Exploring the mechanism of action bitter melon in the treatment of breast cancer by network pharmacology
Kavan Panchal,Bhavya Nihalani,Utsavi Oza,Aarti Panchal,Bhumi Shah
Abstract BACKGROUND Bitter melon has been used to stop the growth of breast cancer (BRCA) cells. However, the underlying mechanism is still unclear.AIM To predict the therapeutic effect of bitter melon against BRCA using network pharmacology and to explore the underlying pharmacological mechanisms.METHODS The active ingredients of bitter melon and the related protein targets were taken from the Indian Medicinal Plants, Phytochemistry and Therapeutics and SuperPred databases, respectively. The GeneCards database has been searched for BRCA-related targets. Through an intersection of the drug’s targets and the disease’s objectives, prospective bitter melon anti-BRCA targets were discovered. Gene ontology and kyoto encyclopedia of genes and genomes enrichment analyses were carried out to comprehend the biological roles of the target proteins. The binding relationship between bitter melon’s active ingredients and the suggested target proteins was verified using molecular docking techniques.RESULTS Three key substances, momordicoside K, kaempferol, and quercetin, were identified as being important in mediating the putative anti-BRCA effects of bitter melon through the active ingredient-anti-BRCA target network study. Heat shock protein 90 AA, proto-oncogene tyrosine-protein kinase, and signal transducer and activator of transcription 3 were found to be the top three proteins in the proteinprotein interaction network study. The several pathways implicated in the anti-BRCA strategy for an active component include phosphatidylinositol 3-kinase/protein kinase B signaling, transcriptional dysregulation, axon guidance, calcium signaling, focal adhesion, janus kinase-signal transducer and activator of transcription signaling, cyclic adenosine monophosphate signaling, mammalian target of rapamycin signaling, and phospholipase D signaling.CONCLUSION Overall, the integration of network pharmacology, molecular docking, and functional enrichment analyses shed light on potential mechanisms underlying bitter melon’s ability to fight BRCA, implicating active ingredients and protein targets, as well as highlighting the major signaling pathways that may be altered by this natural product for therapeutic benefit.
Key Words: Bitter melon; Momordica charantia; Network pharmacology; Molecular docking; Breast cancer; Indian Medicinal Plants, Phytochemistry and Therapeutics
INTRODUCTION
Breast cancer (BRCA) is the highest-escalation cancer in women with incidence rates rising at a rate of 3% annually in recent years around the world[1]. BRCA is the most prevalent cancer in women, with more than 2.3 million cases diagnosed each year[2]. BRCA is the top or second cause of death from cancer in women in 95% of the world’s nations[3]. The age at which BRCA symptoms first appear is also decreasing, which has a severe psychological impact on patients, puts a large amount of financial and life burden on patients and their families, and lowers happiness levels dramatically. BRCA is currently treated with a combination of chemotherapy, radiotherapy, and surgery which have several shortcomings. The psychological and physical side effects include slow wound healing, changes in body shape, liver and kidney damage, changes in metabolism, and severe alopecia[4,5]. As a result, it is important to create medicines that have a wide range of applications and few negative effects. Herbal medicine has a lot of potential because it has few adverse effects and a powerful healing effect. Emergence, expansion, invasion, and metastasis of BRCA are complex processes. Herbal medicine is a useful technique for treating BRCA regardless of stage[6,7].
Bitter melon, also known as momordica charantia, is a member of the Cucurbitaceae family that exhibits anti-inflammatory, potential antibacterial, and antiviral activities[8-10]. bitter melon extracts are also effective against tumors[11] and were also found to be effective for the treatment of ulcers, malaria, pain, psoriasis, hyperlipidemia, and hypertension[12-17]. It includes several different active ingredients, including polysaccharides, anthrone, peptides, proteins, terpenoids, phenolics, and many more. bitter melon anticancer potentials have become more widely known in recent years. According to research, bitter melon suppresses the growth of several malignant tumors, including gastric, liver, and BRCA[18].
