Effects of Differential First-Line Antiretroviral Therapy (ART)Regimens on Mortality among HlV/AlDS Children in Southwest China: A 15-year Retrospective Cohort Study*
CHEN Qiu Li, LIAO Yan Yan, QIN Shan Fang, LU Chun Yan, PAN Pei Jiang,WANG Hai Long, JIANG Jun Jun, ZHENG Zhi Gang, QIN Feng Xiang, HONG Wen,NING Chuan Yi, YE Li,,#, and LIANG Hao,,#
Human immunodeficiency virus (HIV) is a serious public health concern.Globally, 1.7 million (1.3–2.1 million) children aged 0–14 were living with HIV at the end of 2021, 90% of whom lived in low- and middle-income countries.Acquired Immune Deficiency Syndrome (AIDS) remains the leading cause of mortality among children worldwide,resulting in 98,000 (67,000-140,000) child deaths due to HIV-related illnesses worldwide in 2021[1].Over 50% of HIV-infected infants die within two years of age in the absence of treatment[2], indicating rapid disease progression and a high mortality rate.Early initiation of antiretroviral therapy (ART) in infants reduces the risk of severe morbidity by 75%and mortality by 76%[3].Therefore, timely antiretroviral treatment is urgently needed for children with HIV infection.
Expanded access to ART has led to a steep decrease in the number of children dying from HIVrelated causes worldwide.China’s National Free Antiretroviral Treatment Program (NFATP) was fully implemented nationally in 2003 and has significantly reduced deaths in children with HIV.The cost of second-line ART is higher than that of first-line[4], and most children with HIV infection in China receive NFATP-recommended first-line ART regimens.The recommended first-line ART regimens of children with HIV infection comprises two nucleoside reverse transcriptase inhibitors (NRTI) plus either a nonnucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI) (abacavir [ABC] or zidovudine[AZT]) plus lamivudine (3TC) and (efavirenz [EFV] or nevirapine [NVP] or ritonavir-boosted lopinavir[LPV/r]).The International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT)P1060 study conducted a randomized trial of initial therapy with AZT and 3TC plus either NVP or LPV/r in children with HIV infection and compared short-term(2–36 months) or long-term (5 years) outcomes in six African countries.Antiretroviral treatment with AZT and 3TC plus LPV/r resulted in better outcomes than treatment with AZT and 3TC plus NVP[5-7].However,the drug (LPV/r, NVP, or EFV) crucial for reducing mortality, and guidelines to choose the optimal ART regimen in large cohorts of children with HIV infection, according to the recommended first-line ART regimens of NFATP in China, is unclear.
In this study, we retrospectively collected data on children with HIV infection receiving first-line ART in Guangxi from the Guangxi Center for Disease Prevention and Control and electronic medical records from the Check Hospital of Guangxi, and evaluated the effects of different first-line ART regimens on mortality among children with HIV infection in Guangxi.
The study included children no more than 14 years of age with ART initiation, had complete follow-up records, started ART between Nov 16,2004, and Mar 22, 2018, and were treated with the baseline ART regimen: AZT+3TC+LPV/r (LPV/rbased), AZT+3TC+NVP (NVP-based), or AZT+3TC+EFV(EFV-based).Participants were excluded if they were treated with regimens other than LPV/r-, NVP-, or EFV-based regimens.In total, 653 children with HIV infection who were initiated on ART between November 2004 and March 2018 in Guangxi, China,were screened.Of these, 212 children who did not receive AZT+3TC+LPV/r / NVP / EFV were excluded from the study.Finally, 441 children met the inclusion criteria.Of these, 131, 158, and 152 patients received LPV/r-based, NVP-based regimen and EFV-based regimen, respectively.This study was approved by the Human Research Ethics Committee of Guangxi Medical University.The identities and information of all participants were stored in the hospital’s medical record system, which is a secure password-protected system that is not open to the public or unauthorized users.
Data including baseline characteristics, clinical and laboratory information, treatment outcomes,and follow-up times were collected.The monitoring schedule for ART was baseline, half-month, 1-month,and 2-month, then changed to follow-up every three months.The data collected included the CD4+T cell count, World Health Organization (WHO) clinical stage, time from clinical diagnosis to ART initiation,opportunistic infection, and ART regimens.The primary outcome of the analysis was mortality.Death and mortality rates were compared among children with HIV infection who received different first-line ART.Follow-up data were collected until May 25, 2018.The observation period ranged from the ART initiation to the date of death.
