The Value of Prealbumin and its Combination with NT-proBNP for Predicting in-hospital Mortality in Patients with Heart Failure: Real-World Research Based on Propensity Score Matching
LIU Bing and XIE Jia Yi
In recent years, extended life expectancies and lifestyle changes have markedly contributed to the increased incidence of heart failure (HF)worldwide[1].In China, while the age of patients with chronic HF has increased annually, the mortality rate has not decreased significantly[1].Malnutrition, an issue for patients with HF, is associated with a worse prognosis because it can lead to disease progression attributable to loss of skeletal mass, a vicious cycle associated with cytokine activation, and cachexia[2].Prealbumin (PA) and albumin levels are known to reflect the nutritional status of patients and are associated with a poor prognosis for patients with HF[3].The half-life of PA is only 1.9 days, which is shorter than that of albumin[4].In some patients with liver disease or poor nutrition, PA levels decrease while albumin levels remain normal.Previous studies have confirmed that N-terminal pro b-type brain natriuretic peptide (NT-proBNP) can reflect cardiac function in patients with HF and is significantly correlated with prognosis[5].However, to treat such high-risk patients, practitioners tend to pay more attention to cardiac function improvement based on NT-proBNP levels and less attention to nutritional support.
In terms of study design, using propensity score matching (PSM) can effectively reduce confounding biases and is similar to randomized controlled trials.PSM has been shown to balance covariates between groups; thus, non-randomized controlled data can be evaluated for intervention effects[6].In this study based on PSM, we aimed to assess whether PA was an effective prognostic indicator in patients with HF.We compared the predictive accuracy of PA with that of NT-proBNP to determine whether the use of PA could improve predictive accuracy in combination with NT-proBNP.
This study comprised 11,556 consecutive patients with HF (aged > 18 years) as their main diagnosis at the time of admission to the Shengjing Hospital of China Medical University between January 2013 and December 2018.Patient data included baseline characteristics and electronic data system hospitalization records.HF was defined based on modified Framingham criteria[7].Fasting venous blood samples were collected from all patients between 06:00 h and 07:00 h on the second day of admission.PA was assayed using a particleenhanced immunonephelometric assay on an automated analyzer (AU5800; Beckman Coulter Corp., USA).The concentrations of NT-proBNP were determined using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) and an electrochemiluminescence analyzer (Cobas, e601,Roche, Switzerland).The estimated glomerular filtration rate (eGFR) was calculated using the following equation: 175 × SCr-1.154× age-0.203× 0.742(if female).Left ventricular ejection fraction (LVEF)was determined using echocardiography and Simpson’s biplane method during the hospital stay(within the first three days of admission) or upon arrival at the emergency department.The primary endpoint was all-cause mortality during hospitalization.Exclusion criteria comprised a diagnosis of liver disease and/or chronic kidney failure requiring dialysis on admission, a history of cardiac transplantation or chronic alcoholism, and no available PA or NT-proBNP levels.In total, 8,335 study patients with HF were enrolled(Supplementary Figure S1, available in www.besjournal.com).This study complied with the principles of the Declaration of Helsinki and was approved by the Shengjing Hospital of the China Medical University Research Ethics Committee.
To reduce bias owing to imbalances in baseline characteristics dependent on the presence or absence of PA, we used PSM to assemble a matched and balanced cohort.Patients with a PA level ≤15.0 mg/dL (optimal cutoff value, largest Youden's index [sensitivity+ specificity-1]) and those with a PA level > 15 mg/dL were compared.We calculated the PA propensity score (PS) for each patient using a non-parsimonious multivariable logistic regression model.Propensity analysis was used to identify patients with similar probabilities of having a lowerlevel PA based on observed clinical characteristics.In our PS model, 24 baseline characteristics were used as covariates (Table 1).We computed the logit of the estimated PS for each patient to match patients in the different groups.We then used a greedy matching algorithm to match patients using a calipers set to a maximum width of 0.2 standard deviations (SDs) of the estimated PS.Standardized differences for all covariates were used to compare the balance of baseline covariates between the two groups before and after matching.A standardized difference of < 10% suggested inconsequential bias.Continuous variables were presented as mean ± SD or median (interquartile range), depending on whether they were normally distributed.Categorical variables were presented as counts and proportions(%).Logistic multivariate regression analysis was used to assess the prognostic relationship between PA and in-hospital mortality.PA was analyzed as both a categorical and continuous variable.Results are represented as odds ratios (ORs) with associated 95% confidence intervals (CIs).The prognostic potentials of PA, NT-proBNP, and PA+NT-proBNP were analyzed using the following methods.(1) MedCalc statistical software (version 18.1.1;MedCalc Software, Ltd., Belgium) was used to calculate the area under the curve (AUC) with relation to the primary end point through the receiver operating characteristic (ROC) curve.(2) We used category-free net reclassification improvement(NRI) and absolute integrated discrimination improvement (IDI) to examine improvement in the risk estimation efficiency of PA, NT-proBNP, and PA+NT-proBNP.Data analyses were conducted using Statistical Analysis Software (version 9.4; SAS Institute, Inc., Cary, North Carolina) and the level of statistical significance was defined asP< 0.05.
