Resistance risk and molecular mechanism associated with resistance to picoxystrobin in Colletotrichum truncatum and Colletotrichum gloeosporioides
SHI Niu-niu , LIAN Jin-pan, QIU De-zhu CHEN Fu-ru DU Yi-xin #
1 Institute of Plant Protection, Fujian Academy of Agricultural Sciences, Fuzhou 350013, P.R.China
2 Fujian Key Laboratory for Monitoring and Integrated Management of Crop Pests, Fuzhou 350013, P.R.China
3 Crop Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan 750002, P.R.China
Abstract Anthracnose, caused by Colletotrichum truncatum and C.gloeosporioides, is amongst the most serious diseases of soybean in China.Picoxystrobin, a quinone outside inhibitor fungicide, is commonly used for the control of anthracnose.Its resistance risk and mechanism in C.truncatum and C.gloeosporioides are unclear.In this study, the sensitivities of 128 C.truncatum and 121 C.gloeosporioides isolates to picoxystrobin were investigated, and unimodal distributions were observed with average EC50 values of 0.7740 and 1.1561 μg mL-1, respectively.Eleven picoxystrobin-resistant mutants of C.truncatum and six mutants of C.gloeosporioides were acquired, with EC50 values varying from 5.40-152.96 and 13.53-28.30 μg mL-1, respectively.Compared to the parental isolates, mutants showed similar or higher relative fitness in conidial production and germination, and pathogenicity.Collectively, the resistance risk of C.truncatum and C.gloeosporioides to picoxystrobin is moderate to high.There was positive cross-resistance between picoxystrobin and pyraclostrobin, but not between picoxystrobin and fluazinam, difenoconazole, or propiconazole.The G143S mutation in Cyt b protein was detected in seven high-resistant mutants of C.truncatum (RF>100), and G137R occurred in four moderate-resistant mutants (RF<50).Contrastingly, there were no point mutations in Cyt b of any C.gloeosporioides mutants.Molecular docking confirmed that two mutations conferred different resistance levels to picoxystrobin.Under greenhouse trials, picoxystrobin did not control mutants with the G143S mutation, those bearing G137R or no point mutation were somewhat controlled, but at a lower level compared to wild-type isolates.These results showed that integrated management strategies should be implemented to preserve fungicide effectiveness.
Keywords: Colletotrichum truncatum, Colletotrichum gloeosporioides, picoxystrobin, point mutation, Cyt b, molecular docking
1.Introduction
Soybean (Glycinemax(L.) Merr.) is of great significance worldwide, not only as a source of vegetable oil and proteins for human consumption but also as feed for livestock and aquaculture (Masudaet al.2009; Hartmanet al.2011).However, fungal diseases on soybean can lead to considerable yield reduction and financial losses(Silvaet al.2011).Among these, anthracnose caused byColletotrichumspp.is an important disease of the cultivated soybean all over the world (Hartmanet al.2015;Subediet al.2016; Diaset al.2019; Natarajet al.2020).This disease can inflict 100% yield losses with conducive climatic conditions (Padderet al.2017), and about 90 kg ha-1of grain yield is lost for each 1% increment in overall disease incidence in the range of 9 to 17% incidence (Diaset al.2016).
Colletotrichumis one of the most common and important genera of plant-pathogenic fungi, and infects a wide range of crops, especially fruit and vegetable(Shiet al.2021b).Colletotrichumspecies also have a long and distinguished history as model pathogens for fundamental, biochemical, physiological, and genetic studies (Dammet al.2012; Deanet al.2012; Da Silvaet al.2020).Soybean anthracnose is currently recognized as a disease of complex etiology.It has been reported that severalColletotrichumspecies were responsible for this disease, such asC.truncatum(Sharmaet al.2011),C.plurivorum(Barbieriet al.2017),C.gloeosporioides(Mahmodiet al.2013),C.musicola(Boufleuret al.2020), andC.sojae(Dammet al.2019).Among these,C.truncatumis the most widely distributed worldwide,followed byC.gloeosporioides(Boufleuret al.2021).Moreover, these two species are also the main pathogens causing soybean anthracnose in Fujian, China (Shiet al.2022).