According to Rayet al[19], the bitter melon extract reduces cell proliferation and induces apoptotic cell death. The fact that bitter melon extract administration increased p53, p21, and pChk1/2 while inhibiting cyclin B1 and cyclin D1 production suggests yet another mechanism involved in controlling the cell cycle. Muhammadet al[20] also noted that bitter melon extract treatment enhances phospho-adenosine monophosphate activated protein kinase expression in BRCA cells and suppresses the mammalian target of rapamycin (mTOR)/protein kinase B (Akt) signaling pathway. Additionally, they showed that bitter melon extract feeding effectively slowed the development of BRCA in syngeneic and xenograft animal models. Additionally, they noticed that tumors from animals fed bitter melon extract showed enhanced p62 accumulation, autophagy activation, and apoptotic cell death[20]. Shimet al[21] have demonstrated a therapy with bitter melon extract inhibiting the growth of triple-negative BRCA tumors in mouse models more effectively than treatment with estrogen receptor-positive breast tumors. However, the anti-cancer mechanism of bitter melon extract connecting lipid metabolism in BRCA growth is still unknown. They also showed that bitter melon extract feeding reduces acyl-CoA: Cholesterol acyltransferase-1 expression and slows tumor growth in non-obese diabetic scid gamma mice implanted with triple-negative BRCA mammospheres. Their presented study was the first study indicting bitter melon extract’s role in the inhibition of Acyl-CoA: Cholesterol acyltransferase-1 and the proliferation of triple-negative BRCA cells, suggesting it to be beneficial in combination with other therapeutic agents to treat human BRCA[21].
A recently developed analytical technique is network pharmacology. It performs “multitarget and multi-pathway” analysis of pharmaceuticals and diseases through the integration of various databases, and then creates a “drug active ingredient disease” model to visually depict the investigated action mechanism and precisely forecast the targets[22,23]. Even though a significant number of studies have demonstrated bitter melon’s clinical effectiveness in the treatment of BRCA, the exact mechanism by which it works is still unknown[24]. This study aimed to identify potential bitter melon components, targets, and biological pathways against BRCA using network pharmacology. Furthermore, we validated and provided a theoretical foundation for the possible mechanism using molecular docking studies (Figure 1).
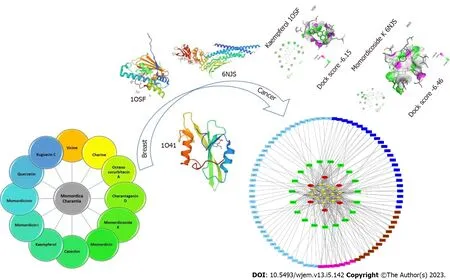
Figure 1 Graphical abstract.
MATERlALS AND METHODS
Collection and screening of active constituents of bitter melon
Chemical constituents were collected from the Indian Medicinal Plants, Phytochemistry and Therapeutics (IMPPAT) 2.0 database (https://cb.imsc.res.in/imppat/)[25]. The bioactive obtained were analyzed and only the chemical constituents of the fruit part were taken by applying the filter. By typing the names of the constituents into the search box on PubChem (https://pubchem.ncbi.nlm.nih.gov/), the canonical smiles of the active constituents were acquired[26]. Moreover, the Molsoft database was used to obtain information regarding the molecular formula, molecular weight, number of hydrogen bond acceptors, number of hydrogen bond donors, MolLogP, and drug-likeness (DL) (https://www.molsoft.com/)[27] to get an idea of whether the compound is following the Lipinski rule. The bioactive violating more than two rules of Lipinski were eliminated. Additionally, the DL and oral bioavailability (OB) thresholds of 0.18 and 30%, respectively, were used to identify the potential active phytochemicals in bitter melon. These two crucial components greatly influence a substance’s potential to have pharmacological effects, and from that the final bioactives were selected, and data were prepared and integrated.
Predicting the targets of the compounds
For identification of the potential targets of the bio-actives, the online platform SuperPred was used (https://prediction.charite.de/subpages/target_prediction.php). The targets of the compound were obtained after entering the canonical smiles[28]. The genes of strong binders and predicted targets were taken, and, on the latter, predicted targets with 75% probability were chosen[29]. The gene’s names corresponding to the proteins were found in the string by putting all the protein’s names, and obtaining the gene UniProt, or official symbol.
