Treatment of patients with multiple brain metastases by isolated radiosurgery: Toxicity and survival
André Vinícius de Camargo,Marcos Duarte de Mattos,Murilo Kenji Kawasaki,Danilo Nascimento Salviano Gomes,Allisson Bruno Barcelos Borges,Vinicius de Lima Vazquez,Raphael L C Araujo
Abstract BACKGROUND Radiosurgery for multiple brain metastases has been more reported recently without using whole-brain radiotherapy.Nevertheless,the sparsity of the data still claims more information about toxicity and survival and their association with both dosimetric and geometric aspects of this treatment.AIM To assess the toxicity and survival outcome of radiosurgery in patients with multiple (four or more lesions) brain metastases.METHODS In a single institution,data were collected retrospectively from patients who underwent radiosurgery to treat brain metastases from diverse primary sites.Patients with 4-21 brain metastases were treated with a single fraction with a dose of 18 Gy or 20 Gy.The clinical variables collected were relevant to toxicity,survival,treatment response,planning,and dosimetric variables.The Spearman’s rank correlation coefficients,Mann-Whitney test,Kruskal-Wallis test,and Logrank test were used according to the type of variable and outcomes.RESULTS From August 2017 to February 2020,55 patients were evaluated.Headache was the most common complaint (38.2%).The median overall survival (OS) for patients with karnofsky performance status (KPS) >70 was 8.9 mo,and this was 3.6 mo for those with KPS ≤ 70 (P=0.047).Patients with treated lesions had a median progression-free survival of 7.6 mo.There were no differences in OS (19.7 vs 9.5 mo) or progression-free survival (10.6 vs 6.3 mo) based on prior irradiation.There was no correlation found between reported toxicities and planning,dosimetric,and geometric variables,implying that no additional significant toxicity risks appear to be added to the treatment of multiple (four or more) lesions.CONCLUSION No associations were found between the evaluated toxicities and the planning dosimetric parameters,and no differences in survival rates were detected based on previous treatment status.
Key Words: Radiosurgery;Brain metastases;Radiotherapy;Survival;Toxicity;Cancer
INTRODUCTION
It is estimated that 19.3 million new cancer cases and 10 million deaths occurred in 2020.Breast (11.7%) and lung (11.4%) cancers are among the most common cancer cases,causing 2.5 million deaths (24.9% of all cancer deaths)[1].Besides being the most prevalent in the population,they are the most prevalent cancer types that evolve into brain metastasis due to their favorable microenvironment for brain metastases development[2,3].
The main radiotherapy technique used in brain metastasis is stereotactic radiosurgery (SRS) performed in a linear accelerator (LA).Thus,it is necessary to determine whether the treatment of multiple brain metastases by isolated radiotherapy is safe and non-inferior to the treatment of one or few lesions,regarding toxicity and survival[4-7] and if previous treatment,such as whole brain radiotherapy (WBRT),is beneficial before radiosurgery[8,9].Moreover,to determine which therapy is appropriate for each patient’s prognosis,it is also important to estimate the survival rate of patients with brain metastasis through prognostic factors such as Karnofsky performance status (KPS),diagnosis-specific graded prognostic assessment (DS-GPA),score index for radiosurgery (SIR),and recursive partitioning analyses (RPA),to determine which therapy is adequate for the prognosis of each patient[10-14].
This work aimed to evaluate the toxicity of isolated radiosurgery in patients with multiple brain metastases (≥ 4 lesions).In addition,overall survival and progression-free survival were evaluated,and survival was correlated with the prognostic index.
MATERIALS AND METHODS
Retrospective data were collected from 55 patients who underwent radiosurgery at Barretos Cancer Hospital from August 2017 to February 2020.Patients who presented with 4-21 brain metastases delineated with the aid of magnetic resonance (MR) were treated in a single fraction with a dose of 18 Gy or 20 Gy.Patients who met the inclusion criteria were included regardless of previous systemic and primary local treatment since all of them received radiation therapy for four or more brain lesions in palliative manners,and the main outcomes were either local or systemic toxicity.A frameless immobilization system was used for simulation and treatment.Simulation computed tomography with a slice thickness of 1.25 mm was used for all plannings.