The implementation of resistant cultivars is the most eco-friendly and economical measure for controlling soybean anthracnose.In China, the identification of disease resistance to soybean has been regarded as a key index for the approval of soybean varieties.From 2011 to 2019, 70 soybean varieties resistant to anthracnose from a total of 590 varieties were screened, however, there were few varieties resistant to anthracnose in spring-sowing vegetable soybeans(Shiet al.2021a).Although screening for identification of anthracnose-resistant soybean varieties has previously been undertaken (Nagarajet al.2014; Natarajet al.2020), no studies have been conducted on the inheritance of anthracnose resistance, which is the first and foremost step in resistance breeding, and even there is no breeding program intended to screen resistance to soybean anthracnose (Yang and Hartman 2015).Hence,fungicides are still a vital solution to the effective control of soybean anthracnose.
Several classes of fungicides have been registered for management of anthracnose, including methyl benzimidazole carbamates (MBCs), demethylationinhibitor fungicides (DMIs), and quinone outside inhibitors (QoIs).Unfortunately, owing to the repeated use of MBCs and DMIs, the decrease in control efficacy have been frequently reported from fields due to the emergence of resistance (Potiet al.2020; Wanget al.2020; Weiet al.2020).QoI fungicides can block electron transport through binding to the quinone oxidation site (Qo) on cytochrome b (Cytb), thereby inhibiting mitochondrial respiration, which in turn turns off the energy of fungal metabolism and growth (Bartlettet al.2002; Gisiet al.2002).In addition, QoI fungicides can also benefit plant health to prolong milk-filling of wheat crops (Owatiet al.2017).However, QoI fungicides are considered to carry a high risk of resistance development because of their site-specific mode of action (FRAC 2021).Resistance to QoI fungicides has been detected in many fungal pathogens (Chechiet al.2019; Fouchéet al.2022).The resistant basis of QoI fungicides is most commonly conferred by (i) point mutation in thecytbgene; (ii) overexpression of the alternative oxidase gene (AOX) in the alternative oxidation pathway; and (iii)enhanced efflux or multidrug resistance (Reimann and Deising 2005).
Picoxystrobin, synthesized by Syngenta Crop Protection, was registered in 2012 in China.It is a typical QoI fungicide and provides preventive and curative activity against many pathogens in cereals, vegetables, and other field crops, includingColletotrichumspp.,Botrytiscinerea,andMacrophomamusae(China Pesticide Information Network; http://www.chinapesticide.org.cn/hysj/index.jhtml).However, to date, no data on the resistance risk and molecular mechanism ofColletotrichumspp.from soybean to picoxystrobin is available in China.Therefore, the objectives of this study were to (i)establish the baseline sensitivities ofC.truncatumandC.gloeosporioidesisolates from China to picoxystrobin,(ii) induce mutants ofC.truncatumandC.gloeosporioidesresistant to picoxystrobin and assess their fitness, and(iii) elucidate the molecular determinant of resistance to picoxystrobin.
2.Materials and methods
2.1.Isolates
ColletotrichumtruncatumandC.gloeosporioidesisolates were collected between 2018 and 2021 from the main soybean-producing areas in seven Chinese provinces:Heilongjiang, Liaoning, Shandong, Sichuan, Hubei,Fujian, and Guangdong.All isolates were isolated using a single spore and identified on the basis of morphology,pathogenic character, and multi-gene phylogeny (ITS,TUB2,ACT,GAPDH, andHIS3sequences).
2.2.Fungicides
Picoxystrobin (95.0% active; DuPont, Wilmington, DE,USA), pyraclostrobin (98.0% active; Jiangsu Huifeng Agrochemical Co., Ltd., Yancheng, China), fluazinam(97% active; Ishihara Sangyo Kaisha, Osaka, Japan),difenoconazole (96.1% active; Jiangsu Gengyun Chemical Co., Ltd., Zhenjiang, China), and propiconazole(95.0% active; Jiangsu Qizhou Green Chemical Co.,Ltd., Zhangjiagang, China) were dissolved in dimethyl sulphoxide (DMSO).All of the above fungicides were prepared at the concentration of 1.0×104μg mL-1and stored at 4°C in the dark until further use.Salicylhydroxamic acid (SHAM, 99% a.i.; Sigma-Aldrich,St.Louis, MO, USA) was dissolved in DMSO to create a stock solution (9.6×104μg mL-1).