Categorical variables are described as frequency while quantitative variables are expressed as the median ± interquartile range (IQR).Chi-square tests for categorical variables were used to compare the characteristics among the three ART regimen groups.Kaplan-Meier analysis was used to assess the cumulative mortality of all children with HIV infection, and statistical testing of differences was performed using the log-rank test or Breslow test.Univariate and multivariate Cox regression analyses were performed to explore factors associated with death.All analyses were performed using the Statistical Package for the Social Sciences (SPSS)version 26.0 (SPSS Inc.Chicago, USA) and GraphPad Prism version 8.0 (GraphPad Software, San Diego,California, USA).All statistical tests were two-tailed.ThePvalues less than 0.05 were considered statistically significant.
Together, these children contributed 2,183.66 person-years of follow-up, with a median of 4.84 person-year (IQR 2.09–7.33).The baseline characteristics of the 441 enrolled patients are summarized in Supplementary Table S1 (available in www.besjournal.com).A total of 39.2% were diagnosed with HIV, 34.7% started ART at 25–60 months, 67.6% were diagnosed with HIV-to-ART initiation at no more than 6 months, and 52.4% were male.More than half of the children (57.4%) had the WHO HIV clinical stage I disease.59.2% children with a CD4+T cell count ≤ 500 cells/μL, 66.2% children with a CD8+T cell count > 1,138 cells/μL.
ART regimens were associated with mortality in HIV-infected children (Table 1).Of the total children,the AIDS-related cumulative mortality rate was 6.1%(27/441) and the mortality density was 12.4 per 1,000 person-years (95% confidence interval [CI]7.8–16.9) in the overall cohort.In children with HIV infection treated with an LPV/r-based regimen, the mortality rate was 3.1% (4/131) or 5.6 per 1000 person-years (95%CI0.2–10.9).In those with NVPbased regimen, the mortality rate was 10.8%(17/158), or 20.2 per 1000 person-years (95%CI10.8–29.5).In those with EFV-based regimen, the mortality rate was 3.9% (6/152), or 9.6 per 1000 person-years (95%CI2.1–17.1).The log-rank test(P= 0.010) showed that mortality was significantly different among the three groups (Table 1).Survival analyses also showed that the cumulative mortality of children with HIV infection in the NVP-based regimen group was higher than that in the LPV/r- or EFV-based regimens at all evaluated time points (log-rank test,P= 0.010; Figure 1).
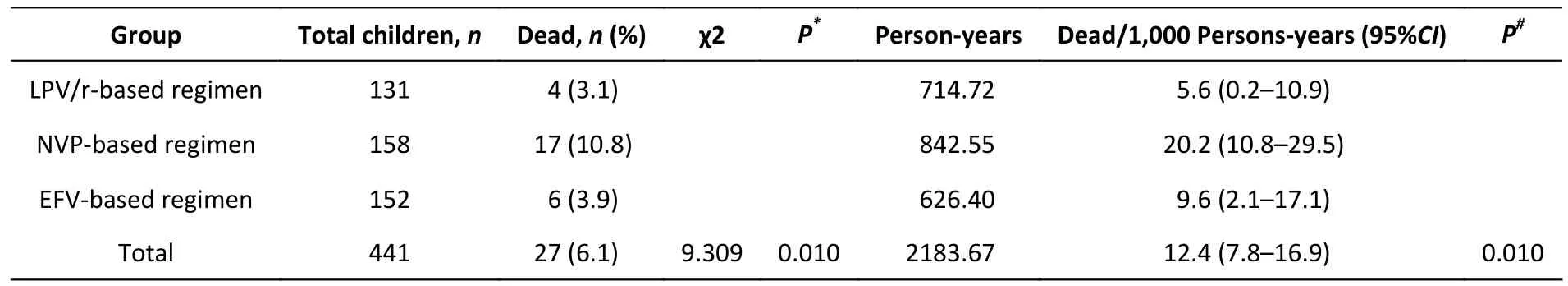
Table 1.Comparison of mortality of children with HIV infection with baseline ART regimen
Univariate and multivariate Cox regression analyses were used to identify factors that affected mortality (Supplementary Table S2, available in www.besjournal.com).As shown in Table 2,univariate Cox regression analysis showed that mortality was significantly higher in the NVP-based regimen than in the LPV/r-based regimen [hazard ratio (HR) = 3.782, 95%CI1.272–11.248,P= 0.017].No significant difference was observed in mortality between LPV/r- and EFV-based regimen (HR = 1.415,95%CI0.399–5.019,P= 0.591).After adjustment for a collection of pre-defined and forward-selection variables (including age at HIV diagnosis, time period between HIV diagnosis and ART initiation, age at ART initiation, sex, WHO clinical stage, baseline CD4+T cell count, baseline CD8+T cell count, baseline ART regimen, TB infection, and opportunistic infection),the NVP-based regimen still had a significantly higher mortality rate than the LPV/r-based regimen[adjusted hazard ratio (aHR) = 4.350, 95%CI1.193–15.860,P= 0.026].There was also no significant difference in mortality between the LPV/r- and EFV-based regimens (aHR = 1.702, 95%CI0.396–7.320,P= 0.475) (Table 2 and Supplementary Table S2).Collectively, these data provide strong evidence of the clinical impact of NVP-based regimens on the mortality of HIV/AIDS children receiving ART compared with LPV/r- or EFV-based regimens.
