Effect of different process conditions on the physicochemical and antimicrobial properties of plasma-activated water
Zhicheng CAI(蔡志成) ,Jiamei WANG(王佳媚),* ,Yuanyuan WANG(王媛媛) ,Xiaohan SANG (桑晓涵) ,Lixian ZENG (曾丽仙) ,Wentao DENG (邓文韬) and Jianhao ZHANG (章建浩)
1 School of Food Science and Engineering,Hainan University,Haikou 570228,People’s Republic of China
2 College of Food Science and Technology,Nanjing Agricultural University,Nanjing 210095,People’s Republic of China
Abstract The physicochemical properties of plasma-activated water (PAW) generated under different process conditions were investigated,and their changes under different storage conditions were also studied.The results showed that increasing the processing time and power,and decreasing generated water volume,could cause an increase in the redox potential,conductivity,and temperature of PAW,and a decrease in its pH.A slower dissipation of the reactive oxygen and nitrogen species in PAW was found on storage at 4°C in a sealed conical flask than on storage at room temperature.The inactivation ability of plasma-activated lactic acid (LA) to Listeria monocytogenes (L.monocytogenes) and Pseudomonas aeruginosa (P.aeruginosa) was higher than that of PAW or LA alone under the same experimental conditions.The results of this study may provide theoretical information for the application of PAW as a potential antimicrobial agent in the future.
Keywords: plasma activated water,physicochemical properties,treatment conditions,disinfection effect
1.Introduction
Plasma,which is considered the fourth state of matter,is usually derived from the ionization of normal gas [1].It has been widely used in the fields of wastewater and medical treatments [2],the food industry [3],and seed germination and plant growth [4].Plasma-activated water (PAW) can be generated with a plasma generator to activate small species above or below the surface of the water [5].The species mainly include the long-lived substances hydrogen peroxide(H2O2),nitrate ions (),nitrite ions (),and ozone(O3),as well as the short-lived substances hydroxyl radicals(·OH),singlet oxygen (1O2),nitric oxide (NO-),and superoxideall of them are considered as reactive oxygen and nitrogen species (ROS and RNS) [6].ROS and RNS are the main reactive ingredients in PAW,which play key roles in the decontamination and/or disinfection of PAW[7].It has been reported that physical properties including redox potential(ORP),conductivity,temperature,and pH are the main parameters of PAW in most practical applications[8].However,the generation parameters including treatment time,the volume of water,power,and the plasma generation equipment [9] determine the physicochemical properties of PAW.Therefore,it is of great significance to explore the effects of different treatments on the physicochemical properties of PAW.
Diseases caused by food-borne pathogens are a serious threat to human life and health.According to the World Health Organization (WHO),more than two million people worldwide die from diseases caused by food infections every year [10].Pseudomonas.aeruginosa (G-) is a zoonotic pathogen with strong drug resistance and is difficult to eliminate after infection.Listeria.monocytogenes(G+)is also a highly lethal zoonotic pathogen that can cause sepsis and meningitis,with strong survivability,and can survive in soil,feces,and hay.L.monocytogenes as a‘refrigerator killer’can grow at refrigerator conditions and is one of the main pathogenic bacteria in refrigerated food [11].Some of the traditional sterilization methods have risks to the environment and human health due to reagent residues[12].PAW is clean,safe,and environmentally friendly,and has been paid more and more attention in the food industry in recent years,and is considered to be a potential technology to replace the traditional chemical and physical disinfection methods [13].
Lactic acid (LA) is a natural organic acid that exists widely in nature and living organisms and is mainly used as a preservative and/or acidulant in the food industry[14,15].It is reported that natural rice gel (NGL) containing 5% LA is effective in decreasing the amounts of total bacteria and Escherichia coli on cows’ teats,and reducing the contamination of milk due to mastitis caused by bacteria on the cows’teats [16].The inactivation effect of LA on Shiga toxinproducing Escherichia coli (STEC) was found to be higher than that of both cellulase and acetic acid [17].However,there are few reports about the synergistic effect of LA and non-thermal treatment.
To improve the inactivation efficacy,the synergistic effect of LA and cold plasma was investigated in this study.Firstly,the changes in the physicochemical properties of PAW under different treatment conditions were studied by varying the PAW treatment time,treatment power,solution volume,storage vessels,storage temperature,and time.Then,the inactivation effect of PAW and LA on P.aeruginosa and L.monocytogenes was investigated.The main results of this study are helpful in providing theoretical foundations and technical support for inactivation technology.
2.Materials and methods
2.1.PAW preparation
PAW was prepared using atmospheric pressure plasma jet experimental equipment (PG-1000ZD,Nanjing Suman Plasma Engineering Research Institute Co.,Ltd,China),as shown in figure 1,to treat distilled water under the water surface.The flow of air was set to 0.9 l min-1.The main parameter tests were carried out as follows:
A.Treatment time condition: 200 ml distilled water was treated at 750 W for 30,60,90,120,150,and 180 s to prepare PAW.