All lesions were treated on a Varian TrueBeam®™ STX Varian Medical Systems LA with high-definition mulitleaf (120-leaf) collimator and planned with an Eclipse®treatment planning system (Varian Medical System Inc,version 13.6).The calculation algorithm used was the anisotropic analytical algorithm with a 1.25 mm calculation grid and heterogeneity correction.VMAT (RapidArc®,Varian Medical System,Inc.) treatment technique was used for all cases with a planning target volume (PTV) margin of 1 mm from the gross target volume contour.Before treatment,a cone-beam computed tomography scan was performed.Planning was carried out by the Department of Radiation Oncology with many physicists and radiation oncologists in who followed the institutional protocol of dose constraints in the organs at risk and of coverage of targets.
The following toxicities were collected: Headache,convulsion,focal deficit,drop in the level of consciousness,fatigue,nausea or vomiting,and mental confusion.They were based on the Common Terminology Criteria for Adverse Events.The patient’s first complaint after radiosurgery was selected.
Prognostic factors were also collected: Initial KPS and that at the first follow-up after radiosurgery,DS-GPA,SIR,and RPA.In addition,age,gender,and the International Classification of Disease of the primary tumor were also surveyed.
Dosimetric variables included were V5Gy,V8Gy,V10Gy,V12Gy,V14Gy,conformity index (CI),heterogeneity index (HI),dmax,and 50% isodose CI (CI_R50).The VxGy represents the volume of the “x” Gy dose that the normal brain minus PTV received.The CI was calculated by the ratio between the volume of the prescription isodose and the volume of the PTVs: Vpresc_isodose/VPTVs.The HI was calculated as (D2%-D98%)/D50%[15].Dmax is the maximum point dose of the plan and the CI_R50 is the ratio between the volume of the 50% isodose line and the volume of the PTVs.
The geometric variables collected were the number of lesions,total target volumes,the smallest and largest target volumes,and the distance between the isocenter and the most distant lesion.The distance between the isocenter and the most distant lesion was determined using the coordinates of the lesion center and its respective isocenter.The calculation was according to equation 1.In cases where there was more than one isocenter,the distance was measured between the isocenter and the most distant lesion that its arcs was treated,as demonstrated in the Supplementary material.
Technical variables included were the total number of arcs,coplanar or non-coplanar arcs,number of non-coplanar arcs if used,and number of isocenters.The correlations between the dosimetric,geometric,and technical variables collected for this work were previously published by our group[16].
We reported the response in treated lesions as complete response,partial response,stable disease,progressive disease,or radionecrosis.Complete response indicated complete remission of all lesions;partial response indicated that some lesions entered complete remission,while others remained stable;stable disease indicated that all lesions remained the same size;progressive disease indicated that at least one of the lesions enlarged in size;and radionecrosis indicated that at least one lesion went through necrosis due to radiation.Information on the location of new lesions was also reported as either parenchymal or meningeal.
The initial date of treatment was used for estimating the overall and progression-free survival rates and for the outcomes,respectively,the date of death or the date of the last information obtained in the medical records after the treatment,and the date of the MR in which the progression of the treated lesions was detected or the date of the MR in which the appearance of new lesions was detected were used.Survival rates were calculated based on the data of 53 patients with assessable clinical records.
In this study,radiosurgery treatment of multiple brain metastasis (≥ 4) delivered in a isolated manner and at a single dose was referred to as radiosurgery.Whenever the patient underwent radiosurgery in more than one course of treatment,we would consider the first radiosurgery with four or more lesions.To evaluate if the previous treatment influenced survival rates,patients were divided into two groups: No previous irradiation (NP) and irradiation before radiosurgery (P).
Comparisons of toxicities between categories or between different groups of patients were made using chi-square tests or Fisher exact tests,and Mann-Whitney tests for continuous variables,and the relation between prognostic factors and age was evaluated using Mann-Whitney or Kruskal-Wallis tests.The results are presented as proportions or median and interquartile when appropriate.Spearman’s correlation coefficient was used to determine if there was a correlation between toxicities and dosimetric and geometric variables.KPS comparison was made using a marginal homogeneity test.Survival was estimated by the Kaplan-Meier method and the curves of each category were compared using the Logrank test.Statistical relevance was considered ifP<0.05.Data were collected and managed using the research electronic data capture platform[17] and analyzed using the software SSPS®(v.20).