The baseline clinical characteristics of the 8,335 patients (mean age, 68.4 years; males, 53.7%) are shown in Table 1.Patients in the lower PA group were older, disproportionately female, had a higher New York Heart Association (NYHA) grading, a higher heart rate on admission, lower systolic blood pressure on admission, and lower proportions of coronary heart disease, hypertension, and atrial fibrillation.The lower PA group also had higher total bilirubin, blood urea nitrogen, uric acid, cardiac troponin I, and NT-proBNP levels and lower albumin,low-density lipoprotein, eGFR, hemoglobin, serum sodium, and LVEF levels.PSM resulted in 1,485 matched patient pairs in the PA groups.The baseline characteristics for all patients (except for PA)showedP> 0.05, and PSM reduced the standardized difference for all variables to < 10% (Table 1).
Several studies have shown that albumin levels are associated with a poor prognosis for patients with HF,whether hospitalized or discharged.However, its long half-life makes it an insensitive indicator.Owing to the considerable challenges in treating patients with HF, a more sensitive indicator may be needed to determine their nutritional status.PA, also called transthyretin,with a half-life of 1.9 days, is mainly synthesized through the liver and excreted by the kidneys[4].PA is directly correlated with dual-energy X-ray absorptiometry results and bioelectrical impedance analysis, which are gold standards for defining malnutrition.Malnutrition is common in patients with HF, varying in prevalence rates of 20%–70%[3]and is strongly associated with a poor prognosis in patients with HF.Masakazu et al.[8]investigated 166 patients with chronic heart failure and found that patients with anorexia were more likely to present with impaired functional capacity and higher all-cause mortality within two years.A multicenter registry study suggested that identifying malnourished patients at hospital discharge was helpful in predicting the longterm prognosis of older adult patients with HF and a preserved ejection fraction[9].In our study, 193 (2.3%)patients died during hospitalization, and the outcome was poor in the lower PA group.The multivariate adjustedORfor all-cause in-hospital mortality was 0.443 (95%CI: 0.305–0.642,P< 0.001) for the category, and 0.92 (95%CI: 0.888–0.954,P< 0.001) for PA per mg/dL increase (Table 2).Logistic regression analysis showed similar results after PSM.In the multivariate model, the risk of in-hospital mortality was 0.438 (95%CI: 0.274–0.700,P< 0.001) and 0.905(95%CI: 0.861–0.951,P< 0.001) when PA was treated as a categorical and continuous variable, respectively(Table 2).
The pre- and post-match PSMROCcurves for PA,NT-proBNP, and PA+NT-proBNP were assessed using C-statistics (Supplementary Figure S2 and Supplementary Table S1, available in www.besjournal.com).NT-proBNP has been reported to be associated with adverse events in patients with HF, whether hospitalized or discharged[5].Themetabolic pathway of NT-proBNP involves the kidney, and its circulating concentration is affected through renal clearance.Renal dysfunction, which abnormally increases the concentration of NTproBNP and limits its clinical utility, is prevalent in patients with HF[10].PA had no such limitations and showed the same predictive accuracy for in-hospital mortality (C-statistic:Z= 1.148,P= 0.251).Furthermore, the novel model consisting of PA and NT-proBNP showed better performance than NTproBNP alone (C-statistic:Z= 2.368,P= 0.018;IDI=0.0176,P< 0.001;NRI= 0.6526,P< 0.001).The prognostic significance was not attenuated after PSM (C-statistic:Z= 2.521,P= 0.012;IDI= 0.0057,P< 0.001;NRI= 0.3645,P= 0.001) (Table 3,Supplementary Figure S2).