B.Volume condition: 100,150,200,250,300,and 350 ml distilled water was treated at 750 W for 90 s to prepare PAW.

Figure1.Schematic diagram of PAW preparation by atmospheric pressure plasma jet.
C.Power condition: 200 ml distilled water was treated at 550,600,650,700,750,and 800 W for 90 s to prepare PAW.
D.Storage condition: optimal conditions based on the experiments above were used to prepare PAW.Store PAW in sealed conical flasks at 4°C and 25°C,and store PAW in unsealed conical flasks under the same conditions.During storage,changes in the physical parameters were measured every hour over 24 h.
Distilled water without treatment was taken as a control at the same time.All treatments were repeated three times.
2.2.Measurement of the physical parameters
The redox potential,conductivity,and pH of PAW was measured using a composite electrode (501-ORP,Lei-ci,Shanghai,China),conductivity meter (DDS-307,Lei-ci,Shanghai,China),and pH meter (E-201F,Lei-ci,Shanghai,China),respectively.The temperature of the PAW was measured with a thermometer (179-UI,Jiangsu,China).
2.3.Measurement of chemical properties
The concentrations of H2O2,NOx,and O3were measured using an Amplex®UltraRed Reagent kit and Amplex®Red/UltraRed Stop Reagent (A3385/A36006,Thermo Fisher,Shanghai,China);a Griess Reagent Kit for NOx Determination (G-7921,Thermo Fisher,Shanghai,China);and an EasyBox®Ozone Rapid Detection Kit (Guangdong Huankai Biotechnology Co.,Ltd,China),respectively.
2.4.Microbial strains culture
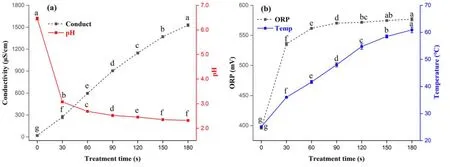
Figure2.Changes in conductivity,pH,ORP,and temperature in PAW for different treatment times.(a)Conductivity and pH,(b)ORP,and temperature.Within the same group,different lowercase letters indicate significant differences (P <0.05).
P.aeruginosa (CICC 21643) and L.monocytogenes (CICC 21635) were purchased from the China Industrial Microorganism Culture Collection and Management Center.They were cultivated in 100 ml nutrient broth medium (NB,Qingdao Haibo Biotechnology Co.,Ltd,China) at 37°C for 15 h.Bacterial cells were harvested by repeating centrifugation (6000 r min-1for 15 min) at 4°C and washing twice with sterile phosphate buffered saline (PBS,pH 7.4).All relevant operations were performed in a sterile environment and the results were measured in log colony-forming unit(CFU)/ml.
2.5.Microbiological analysis of PAW treatment
PAW was mixed with P.aeruginosa and L.monocytogenes solution in a ratio of 10:1(v/v),and samples were taken at 5,10,15,and 20 min.The samples were diluted serially with sterile phosphate buffer,then 0.1 ml appropriate dilutions were inoculated on the plate-counting medium (PCA,Qingdao Haibo Biotechnology Co.,Ltd,China) and were incubated in a biochemical incubator (SPX-80B5H-II,Shanghai Xinmiao Medical Equipment Manufacturing Co.,Ltd,China) at 37°C for 24 h.
2.6.Microbiological analysis of LA treatment
The disinfection effect of LA on both P.aeruginosa and L.monocytogenes was analyzed by different concentration treatments.P.aeruginosa was treated with LA in a gradient of 0.03125%,0.0625%,0.125%,and 0.25% for 1 and 2 min;L.monocytogenes was treated in a gradient of 0.25%,0.5%,0.75%,and 1% for 5,10,15,and 20 min.Bacteria samples were taken and analyzed in the same way as the PAW treatment sample described above.
2.7.Plasma-activated lactic acid microbiological analysis
Optimal conditions for cold plasma treatment and LA concentration,obtained from the above tests,were chosen for creating plasma-activated lactic acid (PALA) and the physical and chemical properties of PALA after activation were also determined.P.aeruginosa was treated with PALA for 1 and 2 min before incubation analysis.L.monocytogenes was treated with PALA for 5,10,15,and 20 min before incubation analysis.
2.8.Statistical analysis
SPSS 24.0 software(SPSS Inc.,Chicago,IL,USA)was used for data analysis,Origin 9.8.0 software and BioRender were used for graphing.All experiments were carried out in triplicate and results are reported as mean ± standard deviation.Duncan’s test was used to determine the significance (P <0.05) for multiple comparisons.
3.Results and discussion
3.1.Physical parameters of PAW under different treatment conditions
As shown in figure 2(a),the conductivity of PAW showed a significant (P <0.05) upward trend with the prolongation of treatment time.The initial conductivity of the solution was 18.97 μS cm-1,which increased quickly to 1529.33 μS cm-1after 180 s treatment.The pH of PAW showed an opposite trend to the conductivity as it decreased sharply to 3.08 after 30 s treatment,and much more slowly as treatment time increased from 30 to 180 s.These results indicated that the PAW was acidic,because more acidic species,such asand H+,were produced with a prolonged preparation time,resulting in a new chemical reaction to acidify the solution [18].However,some studies have shown that the pH of PAW reaches a constant state after a certain long treatment time,and results are easily affected by the equipment used [19,20].