RESULTS
The descriptive characteristics of groups NP and P are displayed in Table 1.Briefly,the most prescribed dosage was 18 Gy (83.6%),and 67.3% of patients were female.Of the 55 patients who underwent radiosurgery,32 (58.2%) declared feeling some toxicity,with headaches (38.2%) being the most frequent.Incidence rates for each toxicity are shown in Table 2.
The number of reported cases of toxicity as a function of time after treatment is shown in Table 3.It was observed that the highest incidence (40.6%) occurred between the first and third month after treatment.To deal with the heterogeneity of patients who had nervous system irradiation more than once,they were divided into four groups: (1) Patients with ≥ 4 lesions who underwent radiosurgery only;(2) patients with previous either WBRT or SRS with less than four lesions or fractionated stereotactic radiotherapy (SRT);(3) patients that underwent irradiation after radiosurgery and reported side effects after the second irradiation;and (4) patients that were irradiated before and after radiosurgery and reported some toxicities after the last irradiation.
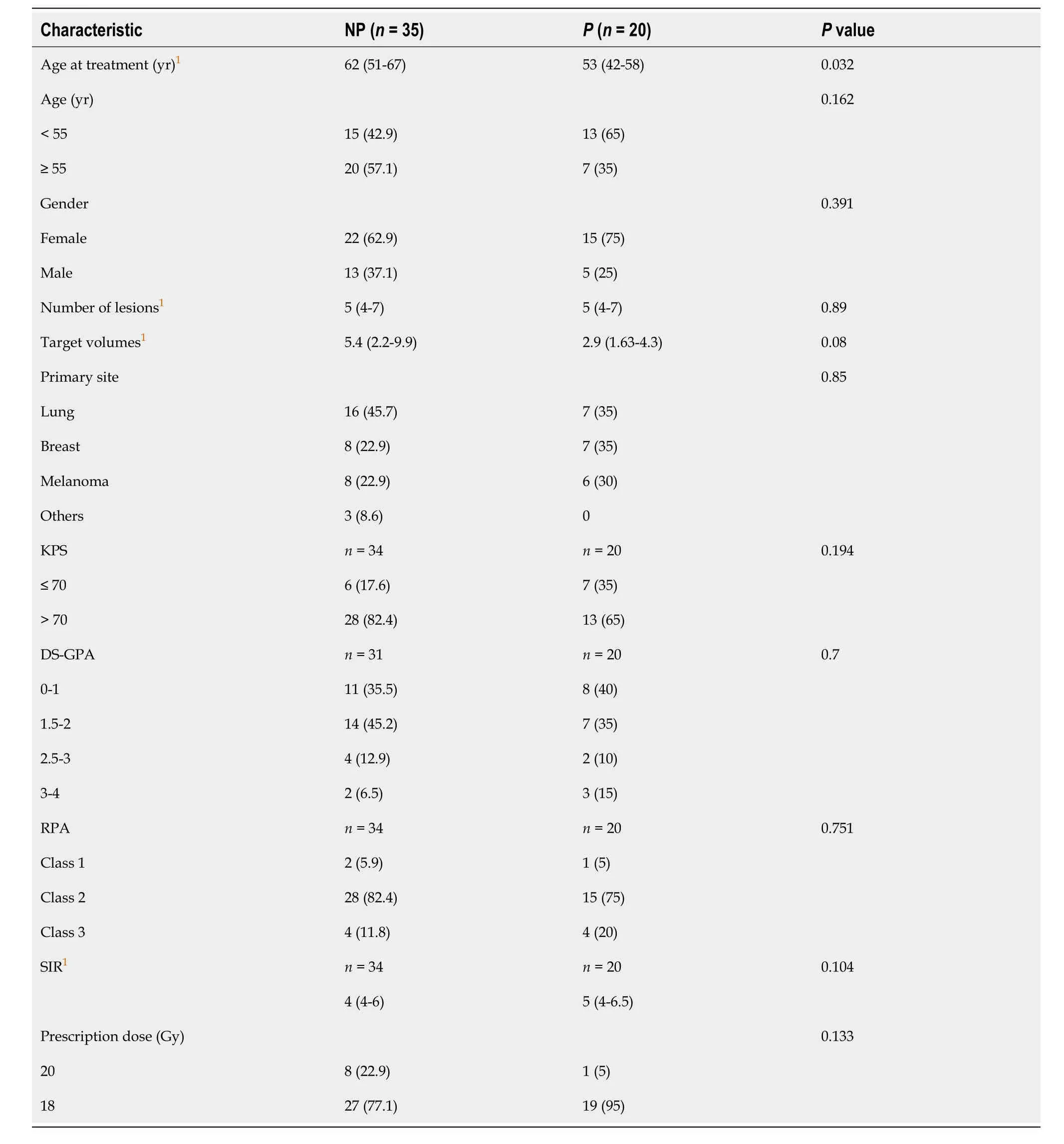
Table 1 Descriptive characteristics of 55 patients treated by radiosurgery for multiple brain metastases (%)
The proportion of patients per technique that had one irradiation before SRS of multiple lesions was ten for WBRT,seven for SRS with less than four lesions,and eight for SRT.Five patients did two previous irradiations before treating multiple brain metastases (≥ 4 lesions) by SRS.The proportion of patients in each group that reported toxicity was 17 of 18 patients (94.4%) in group 1;7 of 11 (12.7%) in group 2;3 of 17 (17.6%) in group 3;and 5 of 9 (55.6%) in group 4.