2.3.Sensitivities of C.truncatum and C.gloeosporioides isolates to picoxystrobin
The sensitivities of 128C.truncatumand 121C.gloeosporioidesisolates to picoxystrobin were investigated by calculating 50% growth inhibition (EC50)value, as suggested previously (Duet al.2021).Fresh plugs (5 mm in diameter) from the growing edge of active mycelia were transferred to PDA plates amended with 0.01, 0.05, 0.1, 0.5, or 1 μg mL-1picoxystrobin.PDA plates containing 0.1% (v/v) DMSO served as controls.SHAM (100 μg mL-1) was added to the medium to inhibit the alternative respiration pathway.The diameter of each colony was measured perpendicularly by the cross method after 7 days incubation at 28°C.The experiment was performed twice with three replicate plates for each treatment.
2.4.Generation of picoxystrobin-resistant mutants
SevenC.truncatumisolates (Ct012, Ct023, Ct036, Ct058,Ct061, Ct081, and Ct095) and sixC.gloeosporioidesisolates (Cg017, Cg035, Cg046, Cg059, Cg087, and Cg105) were randomly selected for picoxystrobin adaption.The origins of 13 parental sensitive isolates were provided in Appendix A.For each parental isolate, mycelial plugs(5 mm in diameter) were first dark-cultured at 28°C on PDA plates containing 10 μg mL-1picoxystrobin.After 15-20 days, the surviving isolates were subcultured on a fungicide-free medium for incubation and then on 20 μg mL-1picoxystrobin-containing medium.After that,the putative mutants were further transferred to plates amended with picoxystrobin: 50, 100, 200, or 500 μg mL-1.Finally, the colonies grown on PDA amended with 200 μg mL-1picoxystrobin were identified as potential resistant mutants.SHAM with 100 μg mL-1was added during the entire process.The mutation frequency was calculated as mentioned previously (Zhanget al.2017).
2.5.Phenotype analyses of picoxystrobin-resistant mutants
Level and stability of picoxystrobin resistanceThe resistance level was represented by the resistance factor(RF): RF=EC50(R)/EC50(S), where EC50(R) was the EC50of the resistant mutants, and EC50(S) was the EC50of their parental isolates.The stability of picoxystrobin resistance was determined as we described earlier (Duet al.2021).In simple terms, picoxystrobin-resistant mutants were transferred 10 times to fresh, fungicidefree PDA plates and dark-incubated at 28°C.The EC50value of each isolate was determined as described above and compared with that of each isolate before subculture.There were three replicates for each treatment, and the experiment was repeated twice.
Temperature sensitivity of mutantsResistant mutants and their parental isolates were transferred to PDA plates and dark-incubated at 16, 20, 25, 28, 30, and 37°C for 7 days.For each treatment, there were three replicates.And the experiment was conducted twice.
Sporulation and conidial germinationFor assessment of sporulation, resistant mutants and their corresponding parental isolates were grown on PDA plates and darkincubated for seven days at 28°C.Then, a conidial suspension was prepared by scraping the surface of the colonies with distilled water, and the concentration was standardized to 1×105conidia mL-1.Conidial germination was assessed under a microscope after 12 h with 100 μL conidial suspension on water agar dishes.The experiment was performed twice with three replicates for each treatment.
Pathogenicity assay and control efficacy in a greenhouseBriefly, soybean seeds (cv.Maodou 75-3) were sown in a greenhouse at (25±2)°C to the early podding stage.The conidia earlier cultured was harvested and the newly developed young pods of each plant was spray-inoculated with a 50 mL conidial suspension (1×105conidia mL-1).Control pods were sprayed with sterilized water.
To determine the control efficacy, the soybean plants at the early podding stage were first treated with 150 g a.i.ha-1picoxystrobin prepared from a commercial formulation (Acanto, 22.5% picoxystrobin SC, Dupont).Plants sprayed with water were used as controls.After 24 h, the soybeans were inoculated with a conidial suspension of each isolate.Then all inoculated plants were placed in a greenhouse (RH>90%) set at (25±2)°C until disease fully developed.The disease severity was assessed by a 0-9 scale standard based on the type and size of lesions and disease index was calculated according to the methods described in our previous study(DB35/T 1574-2016 2016).The control efficacy was calculated by: Control efficacy (%)=(Disease severity of control-Disease severity of treated)/Disease severity of control×100.For each isolate, six plants were inoculated,and the experiment was performed twice.