Figure 2(b)shows that the ORP and temperature of PAW were increased significantly(P <0.05)with the prolongation of treatment time.The ORP increased sharply to 535.66 mV after 30 s of treatment,and grew slowly after 60 s.The initial temperature was increased to 60.90°C from 25.13°C after 180 s of treatment.These results are consistent with reports that the variation of PAW temperature depends on the processing time and voltage [21].
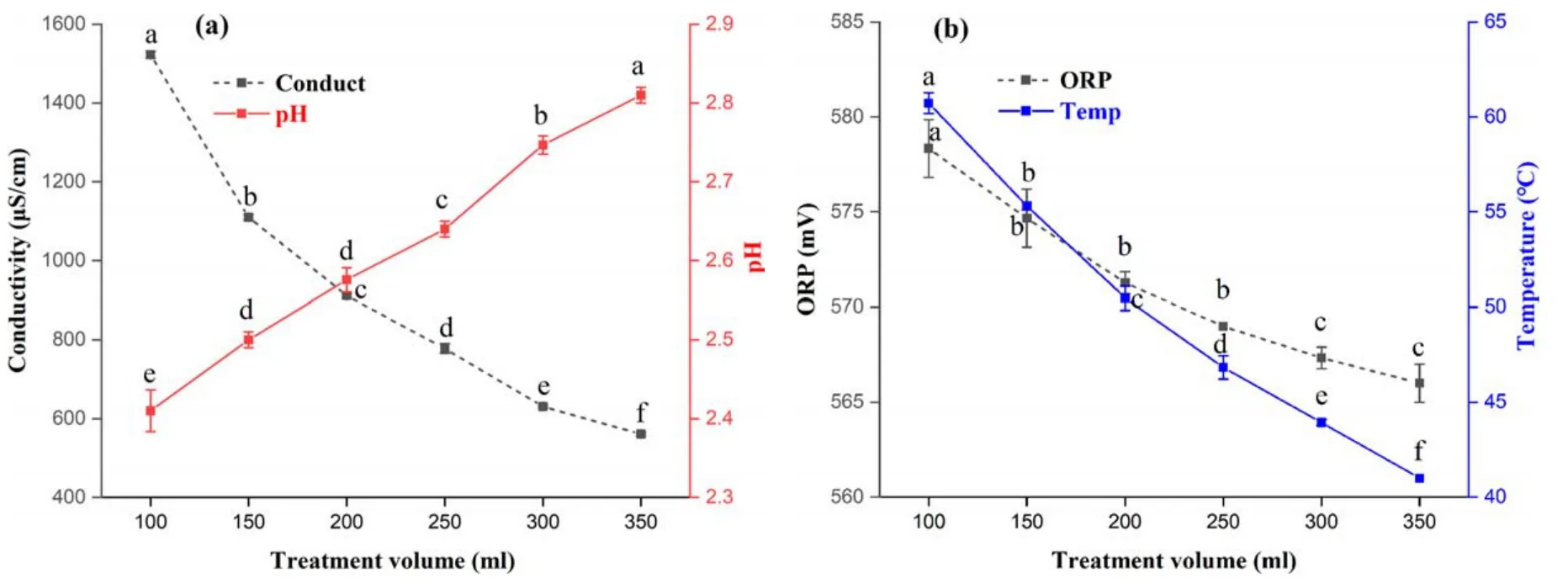
Figure3.Changes in conductivity,pH,ORP,and temperature in PAW with different treatment volumes.(a)Conductivity and pH,(b)ORP and temperature.Within the same group,different lowercase letters indicate significant differences (P <0.05).