Screening for gene targets
The genes related to BRCA were sourced from GeneCards (http://www.genecards.org), using the term “BRCA” as the search query[30]. A Venn diagram was created using the online tool Venny (https://bioinfogp.cnb.csic.es/tools/venny/) to compare the targets of bitter melon and BRCA[31]. The overlap diagram represents the anti-BRCA targets of bitter melon.
Construction and analyses of the protein-protein interactions (PPI) network
The PPI network was constructed by uploading the anti-BRCA targets of bitter melon to the STRING platform (https://string-db.org) with “Homo sapiens” chosen in the organism box[32]. After selecting “Medium Confidence (0.400)” in the basic setting column and “Hide disconnected nodes in the network” in the advanced setting column, the tab-separated values (TSV) format file was exported. The TSV format file was imported into the Cytoscape software to produce the visual analysis of the PPI network[33].
Network construction of active phytoconstituents and anti-BRCA targets
The network of active ingredients in bitter melon and the corresponding targets was constructed and analyzed using the software Cytoscape 3.7.2[34,35]. The nodes in the network represented molecules or genes, which were distinguished by different shapes, and the line between one molecule and one gene meant they were related[36].
Gene ontology (GO) and kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis
The biological role of bitter melon’s anti-BRCA targets was hypothesized using GO and KEGG pathway enrichment analysis. The GO analysis included molecular function, biological processes, and cellular components[37]. The anti-BRCA targets of bitter melon were uploaded to the DAVID platform (https://david.ncifcrf.gov/) to access the database of GO functions and KEGG pathways[38]. Applying a p value of 0.01 and organising the gene count in descending order allowed the top 10 data from the GO analysis to be sorted. The top 10 pathways were then displayed in the form of an enrichment dot bubble by uploading the data to the Bioinformatics Platform (http://www.bioinformatics.com.cn/)[39].
Verification by molecular docking stimulation
The network pharmacology identified possible bitter melon active components as well as the main BRCA-fighting targets and pathways. The bitter melon active compounds with the highest edge counts and which interact the most with targets were chosen as the ligands. Three bioactives were chosen as the ligands, and the Chem 3D program was used to create their 3D structures after searches in the PubChem database (https://pubchem.ncbi.nlm.nih.gov). After adding hydrogens, calculating Gasteiger charges, identifying the root, and selecting rotatable bonds with the help of AutoDock Tools, the ligands were stored in the format of PDBQT. The targets chosen as the protein receptors were those with the top three values in the PPI network, and the 3D structures were taken from the protein data bank (PDB) database (http://www.rcsb.org/)[40]. The specifications of the grid box created around the ligand are shown below.
Hydrogen atoms were added to the receptor structure after the water molecules had been removed, and charges were determined after assigning AD4-type atoms. Thus, prepared receptors were stored in the PDBQT format using the AutoDock tool. All residues remained rigid for the duration of the molecular docking process. The outcomes were shown, and the generation of the binding energy (docking score) was done[40].
RESULTS
Organizing the database of bitter melon
By using the keyword Momordica Charantia in the IMPPAT 2.0 database and reviewing the literature, 304 active ingredients were obtained. By applying a filter from the aerial part of the plant to the fruit part, 43 chemical constituents were obtained, and their canonical smiles were taken to the Molsoft database to demonstrate Lipinski properties and DL. After that, the phytoconstituents that contain DL greater than 0.18, oral OB thresholds of more than 30%, and which do not violate more than 2 Lipinski rules were chosen. So, at last, 14 phytoconstituents’ remains were further analyzed for results.
Active phytochemicals targets
The SuperPred database was used to identify possible protein targets for the bitter melon’s active phytochemicals. Targeted genes for corresponding compounds were determined by taking strong binders, and 75% filtered probability predicted targets were taken. After redundant information was removed, 133 putative protein targets of bitter melon’s active phytochemicals were further examined.
Establishing a database of BRCA
In the GeneCards database, “BRCA” was used as the keyword, and around 15970 genes were obtained. So, to reduce the no. of genes, GeneCards Inferred Functionality Scores filter of > 50 was applied, which resulted in BRCA genes to about 3601.
Acquisition of the venn diagram and construction of the component-target network
A Venn diagram was produced by submitting the bitter melon and BRCA targets to the Venny platform, respectively. The results conclusively showed that 112 instances of 133 genes connected to bitter melon were likewise linked to BRCA (Figure 2).

