a representative structure,the Miura-ori structure contains mutually independent cells programmed by predefine crease patterns to form arbitrary shapes.Because of the cooperation of metamaterials,the Miura-ori structure also exhibits transformation of mechanical properties such as Poisson’s ratio66and modulus.62Direct folding,stiffness modification and kirigami enhancement are typically applied for fabrication.62The Miura-ori based stretchable circuit board has high foldability and stretchability and was used to build a wearable ECG system for integrating stiff electronic components62[Fig.7(c)].
All three smart structures enable rigid components to follow skin deformations by 3D bulking.Compared to origami structures,fractal and kirigami ones have more degrees of freedom in design and optimization,but challenges remain for both manufacturing and stable contact with the skin.
B.Smart micro/nanostructures
Inspired by nature,the fascinating self-adaptive behaviors of organisms are mimicked extensively to fabricate biopotential monitoring devices (Fig.8).To realize adaptation to various environments,the most attention has been given to self-adhesion on both wet and dry substrates,breathability,and sweat drainability.
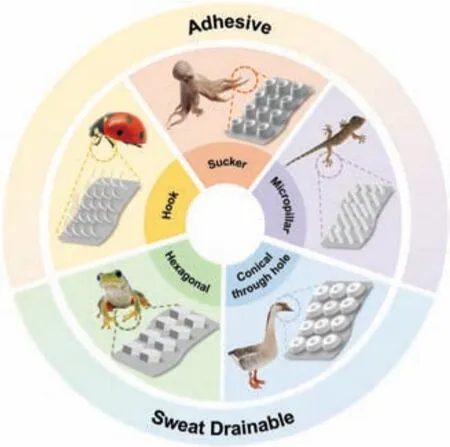
FIG.8. Bio-inspired micro/nanostructures for epidermal electrophysiological electrodes with improved adhesion and drainage.
Inspired by octopi and the forelegs of male diving beetles,suction structures such as convex cups67and wrinkled microgrooves68have been developed.Because of pressure differences,an array of convex cups is intrinsically adhesive in both dry and wet conditions,67and wrinkled microgrooves use friction and capillarity effects for adhesion.68In addition,micropillar structures mimicking the feet of geckos and grasshoppers use van der Waals forces at the tip–skin interface,69thereby offering high-fidelit ECG recording both through hair and underwater.Another self-adhesion pathway underlies mechanical interlocking;for example,microneedles inspired by insects10penetrate the skin to interlock strongly with it.
For sweat drainage,possible solutions include sweat-absorbable sponges in the structures of semi-dry electrodes70and designing structural tunnels for sweat transportation.In structural tunnels,the two main transport directions are vertical and surface:by mimicking the beaks of waterfowls,vertical water transport can be realized by conical through-holes using the Laplace pressure difference;10meanwhile,structures for surface transport such as frog-inspired hexagonal microgroove structures71and wrinkled microgrooves inspired by male diving beetles68are based on the surface capillary effect.Moreover,microchannels between microstructures contribute to permeability.67
However,various environments require the integration of functions and therefore multiple mechanisms.Notably,in 2019 Kimet al.67reported an epidermal ECG patch that was highly permeable,water-drainable,and adhesive when wet.This structure integrated(i)a hexagonal array and microchannels for drainage and(ii)convex cups for adhesion.The liquid in a 3 cm×3 cm area was exuded and evaporated in 3 h,and the patch had a maximum adhesive force of 2.36 N/cm2for dry skin and 1.92 N/cm2for sweaty skin,thereby enabling reliable recording in dry,wet,and running-water conditions.Another example used a combination of conical through-holes and hexagonal microgrooves to drain water,10while the adhesion relied on the mechanical interlocking of microneedles.Therefore,bio-inspired micro/nanostructures learned from natural evolution are having an increasing impact on the design of desirable structures and are showing great potential for combining multiple functions in smart electrodes.
IV.INTELLIGENT ALGORITHMS
A.Overview
Smart electrodes can capture physiological electrical signals such as those of EMG,ECG,EEG,and electrooculography (EOG)at the front end of monitoring.However,to extract valid information,further signal-processing algorithms are required,especially intelligent algorithms based on artificia intelligence.