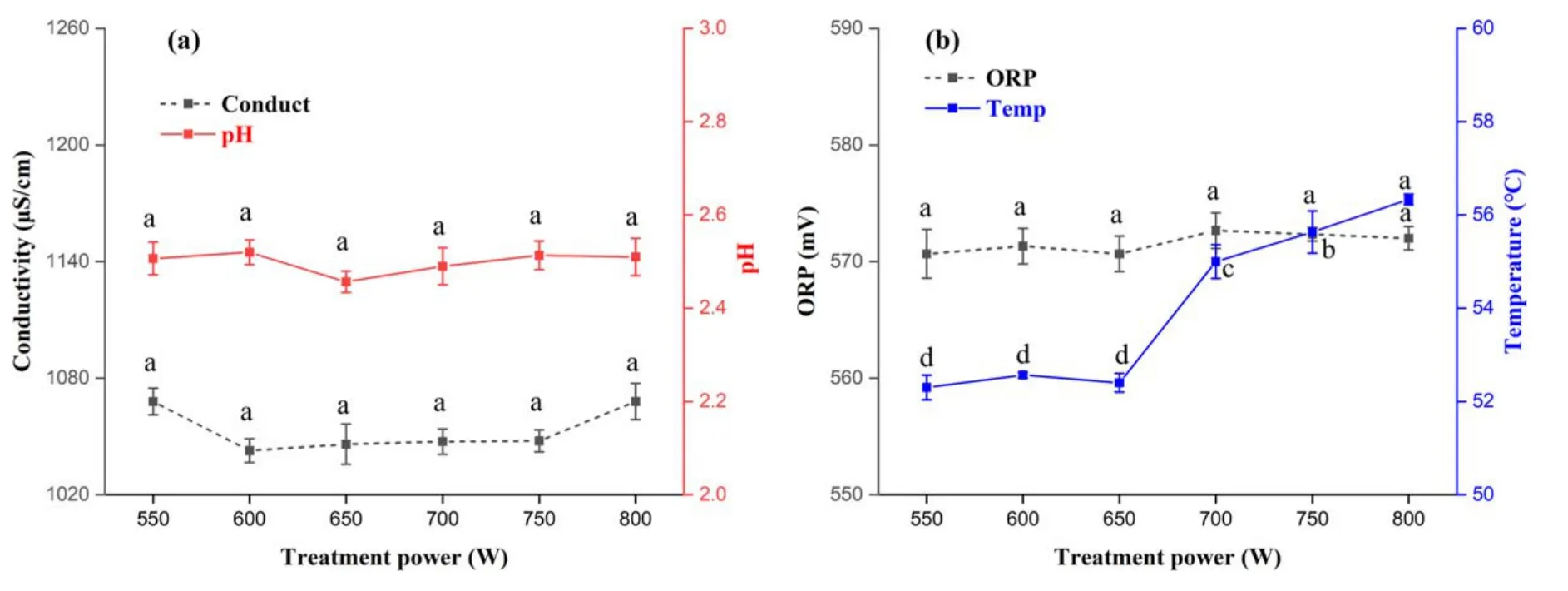
Figure4.Changes in conductivity,pH,ORP,and temperature in PAW at different treatment powers.(a)Conductivity and pH,(b)ORP and temperature.Within the same group,different lowercase letters indicate significant differences (P <0.05).
The conductivity of PAW showed a significant(P <0.05) decreasing trend with the increase in treatment volume(figure 3(a)).The conductivity decreased rapidly from 1522.67 μS cm-1to 560.66 μS cm-1when the volume was increased from 100 ml to 350 ml,while the pH of PAW increased with the volume of the solution.This may be due to an increase in solution volume with constant power and time not producing a higher concentration of active ingredient dissolved in water.Under constant power,the conductivity of PAW decreases with increasing pH as the water volume increases [22].
Figure 3(b)shows that the ORP and temperature of PAW were reduced significantly (P <0.05) with the increase in treatment volume.ORP in PAW is considered to be closely related to the reactive oxygen species H2O2,which can act as an oxidizing agent (E0=1.77 V) or as a reducing agent(E0=-0.7 V),which determines the ability of the solution to oxidize or reduce a substance.It was reported that the higher the solution ORP,the stronger the inactivation ability for microorganisms [23,24].
Figure 4(a)shows that power had no significant effect on either conductivity or pH of PAW.Similarly,it has been reported that PAW prepared under the conditions of 5°C/9.5 W and 50°C/6 W showed no obvious change in the concentration of substances in the solution [25].
As shown in figure 4(b),there was no significant effect of power adjustment on solution ORP.When the power was more than 650 W,the temperature of the solution increased significantly (P <0.05) as the power increased.
All of the results indicated that changing the treatment time and solution volume could significantly(P <0.05)affect the physical parameters of PAW.The conductivity and ORP showed opposite trends to the pH of PAW under the same treatment conditions,because H+has a higher mobility than OH-ions [20,25].
3.2.Effects of storage conditions on the physical parameters of PAW
As shown in figure 5,the conductivity and ORP of PAW stored in a sealed container at refrigerator temperature showed an increasing trend with storage time,while the pH showed a decreasing trend.The conductivity and ORP of PAW stored under unsealed and ambient conditions decreased slightly,and the pH showed a small increasing trend.The conductivity and ORP of PAW increased to 1760.67 μS cm-1and 603.33 mV,respectively,and pH decreased to 2.39 after 24 h storage at 4°C in sealed conical flasks.However,in an unsealed conical flask,the conductivity and ORP of PAW decreased slightly to 1160.67 μS cm-1and 589 mV,respectively,and pH increased to 2.59.The physical characteristics of PAW were better when it was stored in a sealed container at refrigerator temperature.The movement and escape of active gas molecules are limited during sealed storage,and more ionized small molecules of active material can dissolve into the solution increasing the concentration of RONS in the solution.According to Henry’s law[26,27],low temperature decreases the molecular motion in the water and favors the increase in the concentration of RONS in PAW.Therefore,the concentrations of long-lived species such as H2O2and HNO2/in PAW were better maintained in sealed containers [28].In our study,the conductivity,ORP,and pH changes of PAW stored at 4°C were better than those stored at 25°C.The study also proved that low-temperature storage can maintain the concentration of active substances in PAW very well and that the concentration of active substances in PAW decreases significantly when the storage temperature is higher than 25°C [29,30].