2.6.Cross-resistance
All picoxystrobin-resistant mutants and their parental isolates were used to determine the sensitivity to four fungicides with different types of action.The EC50values were calculated according to the method mentioned earlier.The details of each fungicide are listed in Appendix B.The experiment was performed twice with three replicates for each treatment.
2.7.Sequencing the cyt b gene of C.truncatum and C.gloeosporioides
The completecytbgenes ofC.truncatumandC.gloeosporioideswere amplified with primer pairs CtCytb-1/CtACytb-2 and CgFt-1/CgAFt-3, respectively(Table 1).PCR was performed in 25 μL reaction mixtures containing 12.5 μL of 2×Phanta Flash Master Mix(Vazyme, Nanjing, China), 1 μL of each primer, and 30 ng of template DNA.Amplifications were conducted with the following profile: 98°C for 30 s, followed by 35 cycles of 98°C for 10 s, 5 s annealing at 56°C, and 25 s extension forC.gloeosporioides(or 15 s forC.truncatum) at 68°C,followed by 72°C for 1 min.After amplification, PCR products were analyzed in GelRed-stained 1% agarose gels and purified using a TIANgel Purification Kit (Tiangen,Beijing, China).Amplified fragment was cloned into theTrans1-T1 (TransGen, Beijing, China), and at least six clones were sequenced (TsingKe Co., Beijing, China).Sequences of the Cytbproteins were analyzed using DNAMAN (ver.6.0).
2.8.Molecular docking analysis
The 3D structures of proteins wild-type, G137R and G143S were predicted by AlphaFold2 (Jumperet al.2021) respectively.AutoDock Vina (Trott and Olson 2010) was used for molecular docking of picoxystrobin with protein wild-type, G137R and G143S, respectively.During the docking process, the protein structures were converted to a PDBQT file, which contained all the polar residues with hydrogen.The compound picoxystrobin was also converted to a PDBQT file and all the bonds were set to be rotatable.The receptor was set to be fixed while the ligand was allowed to have a certain flexibility,and this flexible docking simulation was carried out using the Lamarcian genetic algorithm.A search of possible conformations of the ligand was performed within the confines of the grid box and the conformation with the lowest binding free energy was finally identified as the best probable conformation.The models of the complex were analyzed using Discovery Studio (BIOVIA Dassault Systèmes 2017).Surflex-Dock scores (total scores) were expressed in kcal/mol units to represent binding affinities.
2.9.Statistical analysis
Data on mycelial growth, sporulation, conidial germination,disease index, and control efficacies of picoxystrobin were processed by Data Processing System Software (ver.7.05; Hangzhou RuiFeng Information Technology Co.,Ltd., Hangzhou, China).Significant differences between resistant mutants and parental isolates were identified using Duncan’s multiple comparisons tests in one-way ANOVA.Spearman’s rank-order correlation analysis was used for analysis of cross-resistance.
3.Results
3.1.Baseline sensitivities of C.truncatum and C.gloeosporioides isolates to picoxystrobin
The EC50values of picoxystrobin against 128C.truncatumand 121C.gloeosporioidesisolates varied from 0.0219-2.0342 μg mL-1and 0.0198-2.9084 μg mL-1, with means of (0.7740±0.0388) and (1.1561±0.0576) μg mL-1,respectively.These results showed thatC.truncatumisolates were more sensitive to picoxystrobin thanC.gloeosporioidesisolates.In addition, the distributions of EC50values forC.truncatumandC.gloeosporioidesisolates displayed unimodally, which suggested that there were no pre-existing picoxystrobin-resistant subpopulations in the fields (Fig.1-A and B).

Fig.1 Frequency distributions of effective concentrations for 50% growth inhibition (EC50) of picoxystrobin against 128 Colletotrichum truncatum (A) and 121 Colletotrichum gloeosporioides (B) isolates.