Traditional approaches commonly rely on hand-crafted features,leading to several problems.First,they make it difficul to capture interpretable and relevant information directly from multiple medical ontologies.72Second,manually designed processes can be subjective or misinterpreted because of individual differences in the study population.However,thanks to the development of DL,deep features are used instead to encode electrical signals,thereby overcoming the above problems more efficiently accurately,and cheaply.In the following subsections,we discuss different processing frameworks based on the effectiveness of intelligent algorithms for analyzing physiological electrical signals.
A fully automated approach to obtaining information from physiological electrical signals can be divided into the following fiv main stages11(Fig.9): (i) data acquisition,(ii) preprocessing,(iii)feature extraction,(iv) feature optimization,and (v) feature classification In the firs stage,physiological electrical signal data are acquired from different sources (e.g.,clinical databases,wearable devices,electrode acquisition).In the second stage,various filterin techniques are used to remove noise and normalize the signals.In the third stage,features are identifie and extracted,which is crucial for correctly classifying various clinical symptoms and basic physiological activities of the human body.In the fourth stage,duplicate and redundant features are removed to improve the classificatio accuracy and efficienc of the model.Finally,in the fift stage,each detected signal is classifie according to the requirements of different tasks.
As algorithms develop,so they perform increasingly better for electrophysiological signal processing.From traditional algorithms to machine learning ones,especially DL techniques,increasingly powerful tools are available for modeling complex signals.Below,we review the aforementioned stages(ii)–(v)as the main processes of self-processing.
B.Stage-based intelligent algorithms
1. Preprocessing
The preprocessing stage involves steps such as filtering resampling,digitization,amplitude normalization,and artifact removal.
Above all,the most critical step is denoising,and in this subsection we focus on denoising algorithms.
a.Types of noise.During data acquisition,the electrical signals acquire various biological and nonbiological artifacts73that contaminate the recorded signal and degrade its quality.The electrical pulses recorded from the human body have very small amplitudes and are susceptible to various types of noise and artifacts,so to prevent incorrect judgments,especially for biomedical diagnosis and treatment,74it is necessary to improve accuracy by preprocessing to eliminate artifacts and noise.
Physiological electrical signals are subject to nonbiological noise,including powerline noise/interference,industrial frequency interference,instrument noise,electrosurgical noise,electrode contact noise,electrode movement or motion artifacts,and muscle noise.Additionally,specifi noises exist for different types of physiological electrical signals.
ECG is influence strongly by baseline drift.This is low-frequency interference that can result from electrode–skin impedance,breathing rhythm,individual body limb movements,and front-end processing circuit design,causing the amplitude of the original signal to drift by up to 0.1 to 0.2 times the maximum value of the R-wave.
For EEG,the main artifacts are ocular ones,which comprise mainly interference from eye drift,blinking,and vertical and horizontal eyelid movements.75Blink artifacts are generally recorded using vertical EOG (VEOG) electrodes,with the waveforms recorded on VEOG and scalp electrodes having opposite polarity[Fig.10(a)].Compared to other artifacts,ocular ones are the most harmful because they alter the amplitude of the actual EEG signal.76Typical “caravan-shaped” saccade artifacts in eye movement artifacts are shown in Fig.10(b).To address this issue,highly effective algorithms have been developed for extracting EOG signals from EEG ones,which is beneficia for obtaining pure EEG signals and analyzing EOG ones if required.
b.Traditional algorithms.The main purpose of filterin is to remove noise and artifacts from the signal,which is a necessary step before signal analysis.Initially,band-pass filter were commonly used in the preprocessing stage to attenuate muscle noise,artifacts caused by electrode movement,powerline interference,baseline drift,and high-and low-frequency noise signals.Common bandpass frequency ranges that were used were 1.5–50 Hz,792–36 Hz,800.1–100 Hz,81and 1–80 Hz.82
For removing unidirectional frequencies only,basic low-pass filter (LPFs) and high-pass filter (HPFs) were sufficient LPFs were mostly used to filte out high-frequency components of the acquired signal,such as those greater than 30 Hz,8332 Hz,84or 40 Hz.85In contrast,HPFs were designed to eliminate lower frequency components to achieve signal enhancement;they attenuated a smaller amount of signal but were affected by phase shift.74The frequencies of the different HPFs compared herein were 1 Hz,83,850.5 Hz,84and 0.1 Hz.86However,the widespread use of these filter frequently interfered with the signal pattern,making accurate diagnostics impossible.Trap filter were used extensively to remove the DC offset in a signal;these filter combined lowpass and high-pass to extend the frequency cancellation range,and most trap filter canceled noise centered on either 50 Hz or 60 Hz.79,86
In addition to the basic conventional filters more-efficien methods related to filte improvements were developed,including heart-signal-enhancement adaptive filter based on the proportionate normalized least mean square (PNLMS) algorithm,87cyclic leakage adaptive algorithms,88reference-free single-tap adaptive filters and low-order moving average(MVA)filters89Another option for noise reduction was the sign regressor normalized version of dead zone least mean square (SRNDZLMS) algorithm used within an adaptive signal enhancement unit (ASEU) filte90(Table I).