Several studies have confirmed an association between mortality and WHO clinical stage.WHO clinical stage III/IV is associated with an increased risk of death[8,9].Factors associated with mortality in children remaining in care included clinical stage(HR = 10.13; 95%CI2.25, 45.58 comparing WHO stage I/II with III/IV)[10].In this study, advanced WHO clinical stage was independently associated with higher mortality (WHO clinical stage III/IV: aHR =8.223, 95%CI2.583–26.180,P< 0.001; reference to WHO clinical stage I/II) (Supplementary Table S2).As shown in Supplementary Table S3 (available in www.besjournal.com), the highest mortality (23.3%,or 45.1/1,000 person-years, 95%CI22.1–68.2,P< 0.001) was observed in NVP-based regimen with WHO clinical stage III/IV.Similarly, in the EFV-based regimen, mortality with WHO clinical stage III/IV(9.8%, or 22.1/1,000 person-years, 95%CI3.2–41.0,P= 0.009) was significantly higher than children with WHO clinical stage I/II.The Kaplan-Meier chart showed that WHO clinical stage III/IV patients had significantly higher cumulative mortality than WHO clinical stage I/II patients in all three baseline ART regimen subgroups (Supplementary Figure S1A–C,available in www.besjournal.com).
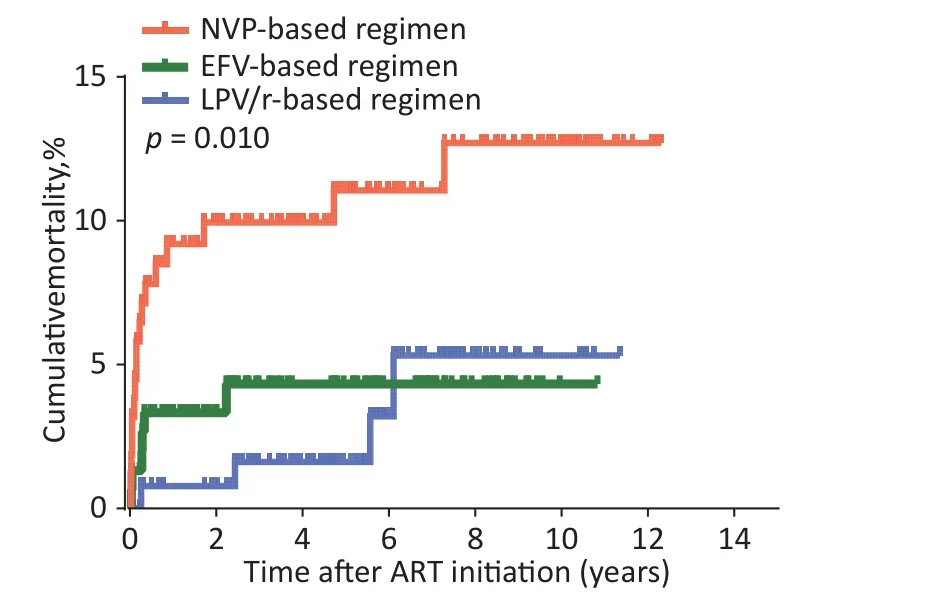
Figure 1.Kaplan-Meier analysis of cumulative mortality of children with HIV infection,grouped by baseline ART regimen.The statistical significance was measured by logrank test.ART, antiretroviral therapy, HIV,human immunodeficiency virus.