Figure 2 Venn diagram of targets of bitter melon and breast cancer where blue color indicates targets of bitter melon while yellow color indicates targets of breast cancer.
Construction and analyses of the PPI network
By inputting 112 anti-BRCA targets into the STRING database and using the Cytoscape program to show them, the PPI network of possible targets was created. There were 428 edges and 112 nodes in the PPI network. The size of map nodes increases as the node degrees rise, and their color shifts from orange to blue. The map edge size increased and the color changed from orange to blue as the total score rose (Figure 3A). On a bar graph, the top 10 genes were displayed in order of their degree (Figure 3B). Each bar was plotted using the degree value associated with each gene. The top 3 potential anti-BRCA targets,i.e., heat shock protein 90 (HSP90) AA1, proto-oncogene tyrosine-protein kinase (SRC), and signal transducer and activator of transcription 3 (STAT3), were chosen for molecular docking studies, with key active phytochemicals described in molecular docking section under result.
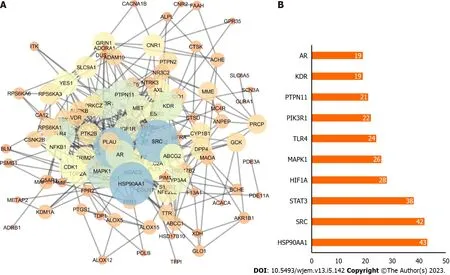
Figure 3 Potential targets. A: Protein-protein interaction network; B: Bar chart of the top 10 genes according to degree where X-axis is the degree value, and the Y-axis is the gene name.
Network of active phytoconstituents and anti-BRCA targets
Using the network analysis program Cytoscape, the active ingredient and BRCA target network was created (Figure 4). There are 369 edges and 126 nodes in the network. The 112 circular nodes surrounding the 14 active components of bitter melon, which were represented by the network’s yellow diamond nodes in the centre, were anticancer targets. Red was used to indicate 6 target nodes linked to more than 10 active compounds, green for 19 target nodes linked to in the range of 5 to 10 corresponding chemicals, pink for 7 target nodes linked to 4 compounds, brown for 13 target nodes linked to 3 active molecules, dark blue for 24 target nodes linked to 2 compounds, and blue for 43 target nodes linked to only 1 active compound. Amongst all, three phytoconstituents, quercetin, kaempferol, and momordicoside K are considered the most important and key active phytochemicals found in bitter melon for BRCA treatment. The findings show that the majority of bitter melon’s active compounds had several targets, and many of these targets related to different active ingredients, demonstrating that bitter melon is a multicomponent, multitarget medication. These outcomes highlight the complex nature of the interactions between many targets and active phytochemicals in bitter melon.

Figure 4 Active ingredient-anti-breast cancer target network of bitter melon.
Enrichment analysis
To further analyze the BRCA targets of bitter melon, GO enrichment was performed on 112 intersecting targets using the web-based DAVID program. The top 10 representative enrichment terms for cellular components, molecular processes, and biological processes, sorted by count in descending order andPvalue < 0.01, are displayed in Figure 5. Orange bars represent a biological process that mainly consisted of G-protein-coupled receptor activity (10/45) and protein kinase binding (9/45). Bars with the green color represented the cellular component, which mainly included the nucleus (42/45), membrane (28/45), and extracellular region (20/45); and lastly, blue colored bars signify the molecular function. Of all 45 potential genes, 9 genes were involved in negative regulation of transcription, DNA template, and cell differentiation.
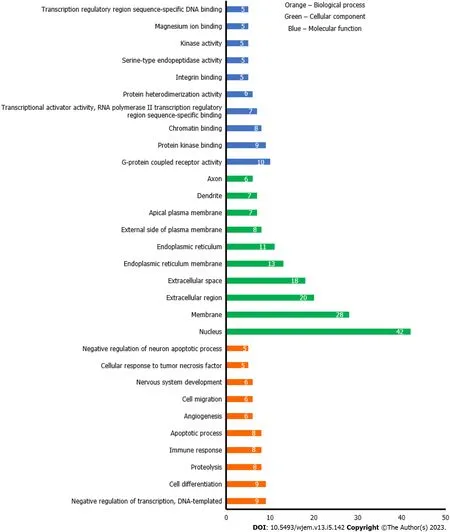
Figure 5 Gene ontology analysis of anti-breast cancer targets of bitter melon where Y-axis is the number of the targets involved in the gene ontology analysis, and the X-axis is the molecular function, biological process, or cellular component.