Besides filters other methods such as wavelet transform(WT)have been used,providing a time-varying decomposition that offers advantages over filtering With time-varying decomposition,different filte settings(i.e.,wavelet coefficients can be selected for different time ranges,thereby allowing event-dependent filte responses to be created.93In 2019,Xuet al.94proposed a method for removing ECG motion artifacts based on empirical WT and wavelet thresholding;the method effectively eliminates motion artifacts,minimizes distortion in re-storing the QRS waveform populationof the original ECG,retains useful information,and improves the signal-to-noise ratio (SNR).In 2019,Berwalet al.95introduced a two-phase technique called steady-state WT with level thresholding(SWT-LT) to effectively remove various in-band motion artifacts,outperforming previous methods with an average correlation coefficien of 0.9337 and an average normalized mean square error of 0.012.
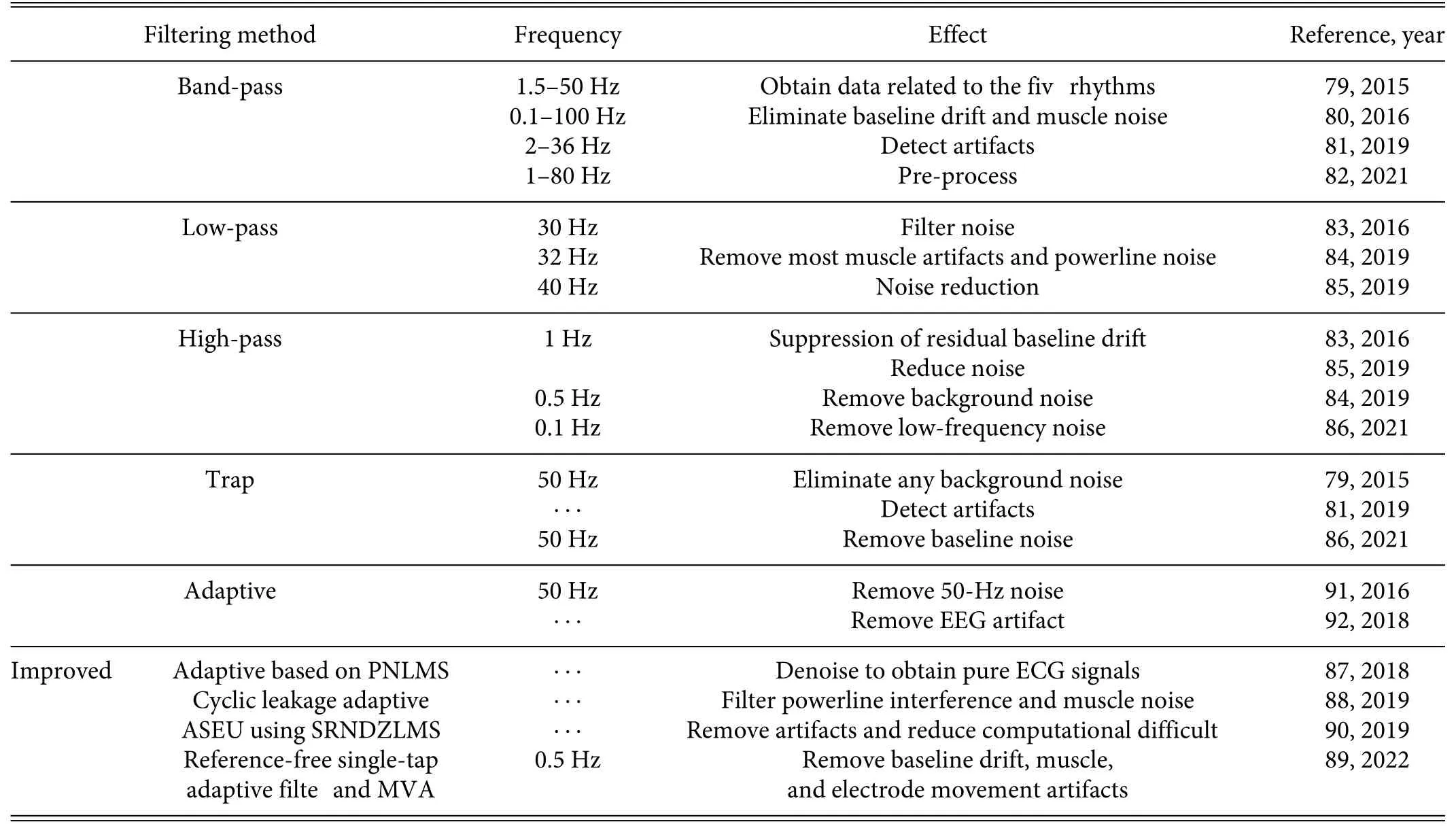
TABLE I.Summary of filte methods for preprocessing.