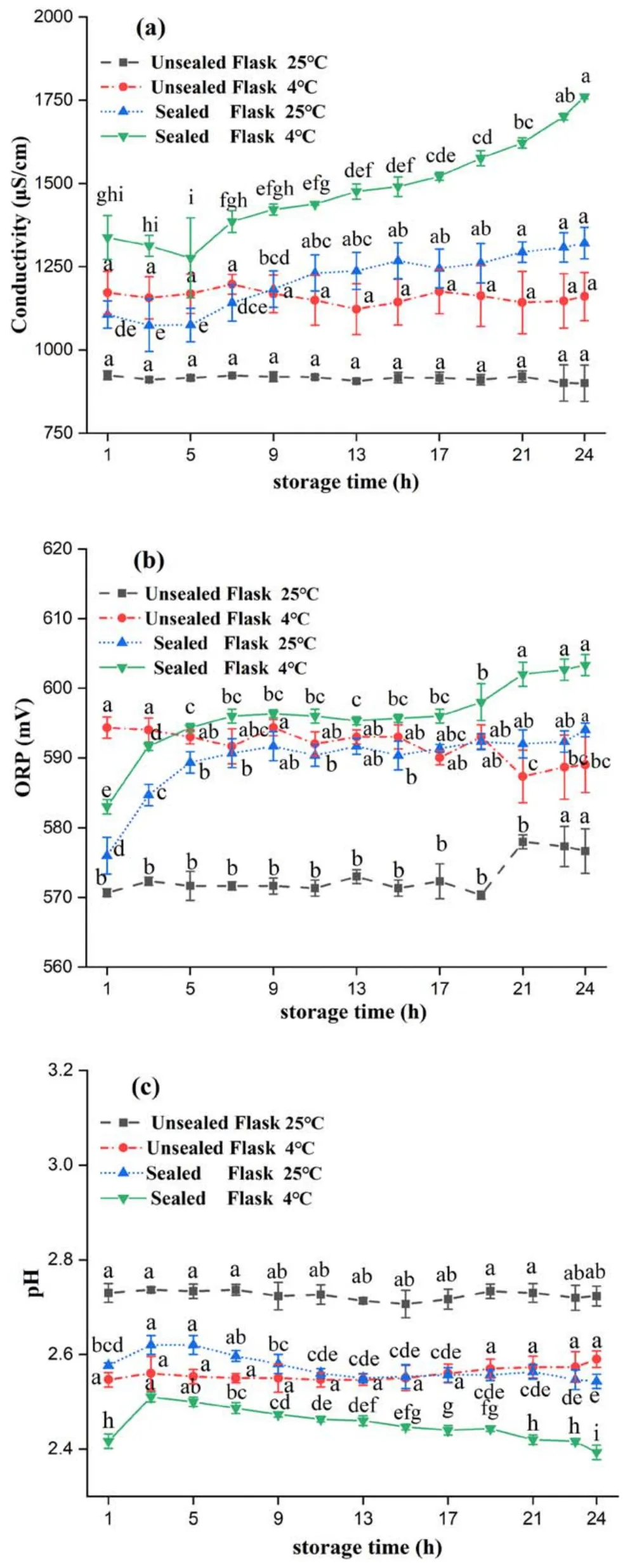
Figure5.Changes in physical parameters of PAW stored at different temperatures:(a)conductivity,(b)ORP,and(c)pH.Within the same group,different lowercase letters indicate significant differences (P <0.05).