KEGG pathway enrichment analysis
Using the DAVID web-based tool, KEGG pathway analysis was carried out on 112 overlapping targets to clarify the molecular mechanism by which bitter melon treats BRCA. By uploading 112 anti-BRCA targets to the DAVID platform, around 90 KEGG pathways were obtained, which were further filtered and sorted byPvalue < 0.01 and gene count in descending order. The top 20 representative enrichment terms were plotted by an enrichment dot bubble in Figure 6. Phosphatidylinositol 3-kinase/protein kinase B (PIK3-Akt) signaling, transcriptional misregulation, axon guidance, calcium signaling, focal adhesion, janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling, cyclic adenosine monophosphate (cAMP) signaling, mTOR signaling, and phospholipase D signaling pathways may play important roles in the mechanism of bitter melon against BRCA. These signaling pathways might work in concert to provide the therapeutic benefits of bitter melon against BRCA.
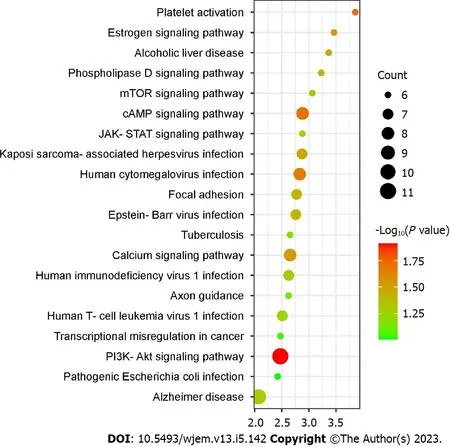
Figure 6 Kyoto encyclopedia of genes and genomes enrichment analysis (top 20) where X-axis is the enrichment gene count, and the Yaxis is the kyoto encyclopedia of genes and genomes pathway. Bubble size represents the number of genes involved in the kyoto encyclopedia of genes and genomes enrichment while color represents -log 10 (P value). PIK3-Akt: Phosphatidylinositol 3-kinase/protein kinase B; JAK-STAT: Janus kinase-signal transducer and activator of transcription; mTOR: Mammalian target of rapamycin.

Figure 7 Docking interactions of kaempferol. A: With protein 1OSF; B: With protein 6NJS.
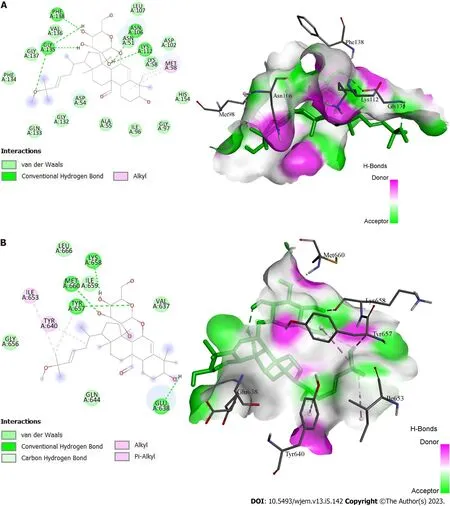
Figure 8 Docking interactions of momordicoside K. A: With protein 1OSF; B: With protein 6NJS.
Molecular docking
HSP90AA, SRC, and STAT3 were employed as the receptors because they have the top three greatest values in the PPI network and the highest level of resolution, while quercetin, kaempferol, and momordicoside k were chosen as the ligands since they each have more than ten gene targets. The PubChem, PDB, Open Babel, and AutoDock tools were used to prepare the ligands and receptors. The three compounds were docked through AutoDock to three different targets to validate the prediction. The result has been displayed as an affinity value (Table 1). Whereas 1O41 had the weakest capacity to attach to the ligands of the three, 1OSF had the easiest time doing so. At a value of -4.47 kcal/mol, quercetin’s affinity for 1O41 was the lowest. Figures 7A and B display the docking interactions of kaempferol with 1OSF and 6NJS, respectively, while Figures 8A and B display the docking interactions o momordicoside K with 1OSF and 6NJS, respectively.