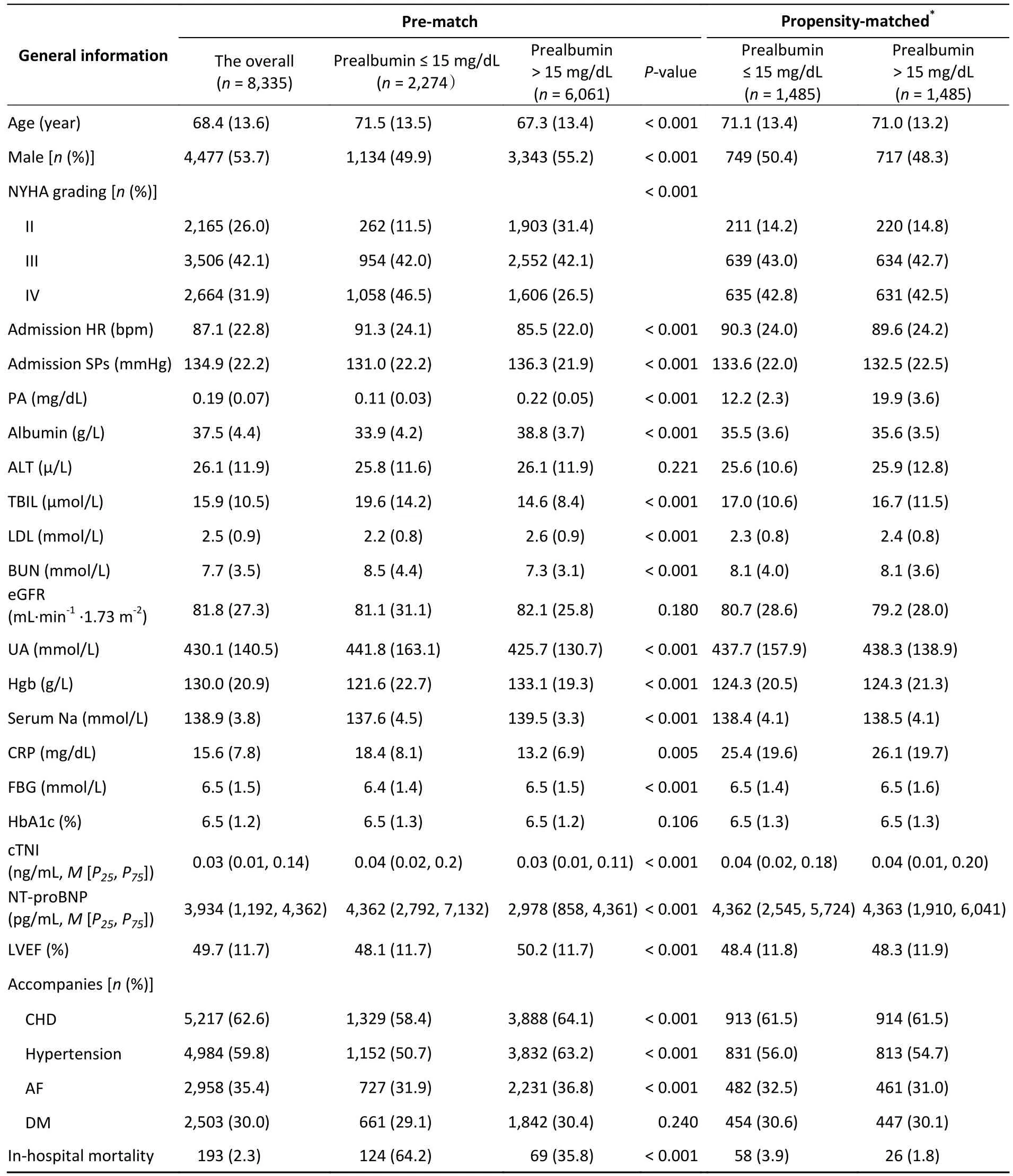
Table 1.Baseline characteristics of patients in the low and high prealbumin groups before and after propensity matching
While the pathophysiological association between malnutrition and HF requires further investigation, numerous mechanisms may underlie this association.A continuous vicious cycle of malnutrition, inflammation, and cachexia syndrome in patients with HF exacerbates disease progression[2].Under conditions of malnutrition,skeletal muscle loss and cytokine activation eventually affect the body’s energy supply.This state of malnutrition represents the initiation and continuation of fat and fat-free mass loss.The result affects left ventricular function and produces a mismatch between protein and energy needs,affecting the heart’s energy supply until it is depleted.Therefore, the increase in PA in response to nutrition in the early acute phase in critically ill patients indicates that at least 65% of their nutritional needs are being met.