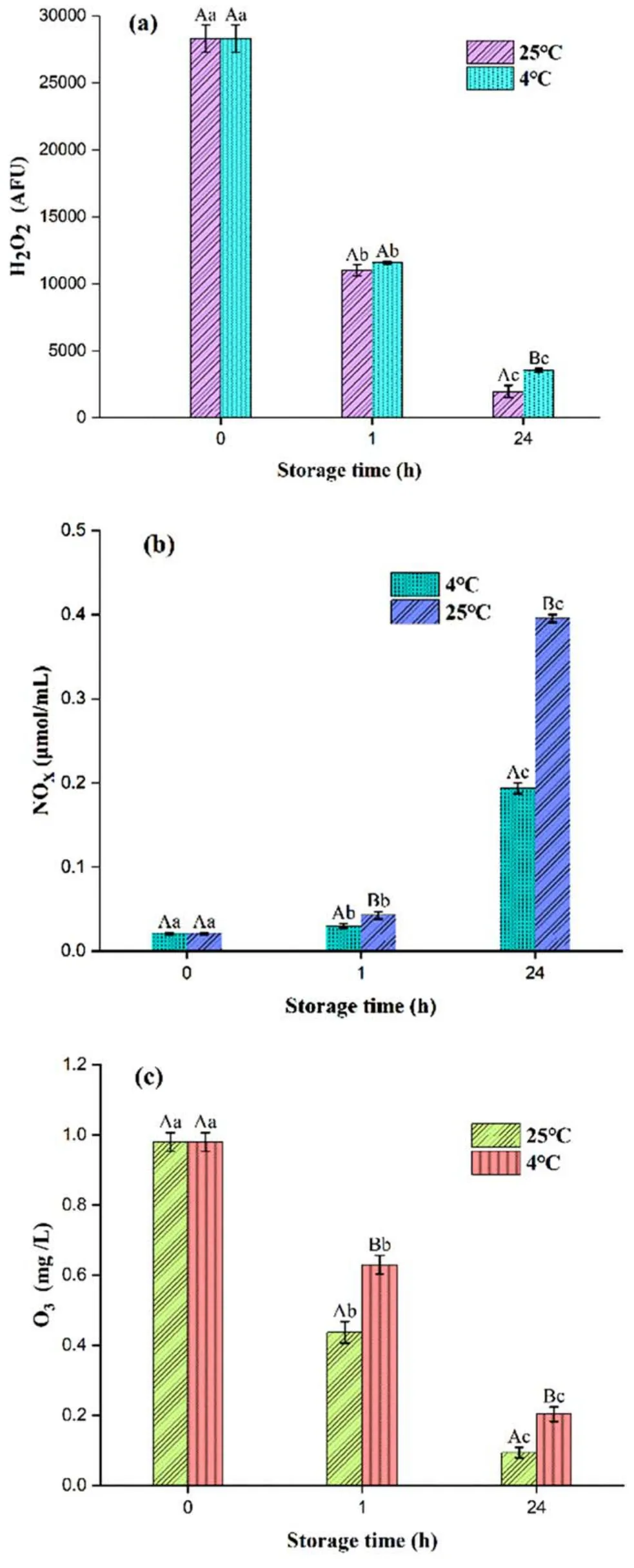
Figure6.Concentration changes of H2O2,NOx,and O3 in PAW with storage time.Within the same group,different lowercase letters indicate significant differences (P <0.05).Among different groups,different capital letters indicate significant differences (P <0.05).
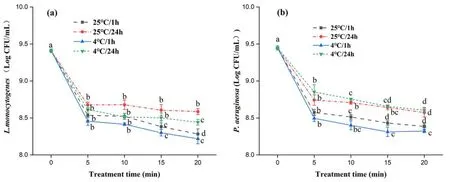
Figure7.Inactivation effect on bacteria of PAW stored at different temperatures: (a) L.monocytogenes and (b) P.aeruginosa.Within the same group,different lowercase letters indicate significant differences (P <0.05).
3.3.RONS concentration in PAW
RONS are considered to play a major role in the application of PAW in agriculture,medical,and food,and in biological sciences [31].As shown in figure 4(a),the concentration of H2O2in PAW was decreased significantly (P <0.05) as storage time was extended.This indicated that storage temperature was not as important as storage time in maintaining the concentration of H2O2in PAW.H2O2is mainly generated by the ·OH generated in the discharge process through the series of chemical reactions,and will be gradually decomposed into H2O and O during the storage process [32].Additionally,reducing the PAW storage temperature was beneficial to the retention of active substances [29].
The concentration of nitrogen oxides (NOx) in PAW showed a significant(P <0.05)increasing trend with storage time (figure 6(b)).The initial concentration of NOxwas increased to 0.19 μmol ml-1and 0.39 μmol ml-1after 24 h storage at 4°C and 25°C,respectively.There are many types of NOx,including NO,NO2,HNO2,NO3,and other active bactericidal species [32].Under acidic conditions,andcan be transformed into each other and PAW is an acidic solution,which is conducive to the mutual conversion ofandand improves the bactericidal performance of the solution.In addition,it has also been reported thatincreases with the extension of the storage time for PAW,which is the result oftransformation [33].
As shown in figure 4(c),the O3concentration in PAW decreased more quickly at 25°C than that at 4°C.The concentrations of bothand O3in PAW showed a significant downward trend as the storage time increased.It has been proved that low-temperature storage could slow down their decrease over two week’s storage [22].
3.4.Inactivation effect of PAW and LA on L.monocytogenes and P.aeruginosa
Colonies of both L.monocytogenes and P.aeruginosa showed a significant decrease (P <0.05) as treatment time increased (figure 7).PAW stored at low temperature for a short time showed a stronger bactericidal property.This may be due to the fact that the short-lived active substance in PAW is not completely dissipated in a short period of time,while prolonged treatment causes persistent damage to the bacterial cell structure and reduces the presence of sublethal bacteria.It was reported that low-temperature storage is conducive to the disinfection ability of PAW,but storage for a long time will weaken it,because more active substances are dissipated during storage [31,34].