Table 1 Affinities of compound to the targets (dock score)
Kaempferol showed many interactions with 1OSF. The hydroxyl group present on the chromen-4-one ring showed hydrogen bonding with Asn106, Lys112, Gly135, and Phe138, while the hydroxyl group present on the phenyl ring showed hydrogen bonding with Asp93. Phenyl ring is also involved in pi-sulfur and pi-alkyl interactions with Met98 and Ala55, respectively. Similarly, the hydroxyl group present on chromen-4-one ring showed hydrogen bonding with Glu638, and Lys658 on 6NJS. The hydroxyl group attached to the phenyl ring showed interactions with Pro639, and Gln644 through hydrogen bonding. Pi-alkyl interactions are observed between chromen-4-one ring and Val637.
Momordicoside K also showed many interactions with 1OSF. Three hydroxyl groups present on the oxane ring form hydrogen bonds with Asn106, Lys112, Gly135, Val136, and Phe138. While the sidechain oxygen atom is involved in hydrogen bond interaction with Gly135. An alkyl interaction is observed between the phenanthrene ring and the alkylsubstituent present on the phenanthrene ring with Met98. As with 1OSF, momordicoside K showed interactions with 6NJS. A hydrogen bond interaction is observed between the phenanthrene ring containing the hydroxyl group and Glu638. Three hydroxyl groups present on the oxane ring form hydrogen bonds with Tyr657, Met660, and Lys658. Also, Van der Waal’s interaction and pi-alkyl interaction are observed between the sidechain oxygen atom and methyl substituent, respectively, with Tyr657. Similarly, pi-alkyl interactions between the sidechain and the methyl substituent are present on the sidechain with Ile653 and Tyr640, respectively.
DlSCUSSlON
In Ayurveda medicine, bitter melon has numerous elements that have therapeutic effects against a variety of ailments. All parts of the plant, including the fruit, are frequently eaten and prepared in many ways, such as stir-frying, stuffing, or using a small amount to add a mildly bitter flavor to soups or beans. Antioxidant, anti-inflammatory, cancer-fighting, anti-diabetic, anti-bacterial, anti-obesity, and immunomodulatory properties are said to be present in the plant. A fascinating discovery showed that bitter melon extract appeared to be beneficial in halting the growth and spread of cancer tumors in studies employing mice models[41]. By causing apoptosis, cell cycle arrest, autophagy, and blocking cancer stem cells, the plant extract reduces the proliferation of cancer cells. The anticancer effects of bitter melon and its active components have been studied; however, the underlying processes have not been comprehensively uncovered. In this study, network pharmacology-an efficient and cost-effective method for conducting systematic therapeutic researchwas used to examine the mechanism by which bitter melon treats cancer.
The 133 relevant targets were found by gathering and screening the 14 active components from bitter melon that were screened out from the IMPPAT 2.0 database. The Venn diagram revealed that 112 of the 133 targets connected to bitter melon were also connected to targets for BRCA, indicating that the majority of the pertinent targets in bitter melon were connected to effects that were anti-BRCA. The interactions between different bitter melon targets were visible in the PPI network. The three targets with the top three greatest degree values, HSP90AA1 (43), SRC (42), and STAT3 (38) may be the primary anti-BRCA targets. To further confirm the potential interaction between the important targets and the active compounds, molecular docking was done.