The incidence of toxicities in each patient group is presented in Table 4.Despite the higher incidence in group 1,no statistical relevance was found between the four groups regarding the seven toxicities.There was also no difference in the toxicities among the different categories of DS-GPA,RPA,and SIR.
Regarding the response of treated lesions and the emergence of new lesions,there were no differences observed between the P and NP groups (P=0.643 andP=0.412,respectively).A single patient had a complete response,17 had partial responses,seven were stable,15 had progression,and six presented radionecrosis (half in each group).
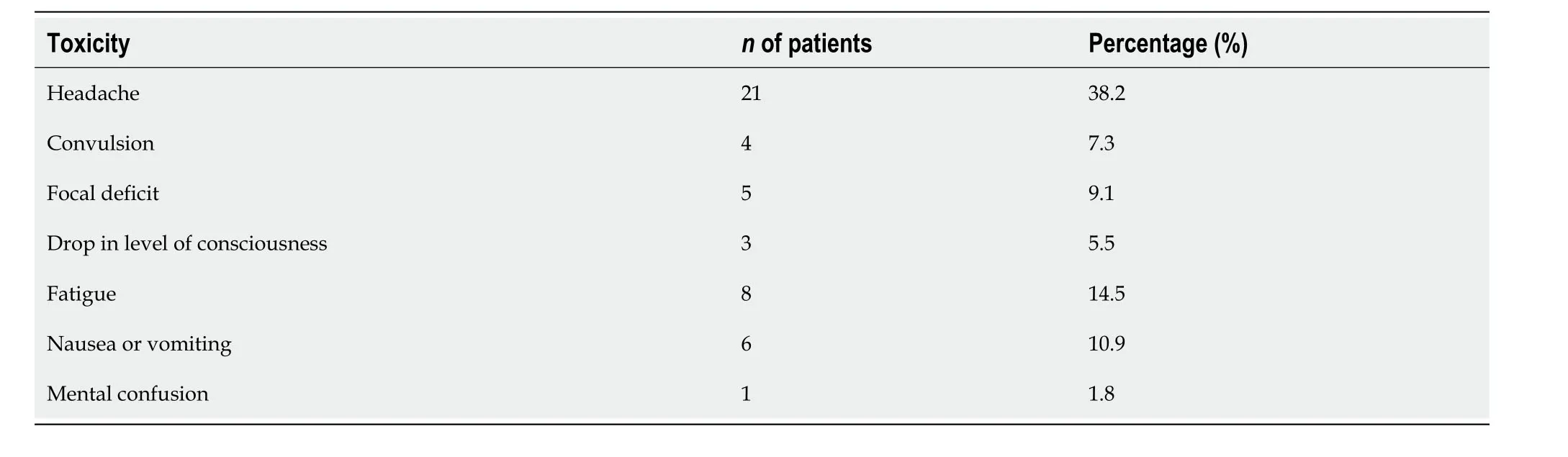
Table 2 Incidence of toxicities
Despite the higher number of patients that presented new lesions in group NP (18) compared to group P (11),there were no differences between the two groups.The number of patients with new lesions was 29,and 17 patients did not develop new lesions.According to location,new parenchymal lesions (26) were more frequent than meningeal ones (3).