3.2.Generation of C.truncatum and C.gloeosporioides mutants resistant to picoxystrobin
In total, eleven picoxystrobin-resistant mutants were generated from fourC.truncatumsensitive isolates(Ct012, Ct023, Ct058, and Ct081) through repeated exposure to picoxystrobin.There were no picoxystrobinresistant mutants obtained from any other parental isolates (Ct036, Ct061, and Ct095).The EC50values of the eleven mutants were 5.40-152.96 μg mL-1(Table 2).Additionally, six picoxystrobin-resistant mutants ofC.gloeosporioideswere derived from three sensitive isolates (Cg035, Cg059, and Cg087); the EC50values of these mutants ranged from 13.53-28.30 μg mL-1(Table 2).

Table 2 Resistance factor and stability of picoxystrobin-resistant mutants of Colletotrichum truncatum and Colletotrichum gloeosporioides
The mutation frequencies ofC.truncatumandC.gloeosporioidesto picoxystrobin were 6.50×10-3and 7.20×10-3, respectively.
3.3.Resistance level and stability of picoxystrobinresistant mutants
The RF values of the 5th and 10th generations of picoxystrobin-resistant mutants inC.truncatumvaried from 18.32 to 580.32, and from 16.99 to 521.77, respectively (Table 2).In general, these RF values decreased slightly to some extent in the 10th generation.On the other hand, the FSC values were 0.89-0.99, indicating that the resistance of all eleven mutants was relatively stable.Furthermore, the RF values of four mutants (Ct012-1, Ct012-2, Ct023-1,and Ct058-2) were more than 10 and less than 50,indicating that they were moderate resistant (MR)mutants; the RF values of another seven mutants were more than 100, indicating that they were high resistant(HR) mutants.
The RF values of the 5th and 10th generations of picoxystrobin-resistant mutants inC.gloeosporioidesvaried from 12.17 to 27.18, and 8.55 to 25.85 (Table 2),respectively, indicating that the resistance levels of these mutants were low to moderate.On the other hand, the FSC values were 0.81-0.98, indicating that the resistance remained stable.
3.4.Effects of temperature on mycelial growth
The optimal temperatures of mutants and sensitive parental isolates ofC.truncatumandC.gloeosporioideswere 28°C and 25°C, respectively (Table 3).Mutant Ct012-1 grew similar rate to its parental isolate at 28°C,but grew more slowly compared to its parental isolate at the other five temperatures.Mutant Ct058-1 grew faster than its parental isolate at 16°C, 20°C, and 25°C.Mutant Cg035-1 grew similar rate to its parental isolate at 16°C.Mutants Ct081-1, Ct081-2, and Ct081-3 grew similar rate to their parental isolates at 37°C.Other mutants grew more slowly relative to their respective parental isolates in all treatments.

Table 3 Effect of temperature on mycelial growth of picoxystrobin-resistant mutants of Colletotrichum truncatum and Colletotrichum gloeosporioides
3.5.Mycelial growth, sporulation, conidial germination, and virulence
The sporulation of six mutants (Ct012-1, Ct012-2, Ct012-3,Ct012-4, Cg059-1, and Cg059-2) was significantly higher or similar to that of their respective parental isolates(P<0.05).However, there were no significant differencein conidial germination between all the mutants and their corresponding parental isolates (P>0.05) (Table 4).As indicated by disease index, most of the picoxystrobinresistant mutants displayed similar pathogenicity compared to their corresponding parental isolates,except Ct012-2, Ct012-4, Ct058-2, and Cg035-1 mutants(P<0.05) (Table 4).

Table 4 Fitness of parental isolates and resistant mutants of Colletotrichum truncatum and Colletotrichum gloeosporioides
3.6.Control efficacies in a greenhouse
As observed, disease index was significantly higher on soybean plants inoculated with resistant mutants than with parental isolates (P<0.05), especially for HR mutants, at 150 g a.i.ha-1picoxystrobin (Table 5).More than 63.64% control efficacy was found in parental isolates, while <38.65 and <52.91% disease control were observed in HR and MR mutants, respectively.That is to say, control efficacy of mutants significantly reduced in the picoxystrobin treatments relative to parental isolates.