c.Deep learning.In addition to the basic methods and their optimization improvements,DL techniques have been applied to denoising.One technique is the denoising autoencoder (DAE),which is a self-supervised learning algorithm that can regenerate the input signal with the highest possible accuracy;it consists of two nonlinear subparts:an encoder and a decoder.
For example,in 2019 Chianget al.96proposed using a DAE based on a fully convolutional network (FCN) for ECG signal denoising.Compared with the denoising models based on convolutional neural networks (CNNs),the FCN achieved better performance with fewer input data,lower root mean square error(RMSE) and percentage root mean square difference,and higher SNR improvement,showing promise for clinical application.In 2021,Samannet al.97trained a DAE with a statistical thresholding approach and demonstrated its reasonably good accuracy in estimating unknown noise levels.Another method based on find ing the highest Akaike information criterion(AIC)weights and the optimal number of neurons was also shown to be effective in reducing the computational burden.98Moreover,in 2016 Xionget al.99proposed a deep neural network (NN) created from an improved DAE reformed by a WT method;it could eliminate residual noise with an unknown distribution in the frequency domain and showed improved performance in SNR and RMSE compared to using WT or DAE alone.
To improve DAE-based denoising of ECGs,in 2021 Singhet al.100proposed using a generator–discriminator model based on a generative adversarial network (GAN);the generator could generate false samples close to the real data,while the discriminator distinguished between true and false samples.Another approach is improving the subunits in CNNs.For example,in 2021 Heet al.101proposed an improved encoder–decoder structural model in which adaptive parametric ReLU and a dual attention module were introduced,outperforming other DL and traditional methods.
2. Feature extraction
After preprocessing,electrophysiological signals require feature extraction for subsequent classification102Feature extraction is the process of transforming the original input signal into a simplifie set of features(feature vectors).A well-selected feature vector contains valid and relevant information about the original signal and can be used to represent the desired action or physiological state.103Different types of feature vectors can be used based on the application requirements,such as time-or frequency-domain features,time-series values,numerical values,spatiotemporal features,and statistical analysis,104with the most used feature types being time domain,frequency domain,time–frequency domain,and spatial patterns.105