As shown in figure 8,the inactivation effect of LA on both L.monocytogenes and P.aeruginosa was increased as the concentration increased.The colonies of L.monocytogenes were decreased by 2.98 log CFU ml-1after 20 min treatment with 1% LA.The decrease of P.aeruginosa colonies was 7.32 log CFU ml-1after 0.25% LA treatment for 2 min,and the colonies were below the detection limit value 2 log CFU ml-1when the treatment time was extended to 3 min[34].The results showed that L.monocytogenes was more resistant to LA than P.aeruginosa,this may be because Gram-positive bacteria have a complex peptidoglycan structure and thick cell walls,while Gram-negative bacteria have a loose peptidoglycan structure and are more resistant to G+than G-in the face of harsh environments [35,36].
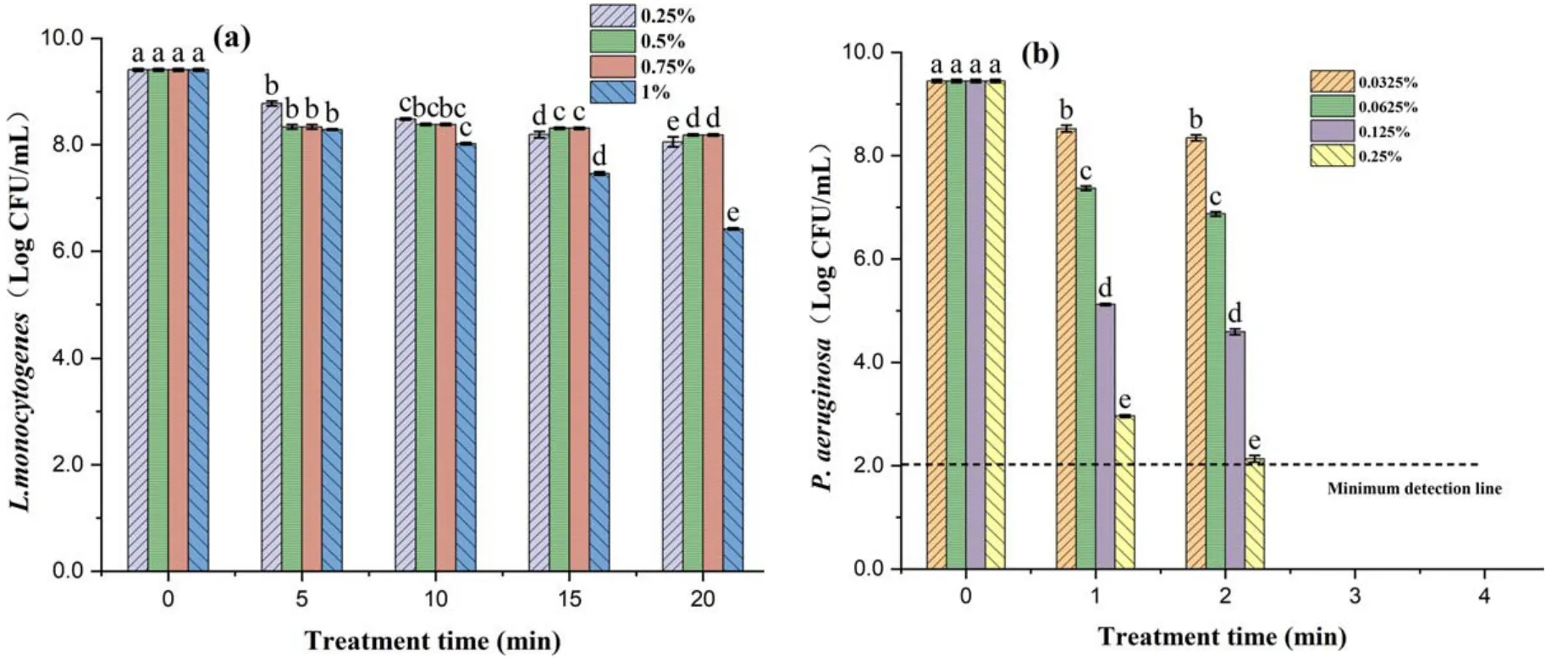
Figure8.Inactivation effect of LA on bacteria at different concentrations: (a) L.monocytogenes and (b) P.aeruginosa.Within the same group,different lowercase letters indicate significant differences (P <0.05).

Figure9.Inactivation effect on bacteria of different solutions:(a)L.monocytogenes and(b)P.aeruginosa.Within the same group,different lowercase letters indicate significant differences (P <0.05).Between groups,different uppercase letters indicate significantdifferences (P <0.05).
3.5.Inactivation effect of PALA on L.monocytogenes and P.aeruginosa
As shown in figure 9(a),the amount of L.monocytogenes was reduced by 4.03 log CFU ml-1after being treated with PALA for 20 min,which was a greater reduction than when treated by LA or PAW alone.