Comparing the initial KPS with that evaluated in the first consult after treatment,a relevant difference was observed between them (P=0.033).The percentage of patients whose KPS decreased after treatment was 39.6%,and 60.4% of patients improved or maintained their KPS.
No statistical correlation was observed between dosimetric and geometric variables and toxicities.The descriptive statistics of dosimetric,geometric,and technical variables have previously been published by our group[16].
The average overall survival (OS) was 13.3 mo,and the median was 10 mo.The life expectancy over time can be observed in Figure 1.It is noteworthy that the survival rate at 12 mo was 42%.Of 53 patients,78% died,and of those,only 10 patients (18.9%) were due to neurological causes.
No differences were observed in the OS rates between groups NP and P.The median survival in group NP was 9.5 mo,and in group P it was 19.7 mo (P=0.110).Considering patients with KPS >70 and KPS ≤ 70,a difference was observed in OS (P=0.047) (Figure 1B).The median survival of the group with KPS >70 was 8.9 mo,and in the group with KPS ≤ 70 it was 3.6 mo.No differences were observed in the survival of DS-GPA (P=0.547),RPA (P=0.113),and SIR categories 0 to 4 and 5 to 10 (P=0.586).
The OS of patients was categorized into two groups for each variable for analysis.No difference between them was found: Number of lesions (P=0.840),n<6 (10.5 mo) andn≥ 6 (9.3 mo);volume of targets (P=0.786),v <5 cc (10.5 mo) and v ≥ 5 cc (9.5 mo);V12Gy ≤ 10 cc (11.1 mo) and V12Gy >10 cc (9.6 mo) (P=0.693);CI_R50 ≤ 8 (13.2 mo) and CI_R50 >8 (9.6 mo) (P=0.655).
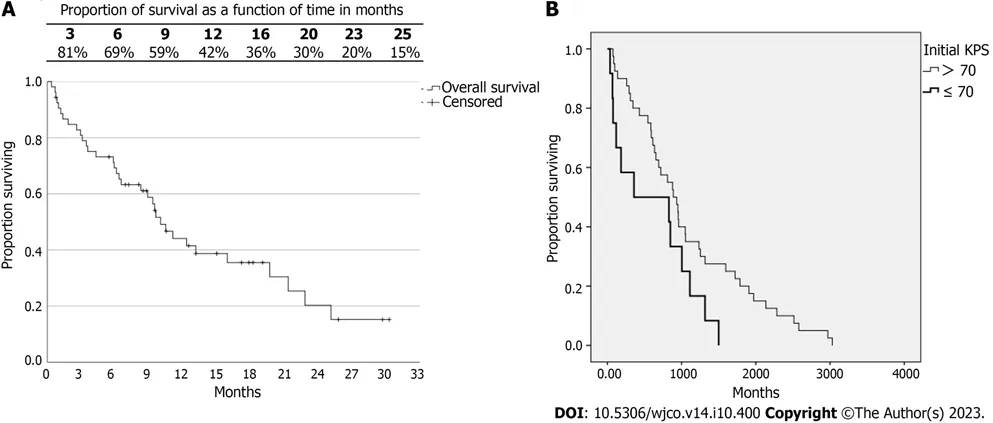
Figure 1 Kaplan-Meyer curves for overall survival of 53 patients treated by radiosurgery for multiple brain metastases. A: Overall survival (OS);B: OS according to Karnofsky performance status (KPS) >70 and KPS ≤ 70.
The median progression-free survival of patients with treated lesions (PFSL) was 7.6 mo.No differences (P=0.293) were found between groups NP and P.The median PFSL of group NP was 6.3 mo,and in group P it was 10.6 mo.The curves for the PFSL of both groups are displayed in Figure 2A.The median survival free from the appearance of new lesions was 6 mo.No difference was observed between groups NP and P (P=0.188).The median for group NP was 4.5 mo,and for group P it was 8.9 mo.The curves of survival free from the appearance of new lesions in both groups are displayed in Figure 2B.
DISCUSSION
It was observed that in group 1,a higher proportion (94.4%) of patients reported grievances and a higher number of different toxicities.Nevertheless,no difference was observed between groups when comparing their toxicity incidence.Besides,the toxicities reported varied regarding their start point.One of the patients reported a grievance a year after treatment,thus rendering it difficult to classify it as a side effect of radiosurgery.