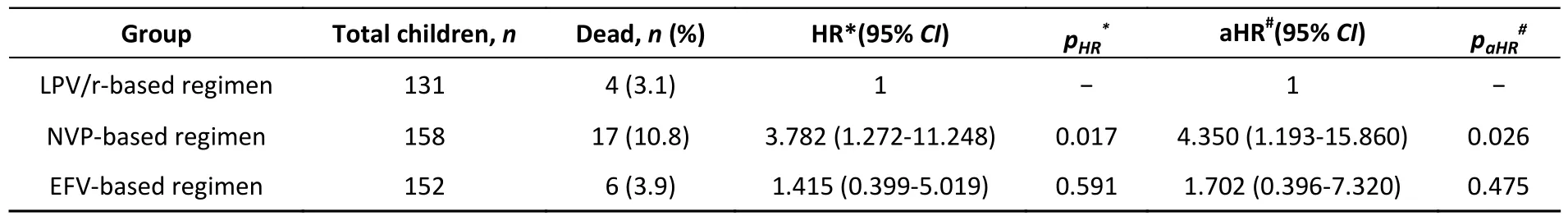
Table 2.Effect of baseline ART regimen on mortality among children with HIV infection receiving ART
As the baseline WHO clinical stage was a major factor influencing mortality, we evaluated the effect of different baseline ART regimens on mortality in the two subgroups of WHO clinical stages I/II or III/IV using the Kaplan-Meier method.In WHO clinical stage III/IV, the cumulative mortality in the NVPbased regimen was significantly higher than that in the LPV/r- or EFV-based regimens(Supplementary Figure S2(A–B), available in www.besjournal.com,P= 0.018).As shown in Supplementary Table S4–S5 (available in www.besjournal.com), in WHO clinical stage III/IV, the highest death rate (23.3%, or 45.1/1,000 personyears, 95%CI22.1–68.2,P= 0.018) was found in NVP-based regimen compared with LPV/r- or EFVbased regimen.There was also no significant difference in mortality between the LPV/r- and EFVbased regimens (P> 0.05), indicating that the WHO clinical stage does not affect the relationship between the baseline ART regimen and mortality.Notably, different baseline ART regimens were most significant in the WHO clinical stage III/IV subgroup.This provides further support for the finding that if different baseline ART regimens have an effect on mortality, the highest effect of the NVP-based regimen would be expected to be most profound in the patient group with baseline WHO clinical stage III/IV.In addition, we found that the mortality rates between LPV/r-based regimen and EFV-based regimen were similar, which means that we could choose LPV/r- or EFV-based regimens regardless of the baseline WHO clinical stage.
In addition to the inherent limitations of this retrospective study, there were other limitations to our research.First, this study was conducted only in Guangxi and thus may not be representative of other regions in China.Second, AZT-based ART regimens(AZT+3TC+LPV/r / NVP / EFV) and ABC-based ART regimens (ABC+3TC+LPV/r / NVP / EFV) are first-line ART regimens according to the NFATP.Only children with HIV/AIDS who received AZT-based ART regimens (AZT+3TC+LPV/r / NVP / EFV) were included in the study, whereas those who received ABC-based ART regimens (ABC+3TC+LPV/r / NVP /EFV) were excluded.Therefore, we could not estimate the treatment effects of ABC-based ART regimens.Finally, there may be a possibility of regimen changesduring treatment; this study was based on ART regimen initiation and did not analyze children with regimen changes.
In this large retrospective cohort study, we estimated the mortality associated with different baseline ART regimens in children with HIV infection who started ART in Southern China.Our study highlights the important contribution of higher mortality associated with NVP-based regimens in children with HIV/AIDS in WHO clinical stage III/IV.Simultaneously, EFV-and LPV/r-based regimens have the same security.This provides a basis for the selection of baseline ART regimens that are associated with fewer deaths in children with HIV/AIDS, especially in those with WHO clinical stage III/IV.In future, we will increase the number of ABCbased ART regimens to identify the safest first-line drugs for children with HIV.
We express our gratitude to all staff from the Guangxi Center for Disease Prevention and Control,and Check Hospital of Guangxi, Guangxi, China, for their collection and provision of epidemiological data on local HIV/AIDS children.
The authors declare that they have no conflict of interest.
&These authors contributed equally to this work.
#Correspondence should be addressed to NING Chuan Yi, E-mail: ningchuanyi@gxmu.edu.cn; YE Li, E-mail:yeli@gxmu.edu.cn; LIANG Hao, E-mail: lianghao@gxmu.edu.cn
Biographical notes of the first authors: CHEN Qiu Li,female, born in 1993, PhD, majoring in viral immunology and epidemiology-related research; LIAO Yan Yan, female,born in 1987, PhD, Assistant Research Fellow, majoring in the etiology and epidemiology of emerging infectious disease; QIN Shan Fang, female, born in 1973, Master,majoring in HIV anti-retroviral treatment and opportunistic infection-related research.
Received: May 10, 2023;
Accepted: October 11, 2023
 Biomedical and Environmental Sciences2023年11期
Biomedical and Environmental Sciences2023年11期
- Biomedical and Environmental Sciences的其它文章
- Decontamination Efficacy of Disinfection and Doffing of Medical Protective Clothing: A Combined Mannequin and Personnel Study*
- SARS-CoV-2 lnfection Risk Factors in Beijing during November 2022: A Case Control Study*
- Association between Temperature Changes and Cardiovascular Mortality Risk in A High-latitude City in Northeast China*
- The Value of Prealbumin and its Combination with NT-proBNP for Predicting in-hospital Mortality in Patients with Heart Failure: Real-World Research Based on Propensity Score Matching
- Mendelian Randomization Analysis to Analyze the Effect of Emergency Caesarean Section on Different Allergic Diseases and Related Blood Markers*
- The Psychosomatic Traits of “People with the Five Elements in Traditional Chinese Medicine”: A Qualitative Study*