For P.aeruginosa,PALA also showed a higher inactivation effect than both LA and PAW (figure 9(b)).The amount was decreased by 2.84 log CFU ml-1after 2 min treatment with PALA,and the amount was less than the minimum detection level after 5 min treatment [33].
PALA included the main components contained in PAW and LA,though the acidity was more stable with a low pH which was beneficial for inactivating microorganisms[37].Salmonella enteritidis inoculated on fresh beef slices was decreased by 1.24-3.52 log CFU g-1after 20 s treatment with PALA [38].This is mainly due to the reaction ofwith H2O2and the decomposition of NaNO2under acidic conditions,and the decomposition of NO2to produce acidified nitrite NO· and NO2· which may be inactivated by PALA on microorganisms [39].
PALA exhibited better bactericidal properties than PAW or LA due to the lower and more stable pH of the activated lactic acid solution and a significant increase in the active substance content (P < 0.05),improving the bactericidal properties of the solution (table 1).As shown in figure 10,ROS including ·OH,O3,and H2O2in PALA can attack cellular peptidoglycan bonds,disrupt cell walls and cell membranes,and then enter the cytoplasm to destroy intracellular proteins,DNA,and other intracellular substances through oxidation,leading to bacterial cell death[40,41].
4.Conclusions
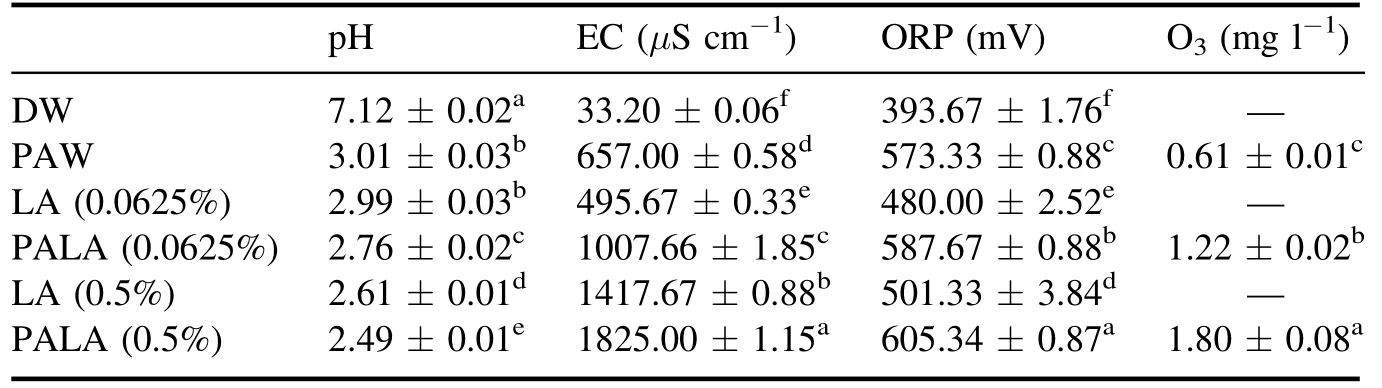
Table 1.Physicochemical properties of different solutions.
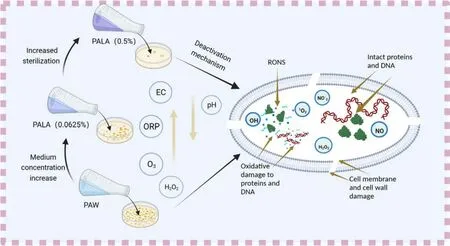
Figure10.Schematic diagram of the action of active substances in PALA on bacterial cells.
The ORP,conductivity and temperature in PAW were positively correlated with treatment time and treatment power,and negatively correlated with treatment solution volume,while pH showed the opposite trends.Storage for a short time under sealed,low-temperature conditions can effectively delay the dissipation of active substances in PAW.Lactic acid can significantly increase the content of active substances in the solution,and the inactivation effect of PALA on L.monocytogenes and P.aeruginosa was better than that of PAW or LA alone.P.aeruginosa was more sensitive to PALA,and its colony count was below the minimum detection value after 2 min of treatment.This study suggests the potential use of PALA in disinfection applications.
Acknowledgments
The authors thank National Natural Science Foundation of China (No.32260643) for financial support of this study.
 Plasma Science and Technology2023年12期
Plasma Science and Technology2023年12期
- Plasma Science and Technology的其它文章
- Automatic recognition of defects in plasmafacing material using image processingtechnology
- Physics design of 14 MeV neutron generator facility at the Institute for Plasma Research
- Numerical simulation of ultrashort-pulse reflectometry (USPR) on EAST
- Matrix effect suppressing in the element analysis of soils by laser-induced breakdown spectroscopy with acoustic correction
- Minimum inhibitory but maximum nonhemolytic concentration of plasma-treated catheters coated with L.tridentata and O.vulgare extracts
- Plasma synthesis of various polymorphs of tungsten trioxide nanoparticles using gliding electric discharge in humid air:characterization and photocatalytic properties