Analyzing the responses of treated lesions,six patients developed radionecrosis.As discussed by Blonigenet al[18],V10Gy and V12Gy can be predictors of radionecrosis.The median of V10Gy and V12Gy of those six patients was 27.8 cc (9.7-45.5 cc) and 17.6 cc (6.2-27.4 cc),respectively (only a single patient had the dosage of 20Gy as prescription).
The median OS found by Changet al[8] was 9.2 mo and the median survival of patients treated only by SRS was 15.2 mo.Aoyamaet al[9] obtained a median OS of 8 mo on the arm of patients treated only by SRS.Brownet al[19] found a median OS of 13.5 mo and a median survival of 10.4 mo for patients treated only by SRS.
Sahgalet al[20] found a median OS of 10 mo for the group that only received SRS.The median time to local failure and development of new lesions was 6.6 mo and 4.7 mo,respectively.This last result matches the PFSL and the development of new lesions in this study.The four aforementioned studies compared patients who underwent SRS alone with patients treated by SRS+WBRT.
Scorsettiet al[21] observed a median OS of 16.2 mo and a 12-mo survival rate of 65.3% in the group of patients who underwent only SRS with an LA.They also indicated that 27 of the 130 patients (20.8%) included in that study presented symptomatic radionecrosis.The incidence obtained in that study was 10.9%.
Differently from the studies mentioned before,in which treated patients had up to four lesions,the current study evaluated patients with 4 to 21 lesions and,despite the underestimated OS (group P began treatment of metastasis before the studied SRS),it was observed that survival values are similar to studies with up to four lesions,especially when analyzing the global survival of all patients,and the survival of patients without previous irradiation,whose comparison is possible with the aforementioned studies.
Among the prognostic indexes,despite the predictive power of survival from DS-GPA,RPA,and SIR[11-14,22] being better than KPS for patients with brain metastasis,only KPS showed a difference in OS (P=0.047) between patients with KPS >70 and KPS ≤ 70.This likely occurred due to KPS considering only the clinical condition of patients,whereas other indexes also consider specific parameters of patients with brain metastasis that were not discretized in the analysis,such as the primary site of disease,number of lesions,and systemic diseases,among others.
Regarding the number of patients whose KPS decreased,it is important to note that metastatic patients have systemic diseases that worsen the clinical outcome.Therefore,we cannot contribute the decline of KPS to SRS,which is corroborated by the low death number due to neurological causes.
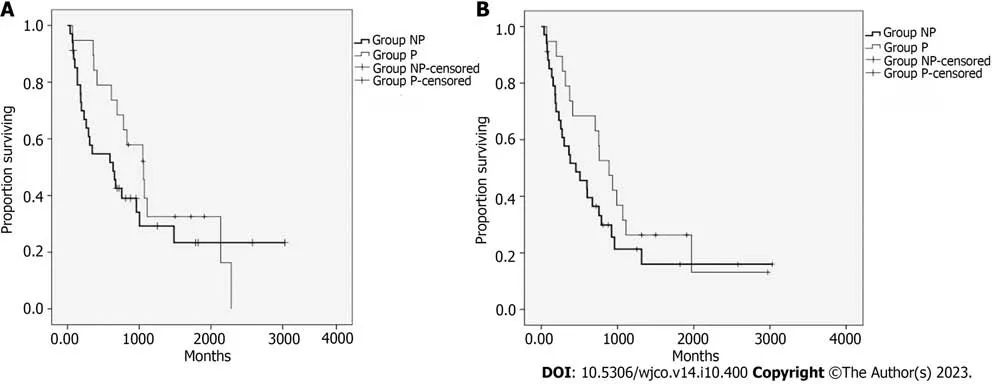
Figure 2 Kaplan-Meyer curves for progression-free survival.A: Progression-free survival (PFS) of 34 patients with treated lesions who did not undergo previous irradiation (NP group) and 19 patients who underwent prior irradiation (P group);B: PFS addressing the appearance of new lesions in both NP and P groups.
According to dosimetric,geometric,and technical variables,the lack of correlation with toxicities does not imply they do not impact each other,especially considering dosimetric variables used for planning approval.It is known that the volume of targets,number of lesions,distance between lesions,and the isocenter impact these plan evaluation indexes[16,23-25].What can be observed is that the indication of isolated radiosurgery for multiple brain metastases was safe,considering that the technique achieved dosimetric values good enough not to cause collateral effects.