Table II summarizes the traditional methods of feature extraction according to different feature types,and we note the following specifically (1) Time-domain analysis is based on the geometric properties of the physiological signal,such as amplitude,mean,and variance,and its advantages are that it is simple and intuitive.(2)Frequency-domain analysis relies on the properties of each frequency,and one of the most widely used methods is power spectrum estimation,which establishes the correspondence between power and frequency through signal transformation.(3) Time–frequencydomain analysis is based on a comprehensive analysis of time-and frequency-domain characteristics.106
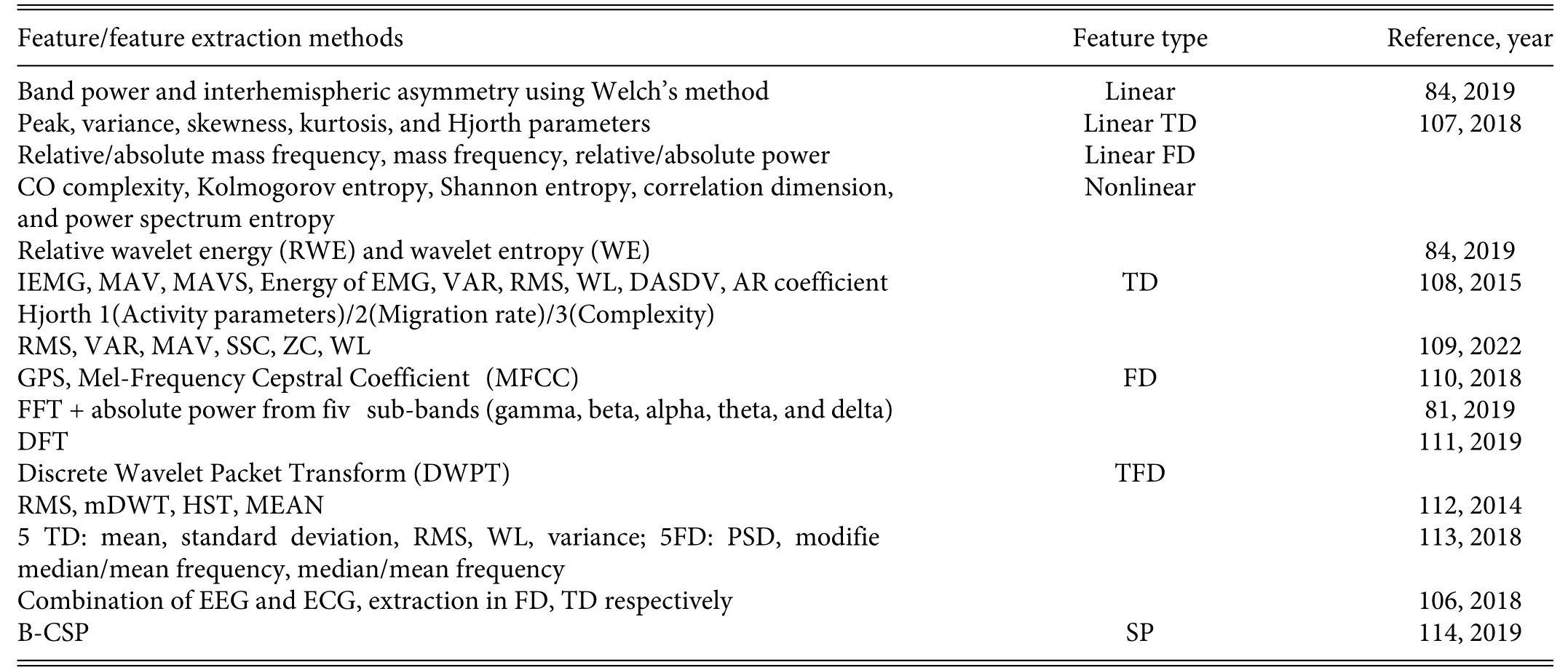
TABLE II.Summary of traditional methods for feature extraction.Abbreviations:TD—time domain,FD—frequency domain,TFD—time–frequency domain,SP—spatial patterns.
For ECG,its waveform specificit means that there are also independent features that must be extracted with attention,mainly the R-R interval,QRS complex,J point,P-R interval,ST segment,and T wave.In particular,the J point and heart rate play important roles in ECG beat classificatio and help in better identifying ST segment changes,which are key indicators of life-threatening conditions such as ischemic disease,infarction,and angina pectoris.Deviations of these features from their normal ranges can indicate the presence of certain diseases.
Traditional methods of feature extraction are time-consuming and are limited by computing power.In recent years,with the advancements in DL,feature extraction using NNs and their improvements has become increasingly popular,with CNNs being the underlying framework that is used most and is the most effective.Table III summarizes some CNN-based algorithms and their performance,demonstrating their satisfactory results in feature extraction.
3. Feature optimization
Features are often encoded as high-dimensional vectors,and if the classifie uses a large number of features for classification then this leads to high computational costs and low efficiency84Feature selection and dimensionality reduction are imperative for three reasons: (i) repetitive patterns in periodic signals are redundant and unnecessary;(ii) the subjects are in a stationary or normal physiological state for most of the time;(iii) the effectiveness of information acquisition and the performance of the classifie in high-dimensional matrices with fewer data need improvement.Genetic algorithms(GAs)and principal component analysis(PCA)are commonly used for this,and some typical examples are given in Table IV.However,DL methods often neglect feature dimension reduction because they can handle large amounts of data.11
In 2012,Martiset al.120proposed using PCA to reduce the dimensionality in classification they computed the principal components and then projected the data onto the direction with the highest variability,thus converting the high-dimensional input vector into an orthogonal low-dimensional component vector.Another commonly used dimension-reduction method is linear discriminant analysis(LDA),121which generally transforms a large original dataset into a smaller one.Also,independent component analysis(ICA)122is useful for transforming multi-dimensional data into statically independent features.