Despite the large sample size, this study had some limitations.First, we used PSM to eliminate confounding factors and selection bias; however, we did not include substantial real-world data.Second,PA data were obtained only at admission with no dynamic monitoring; therefore, we could not assess the extent of the PA increase in terms of improving patient prognosis.Finally, potential confounders likely remained despite including as many clinically relevant variables as possible in the multivariateanalysis.Undernutrition in HF is multifactorial, and the chronic low-grade inflammatory state is considered to result from bowel wall edema because of hyperhydration and bacterial translocation.We only included C-reactive protein levels to represent inflammation and did not include indicators such as procalcitonin, which may have led to a selection bias.
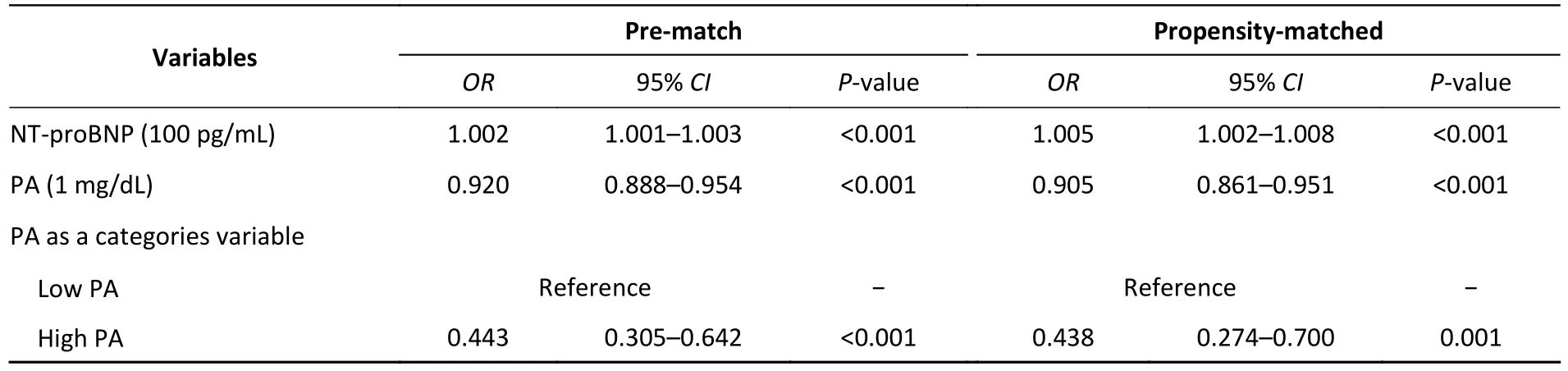
Table 2.Effects of multiple variables on clinical outcomes in multivariate analysis

Table 3.Comparisons of the predictive performance of PA, PA +NT-proBNP, and NT-proBNP for the prognosis prediction
The results obtained through PSM confirmed that PA is a potential prognosticator of in-hospital mortality in patients with HF.PA did not have a lower predictive performance than NT-proBNP.The addition of PA could enhance the predictive significance of NT-proBNP.
AcknowledgementsAll authors who contributed to this article met the criteria for authorship.
Conflict of InterestThere was no conflict of interest.
#Correspondence should be addressed to XIE Jia Yi,Tel: 86-18940255880, E-mail: xiejiayi588@126.com
Biographical note of the first author: LIU Bing, female,born in 1985, majoring in research on the development of cardiovascular disease and heart failure.
Received: May 13, 2023;Accepted: July 11, 2023
 Biomedical and Environmental Sciences2023年11期
Biomedical and Environmental Sciences2023年11期
- Biomedical and Environmental Sciences的其它文章
- Decontamination Efficacy of Disinfection and Doffing of Medical Protective Clothing: A Combined Mannequin and Personnel Study*
- SARS-CoV-2 lnfection Risk Factors in Beijing during November 2022: A Case Control Study*
- Association between Temperature Changes and Cardiovascular Mortality Risk in A High-latitude City in Northeast China*
- Mendelian Randomization Analysis to Analyze the Effect of Emergency Caesarean Section on Different Allergic Diseases and Related Blood Markers*
- Effects of Differential First-Line Antiretroviral Therapy (ART)Regimens on Mortality among HlV/AlDS Children in Southwest China: A 15-year Retrospective Cohort Study*
- The Psychosomatic Traits of “People with the Five Elements in Traditional Chinese Medicine”: A Qualitative Study*