This study has limitations inherent to the retrospective cohort model where selection and information biases cannot be discarded.There were patients subjected to multiple irradiation techniques before the SRS in this study,and many of the patients were also under systemic treatment,which may interfere with the clinical results.In addition,all of them received some or many kinds of local and systemic treatment for many types of tumors,since in our institution the radiosurgery for four or more lesions was reserved for local control in a palliative manner and usually failed for previous treatments.Regarding the toxicities,precise graduation was not possible to obtain and therefore,they were not differentiated.Although our study had some missing data for clinical variables,they represent less than 4%,which seems acceptable for a retrospective study[26].If factors were significantly associated with outcomes in univariate analysis,and they were not as demonstrated in Table 1,they would be entered into a multivariate analysis,but it was not possible.
The planning was performed by different personnel,with distinct dose prescriptions and,in some patients with one or more lesions (more significant volumes),planned with three fractions but,even in these cases,there were also four or more lesions treated with a single dose.Considering the prescription,we tested the difference between the doses of 18 and 20 Gy regarding geometric,dosimetric,and technical variables,and no differences were observed.
CONCLUSION
Our data demonstrate the safe use of isolated SRS to treat patients with four or more brain metastases,with no significant association between dosimetric,geometric,or clinical parameters and the related toxicities.
ARTICLE HIGHLIGHTS
Research background
Radiosurgery for multiple brain metastases has been more reported recently without using whole-brain radiotherapy,but mainly for oligometastatic scenarios (up to 3-4 lesions).Nevertheless,the sparsity of the data still claims more information about toxicity and survival and their association with both dosimetric and geometric aspects of this treatment,especially for the presence of more lesions or in patients with previous irradiation.
Research motivation
To evaluate the toxicity of treatment offered for patients with four or more lesions.
Research objectives
To assess associations of toxicity and survival outcome of stereotactic radiosurgery (SRS) among patients with four or more brain lesions with or without previous brain irradiation.
Research methods
Retrospective cohort.
Research results
Neither difference in toxicity nor survival was detected when comparing patients who underwent SRS for four or more brain lesions with or without previous brain irradiation.
Research conclusions
This retrospective study did not detect differences in toxicity for this population with or without previous irradiation,suggesting that the use of SRS for four or more brain lesions with or without previous brain irradiation is safe.
Research perspectives
This study claims for more data in larger studies in a prospective manner to better address this question.
FOOTNOTES
Author contributions:de Camargo AV,Borges ABB,Vazquez VL,and Araujo RLC contributed to conceptualization;de Camargo AV,de Mattos MD,Kawasaki MK,Gomes DNS,and Borges ABB contributed to data collection;de Camargo AV and Araujo RLC contributed to data analysis;all authors have read and approved the final manuscript.
Institutional review board statement:This study was reviewed and approved by the Ethics Committee of the Barretos Cancer Hospital.
Informed consent statement:All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement:We have no financial relationships to disclose.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Brazil
ORCID number:André Vinícius de Camargo 0000-0001-7153-6724;Marcos Duarte de Mattos 0000-0002-5069-8051;Murilo Kenji Kawasaki 0000-0001-5151-5221;Danilo Nascimento Salviano Gomes 0000-0002-7909-0728;Allisson Bruno Barcelos Borges 0000-0003-3868-4420;Vinicius de Lima Vazquez 0000-0002-0325-5514;Raphael L C Araujo 0000-0002-7834-5944.
S-Editor:Qu XL
L-Editor:Wang TQ
P-Editor:Cai YX
 World Journal of Clinical Oncology2023年10期
World Journal of Clinical Oncology2023年10期
- World Journal of Clinical Oncology的其它文章
- Comprehensive analysis of disulfidptosis related genes and prognosis of gastric cancer
- What should be the future direction of development in the field of prostate cancer with lung metastasis?
- Splenic lymphangioma masquerading as splenic abscess managed by laparoscopic splenectomy: A case report
- Hub genes and their key effects on prognosis of Burkitt lymphoma
- Classification of patients with metastatic colorectal cancer into consensus molecular subtypes into real-world: A pilot study