GAs are used widely for feature selection in machine learning.During each iteration,the GA generates a new population,also known as a new generation.To do so,the GA selects certain individuals in the current population (parents) to contribute genes to the next generation (children),123and the selection of individuals for reproduction is determined by a fitnes function that evaluates their fitnes scores.Experimental studies have shown superior optimization performance using GAs in feature selection.
4. Classification and applications
Having extracted and optimized the important features,they are fed into a suitable classifie that meets the requirements of speed,adequacy,accuracy,and the ability to handle differences in feature values128to map and match various application requirements.Manyalgorithms have been designed for signal prediction and classifica tion,ranging from(i)traditional algorithms such as time–frequency domain transforms129to (ii) machine learning algorithms such as support vector machines(SVMs),Bayesian classifiers LDA,random forests,multilayer perceptrons,Knearest neighbors to(iii)the more popular DL-based algorithms such as CNNs,recurrent NNs,and graph convolutional networks.
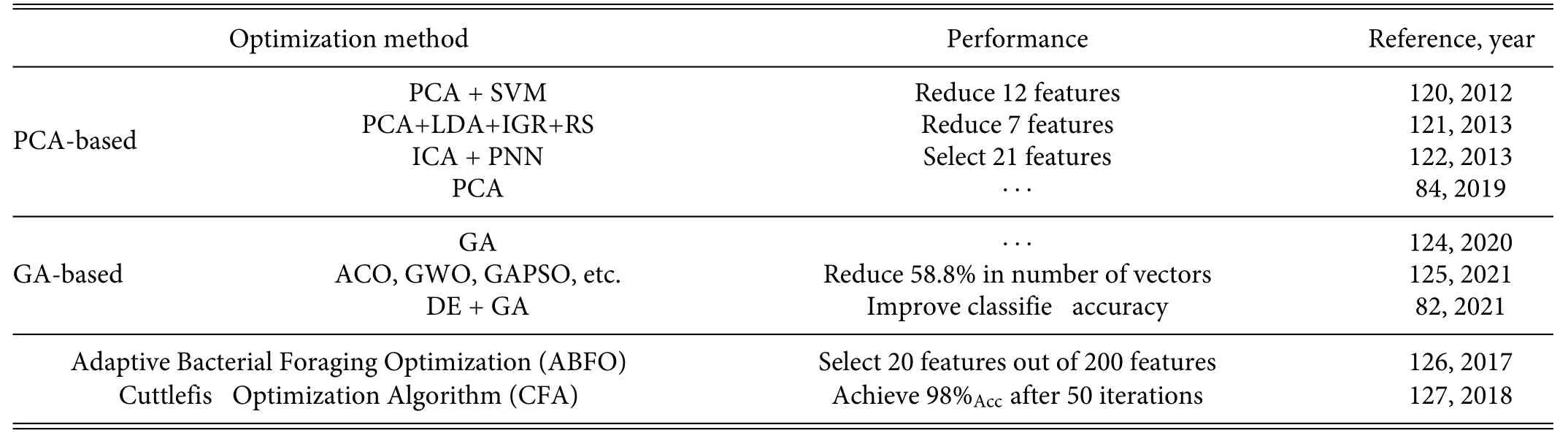
TABLE IV.Summary of feature optimization methods.Abbreviations:IGR—information gain ratio,RS—rough set,DE—differential entropy.
Machine learning is a subset of artificia intelligence,and DL is a subset of machine learning.DL-and ML-based algorithms perform classificatio using various intelligent supervised or unsupervised learning algorithms.Supervised learning methods classify data on labeled/structured data,while unsupervised learning involves a form of clustering/classificatio based on unstructured data.
The most widely used algorithm in ML is the SVM,130–132a classifie that linearly separates classes by utilizing the inner product kernel function instead of nonlinear mapping data to high-dimensional spaces.However,it can only handle binary classificatio tasks.In addition,some ML algorithms require manual selection of features,which can easily lead to information loss.74
To overcome these limitations,DL algorithms are promising for structural design.DL differs from traditional technologies in three aspects: (i) it jointly optimizes the representation and classificatio of features;(ii)deep NNs have massive parameters,so they have great potential for processing complex data;(iii)those deep features capture higher-level variations of data and so can be further used in various applications.Notably,CNNs rely on attention-based architectures to improve the interpretability of their classifications with the attention module ultimately selecting only the most informative features that are needed.DL has been used successfully in various applications such as biometric applications,133–136object detection,image classification137disease classification138–142emotion recognition,135,138,143,144sleep monitoring,145,146and computer vision.