One of the most prevalent proteins in mammalian cells, HSP90, together with the oestrogen receptor, progesterone receptor, key HER2 signaling molecules (HER2, AKT, c-SRC, RAF, and HIF-1), and epidermal growth factor receptor, are crucial for the growth and survival of cancer. Moreover, HSP90 has an N-domain ATP binding site, and all its cellular functions depend on its ATPase activity[11]. HSP90 activation may sustain the malignant phenotype, enhance metastasis, and foster treatment resistance in a variety of BRCA subtypes because it functions as a key integrator of numerous pathways. According to research findings, treating BRCA with up-regulated HSP90 may lessen the chance of fatal recurrence and distant metastases. The transcriptional program that are triggered by various cytokines, growth factors, and carcinogenic stimuli are mediated by the STAT3 protein, which is a signal transducer and transcription activator. Its expression and activity are frequently associated with cellular transformation, tumor development, and tumor initiation. The interleukin 6 (IL-6)/JAK/STAT3 signaling pathway is an actionable target with preclinical and clinical studies demonstrating therapeutic potential in both primary and metastatic BRCA, according to recent findings elucidating the role of IL-6 in BRCA progression, metastasis, and anti-cancer immunity. Importantly, direct targeting of IL-6, IL-6R, gp130 receptor, JAKs, or STAT3 has been examined as a means of blocking the IL-6/JAK/STAT3 signaling axis. Numerous cancers have dysregulated SRC, a non-receptor tyrosine kinase. SRC plays a significant role in a variety of features of tumor formation, including growth, survival, adhesion, migration, invasion, and, most significantly, metastasis in a variety of tumor forms. SRC boosts proliferation, diminishes apoptosis, and promotes metastasis.
The molecular docking results showed that the binding energies of kaempferol to STAT3 and HSP90AA are the highest. Furthermore, momordicoside K and quercetin have good STAT3 and HSP90AA binding properties. The most significant anti-BRCA targets of bitter melon may be STAT3 and HSP90AA. SRC, a member of the family of nonreceptor protein tyrosine kinases, is important for many signaling pathways that are involved in cell growth, survival, motility, and angiogenesis. Compared to STAT3 and HAP90AA, SRC had poor binding activity to kaempferol, momordicoside K, and quercetin, which suggested that SRC may not be the direct target of bitter melon in BRCA treatment.
The findings of the KEGG pathway study identified bitter melon’s anti-BRCA pathways. Ten out of the top 20 pathways were directly linked to cancer. These pathways, which include PIK3-Akt signaling, transcriptional dysregulation, axon guidance, calcium signaling, focal adhesion, JAK-STAT signaling, cAMP signaling, mTOR signaling, and phospholipase D signaling, may be crucial in the bitter melon’s defense against BRCA. According to the findings, bitter melon’s targets are crucial in the management of BRCA.
In the PIK3-Akt signaling pathway, which is involved in the control of numerous cellular physiological processes by activating downstream matching effector molecules and is critical for the cell cycle, growth, and proliferation, bitter melon has 11 anti-BRCA targets. Eight anti-BRCA targets in bitter melon are connected to the calcium and cAMP signaling pathways. By the activation of cAMP-dependent protein kinase, the cAMP signaling pathway controls a wide range of intracellular events that are connected to the regulation of cellular proliferation, differentiation, and apoptosis. BRCA development and chemotherapy resistance are both influenced by essential processes regulated by Ca2+signaling pathways, ranging from inflammation to apoptosis. There are seven anti-BRCA targets in the focal adhesion pathway. bitter melon has six anti-BRCA targets that are involved in the mTOR, JAK-STAT, and phospholipase D signaling pathways. A range of biological processes, including cellular proliferation, survival, metabolism, autophagy, and immunity, are aided by the mTOR signaling pathway. While the phospholipase D signaling system has been linked to survival signaling and the control of cell cycle progression, the JAK-STAT pathway plays critical roles in the carcinogenesis, maintenance, and metastasis of BRCA. As a result, bitter melon has significant therapeutic potential in the treatment of BRCA and functions in several ways.
CONCLUSlON
We have successfully investigated the main phytochemicals and molecular processes in bitter melon that are thought to be involved in the therapy of BRCA. This study found 14 major active phytoconstituents as well as 112 anti-BRCA targets in bitter melon. Our research demonstrated that the anti-BRCA benefits of bitter melon are likely caused by negative regulation of transcription, cell differentiation, apoptosis, proteolysis, negative control of neuron apoptosis, and cell migration. Further, we discovered that nine important pathways, including PIK3-Akt signaling, transcriptional dysregulation, axon guidance, calcium signaling, focal adhesion, JAK-STAT signaling, cAMP signaling, mTOR signaling, and phospholipase D signaling, are likely to be involved in the mechanism of action of bitter melon in the treatment of BRCA. Our results support the hypothesis that bitter melon’s potential anti-BRCA properties may be a result of the direct or indirect synergistic effects of multitarget and multi-pathway activities. According to molecular docking research, the principal active phytochemicals in bitter melon may be able to bind to HAP90AA1, STAT3, and other BRCA-related targets. The results of this study offer suggestions for additional investigation into the phytochemicals and mechanisms of bitter melon’s anti-BRCA activity. This serves as a foundation for creating cutting-edge anti-BRCA medications based on phytochemicals found in bitter melon but experimental validation is necessary to confirm the predicted therapeutic effects of bitter melon on BRCA. Without experimental evidence, the clinical relevance of these findings remains uncertain.