DL models are becoming increasingly popular because of their high accuracy for classification However,these models require special hardware for training and are time-consuming,and in practice the amount of data to be processed is generally small so there is no need to use a DL algorithm.Therefore,ML is still dominant for processing electrical signals.DL models are more suitable when dealing with large datasets,as they have a higher risk of overfittin with small datasets.Meanwhile,researchers have been actively searching for novel methods to alleviate the disadvantage of DL models.This can be seen in Table V,where the accuracies of SVM and NN algorithms differ significantl in one example108but are nearly the same in another example147because of the different sizes of datasets.
Table V summarizes ECG,EEG,EOG,EMG,and multimodal fusion signals based on their applications and compares them to algorithm models,data sources,and performance results both crosssectionally and vertically.The methods covered in Table V provide a reference and pave the way for widespread application of ML/DL algorithm models for electrophysiological signals in both clinical applications and neuroscientifi research.
V.CONCLUSIONS
In conclusion,this review summarizes the current trends of developing smart electrodes by rendering materials,structures,and algorithms intrinsically intelligent.Materials for self-adhesion or self-repair represent two central directions of development.Designing meso-structures or biomimicry structures are effective strategies as well.Additionally,the state-of-the-art DL frameworks for feature extraction and classificatio bring new opportunities for effective signal processing.Generally,materials,structures,algorithms,hardware circuits,and integrated systems constitute mature electrodes;materials and structures are fundamental for signal acquisition,algorithms and hardware circuits contribute the signal processing and transition,and integrated systems play an important role in realizing intelligence for the user end.However,although great progress has been made by efforts from all fields remaining challenges in these aspects limit the application of smart electrodes,and below we discuss in-depth the challenges and opportunities that are involved.
A.Materials and structures
Stability,adaptation,and balancing performances are major challenges for designing suitable materials and structures.
(1) Insufficien stability of smart functions.Although smart functions bring more opportunities for electrodes,the stability of these smart functions remains to be further developed and thoroughly examined in more cycles.Central issues including repeatable control of adhesion and detachment,the repeated times for high-quality self-healing,and linear sensitivity under a wider strain range require more attention in the future to greatly improve the repeatability and stability.
(2) Limited adaptability to various environments.At present,although many smart electrodes have been developed with strong adhesion to skin for specifi scenarios,changes in the environment involve the complex coupling of different cases.However,the performances of smart electrodes are not satisfactory when there is a mechanical,thermal,and hydrous coupling in the environment.Moreover,the condition for triggering smart functions is not mild enough,especially for self-healing materials.Stimulating conditions that damage the human body—such as high temperature and ultraviolet light—should be avoided,and more effort is needed toward a higher recovery rate and efficienc relying on the human body.An example at room temperature is self-healing stimulated by body temperature.50
(3) Imbalanced performances.The electrode design should give comprehensive consideration to stretchability,conductivity,biocompatibility,gas permeability,adhesion,and other smart functions.However,because of contradictions between different performances,a balance is needed to ensure the general function of an epidermal electrode.
In view of the above issues,the possible development directions for smart materials and smart structures can be divided into the following three aspects: (i) enhancing the reproducibility of smart functions to ensure smart electrodes with better stability;(ii)developing new materials and structures to realize adaptability to various environments with high sensitivity,robustness,and userfriendliness;(iii) optimizing the comprehensive properties of new materials and structures for more practical applications.Promising pathways for this issue are bio-inspired structures,10which are worth exploring further.
B.Algorithms
The intelligence of algorithms can be further improved in the aspects of generalized classifiers interpretability,security,deployment,etc.
(1) Lack of generalized classifiers While smart electrodes can be used for multiple tasks,classifier may appear to perform well on a given dataset and perform poorly on another dataset,especially when the data distribution is highly variable.171Furthermore,in practical medicine,disease is a smallprobability event,making it challenging to obtain a sufficien amount of data for DL to achieve optimal results.Therefore,there is an urgent need to develop general-purpose classifier that can perform across different small-scale datasets.