ARTlCLE HlGHLlGHTS
Research background
Bitter melon has been used to stop the growth of breast cancer (BRCA) cells. However, the underlying mechanism is still unclear.
Research motivation
The motivation of this research is to explore the underlying pharmacological mechanisms.
Research objectives
The goal of this study was to predict the therapeutic effect of bitter melon against BRCA using network pharmacology.
Research methods
The active ingredients of bitter melon and the related protein targets were taken from the Indian Medicinal Plants, Phytochemistry and Therapeutics and SuperPred databases, respectively. The GeneCards database has been searched for BRCA-related targets. Through an intersection of the drug’s targets and the disease’s objectives, prospective bitter melon anti-BRCA targets were discovered. Gene ontology and kyoto encyclopedia of genes and genomes enrichment analyses were carried out to comprehend the biological roles of the target proteins. The binding relationship between bitter melon’s active ingredients and the suggested target proteins was verified using molecular docking techniques.
Research results
Through the active ingredient-anti-BRCA target network analysis, three major components were found to be important in mediating the putative anti-BRCA actions of bitter melon: momordicoside K, kaempferol, and quercetin. In the proteinprotein interaction network analysis, the top three proteins were determined to be heat shock protein 90 AA, protooncogene tyrosine-protein kinase, and signal transducer and activator of transcription 3 (STAT3). According to molecular docking research, the principal active phytochemicals in bitter melon are able to bind to HAP90AA1, STAT3, and other breast cancer-related targets.
Research conclusions
Overall, the integration of network pharmacology, molecular docking, and functional enrichment analyses shed light on potential mechanisms underlying bitter melon’s ability to fight BRCA, implicating active ingredients and protein targets, as well as highlighting the major signaling pathways that may be altered by this natural product for therapeutic benefit.
Research perspectives
Database resources were used to examine active compounds found in bitter melon. Network pharmacology and molecular docking techniques were used to explore the mechanism through which bitter melon was used to treat BRCA. Momordicoside K, kaempferol, and quercetin were identified as potentially important active ingredients of bitter melon showing anti-BRCA actions. The study identified several possible molecular targets as well as signaling pathways involved in bitter melon’s anti-BRCA actions, like phosphatidylinositol 3-kinase/protein kinase B signaling and janus kinase-signal transducer and activator of transcription signaling. The study suggests further experimental verification to confirm the potential findings of bitter melon in the treatment of BRCA.
FOOTNOTES
Author contributions:Panchal K designed, performed and wrote the paper; Nihalani B designed, performed and wrote the paper; Oza U edited the paper; Panchal A edited the paper; Shah B designed, supervised and edited the paper.
Conflict-of-interest statement:The authors declare no conflicts of interest in this paper.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORClD number:Bhumi Shah 0000-0002-7520-7132.
S-Editor:Qu XL
L-Editor:A
P-Editor:Yuan YY
 World Journal of Experimental Medicine2023年5期
World Journal of Experimental Medicine2023年5期
- World Journal of Experimental Medicine的其它文章
- Altered expression of miR-125a and dysregulated cytokines in systemic lupus erythematosus: Unveiling diagnostic and prognostic markers
- Red cell distribution width: A predictor of the severity of hypertriglyceridemia-induced acute pancreatitis
- Ground level utility of Access, Watch, Reserve classification: lnsights from a tertiary care center in North lndia
- In vitro study on the transmission of multidrug-resistant bacteria from textiles to pig skin
- Research on nanosciences involvement in pharmaceutical education should be reinforced