(2) Insufficien interpretability and security.Deep models are often blamed for being black boxes,as their internal structures are complex,making it difficul to understand the reasoning for a particular result.In particular,in the absence of big data,overfittin problems can be encountered,influ encing the generalizability of new samples.When smart electrodes are used for medical tasks,this is a very noteworthy issue,as a sound clinical interpretation is necessary to guarantee safety.There are two valuable development options:(i)using relatively simple models to explain complex DL models;172(ii)directly building deep models that can be explained by introducing mechanisms that are more easily understood by humans.173
(3) Difficultie in deployment.Because of the complex structure of deep models,deploying large models on wearable smart electrodes in practical applications can be challenging.Doing so will consume a lot of computing resources,but more importantly,it is difficul to deploy large models on wearable smart electrodes in practical applications.To address this issue,research and development of model compression techniques are needed to improve the efficienc of model utilization.For example,knowledge distillation is often used to convert large and powerful models into simpler ones,but with a slight loss of accuracy.174
(4) Poor performance under specifi conditions.Most existing deep models have long processing times and thus cannot display results in real time,resulting in severe delays and poor performance in tasks requiring high sensitivity.In addition,because of the weak characteristics of physiological electrical signals,the self-processing process often suffers from low accuracy.These issues require further model development to achieve faster speed and higher accuracy,and an effective strategy is the effective integration of traditional methods with intelligent DL frameworks.
C.Hardware circuits
Intelligent circuits act as a bridge between smart materials and intelligent algorithms,and the design of an intelligent circuit should correspond to that of the intelligent algorithm used for the initial processing of the acquired raw signal.Intelligent circuits include active circuits,signalin situamplificatio circuits,electrode pointin situnoise reduction circuits,etc.Meanwhile,the lightweight design of circuits is equally important to meet the requirements for wearable applications,involving key issues such as level of integration,service life,and power consumption.
D.System integration
The system integration of smart components is the ultimate step in electrode implementation,but limited cases have been reported to date.The sensing,signal-processing,communication,and power units and the micro-integration of stretchable modules all must be considered fully for a wearable smart device for end users.To date,Bluetooth,near-fiel communication (NFC),and GSM (Global System for Mobile Communications) modules have been integrated to transmit the processed signals wirelessly.Now,interdisciplinary scholars,clinical professionals,and end users must cooperate to promote the devices toward clinical customization.Based on the intelligent system,the functions of smart electrodes can be further expanded to multimodal measurement and even self-treatment.
E.Commercialization
To realize the commercialization and improve the market share of smart electrodes,three key issues must be solved.First,the biocompatibility and toxicity of new materials should be thoroughly evaluated to ensure their safety when directly attached to the human body for a long time.Applying natural materials may also be an alternative pathway.Second,the smart functions must be applicable in various conditions,and their development must be focused on consumer needs.Third,high-throughput and large-scale manufacturing is still in its infancy,and current methods cannot fully consider sensor accuracy,mass production,and cost.Therefore,more-advanced manufacturing methods must be developed to balance the functionality and economy of smart electrodes.
Overall,despite existing obstacles,smart electrodes are promising devices that will bring welfare to health monitoring and play a greater role in disease diagnosis in the future.
ACKNOWLEDGMENTS
This work was supported by Science and Technology Innovation 2030-Major Project(Grant No.2022ZD0208601),the National Natural Science Foundation of China (Grant Nos.62104056,62106041,and 62204204),the Shanghai Sailing Program (Grant No.21YF1451000),the Key Research and Development Program of Shaanxi (Grant No.2022GY-001),and the Fundamental Research Funds for the Central Universities (Grant No.223202100019).We especially acknowledge the NPU USRI-NEURACLE Tech.Joint Lab for Brain–Computer Interface.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflict to disclose.
Author Contributions
Y.Ye and H.Wang contributed equally to this paper.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
- 纳米技术与精密工程的其它文章
- Nanostructure enabled extracellular vesicles separation and detection
- An image mosaic technique with non-overlapping regions based on microscopic vision in precision assembly
- Electrical characterization of an individual nanowire using flexible nanoprobes fabricated by atomic force microscopy-based manipulation
- An advanced cost-efficient IoT method for stroke rehabilitation using smart gloves
- Design and analysis of longitudinal–flexuralhybrid transducer for ultrasonic peen forming
- Effects of simulated zero gravity on adhesion,cell structure,proliferation,and growth behavior,in glioblastoma multiforme