Table 5 Control efficacy of picoxystrobin against soybean anthracnose in a greenhouse
3.7.Cross-resistance
Not surprisingly, the cross-resistance test and Spearman rank correlation analysis showed that picoxystrobin had positive cross-resistance with pyraclostrobin(ρ=0.936,P<0.01).Nevertheless, there was no crossresistance between picoxystrobin and any of the other three fungicides, i.e., fluazinam (ρ=-0.413,P=0.505),difenoconazole (ρ=0.359,P=0.085), and propiconazole(ρ=0.044,P=0.837) (Fig.2).

Fig.2 Spearman rank correlation for cross-resistance in Colletotrichum truncatum and Colletotrichum gloeosporioides between picoxystrobin and four other fungicides, including pyraclostrobin (A), difenoconazole (B), fluazinam (C),and propiconazole (D).Data points were the logarithmic values of effective concentrations for EC50 (logEC50) among the tested isolates for the indicated fungicide combinations.
3.8.Cyt b gene of C.truncatum and C.gloeosporioides
The fullcytbgene ofC.truncatumwas 1 164 bp, and encoded 387 amino acids.Sequencing results of thecyt bgene revealed two point mutations.In four MR mutants,a G-to-A transversion occurred in codon 409, resulting in glycine being replaced with arginine in position 137(G137R) (Fig.3-A).The other seven HR mutants had aG-to-A transversion in codon 427, where a glycine was replaced by serine (G143S) (Fig.3-B).The fullcytbgene ofC.gloeosporioideswas 3 695 bp and encoded 391 amino acids.However, no point mutations were found in any of theC.gloeosporioidesmutants.
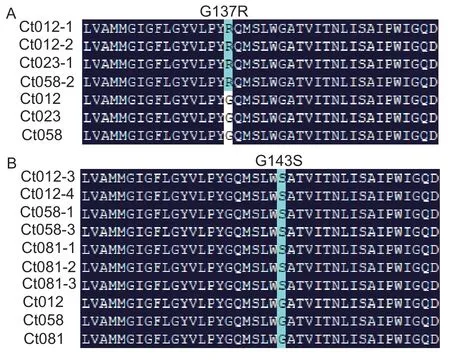
Fig.3 Multiple sequence alignment of the Cyt b protein from picoxystrobin-resistant Colletotrichum truncatum mutants and the corresponding sensitive parental isolates.Ct012, Ct023,Ct058, and Ct081 correspond to the wild-type parental isolates.Others are picoxystrobin-resistant mutants generated from the four sensitive isolates.A, four resistant mutants had a point mutation at amino acid codon 137.B, seven mutants had a point mutation at amino acid codon 143.
3.9.Molecular docking between picoxystrobin and the Cyt b protein in C.truncatum
Models were built of picoxystrobin docking into the Qobinding pocket: wild-type, G143S or G137R (Fig.4).In addition, the docking scores of the predicted models are shown in Fig.4-A.All of the important amino acid residues were marked in Fig.4-B-D.In the wild type,picoxystrobin created a suitable steric complementarity with the binding site of the Cytbprotein.Hydrogen bonds, Pi-sulfur, halogen (fluorine), hydrophobic and Van der Waals (VDW) interactions were formed among the wild-type and picoxystrobin (Fig.4-B).For example, the nitrogen atom of picoxystrobin formed one conventional hydrogen bond interaction with Ile147, and the oxygen atom of picoxystrobin formed one carbon hydrogen bond interaction with Ile147.The two carbon atoms of picoxystrobin formed two carbo hydrogen bond interactions with Thr148 and Glu273, respectively.The benzene ring of picoxystrobin formed a Pi-sulfur with Met296, and the two fluorine atoms of picoxystrobin formed two halogens with Ile147, respectively.
Compare with the wild-type and picoxystrobin, the mutation G137R eliminated carbo hydrogen bond interaction between Thr148 and picoxystrobin, and two halogens between Ile147 and picoxystrobin, causing a docking score decrease from 6.59 to 5.61 (Fig.4-C).As indicated in Fig.4-D, carbo hydrogen bond interactions between picoxystrobin and Thr148 or Glu273 were missing.Moreover, the formation of a Pi-sulfur with Met296 was prevented by the G143S mutation.Finally,the docking score decreased from 6.59 to 5.37.These results were consistent with fungicide sensitivity assays, in which the mutation G143S resulted in high resistance to picoxystrobin but G137R showed moderate resistance.
4.Discussion
Anthracnose is one of the main limiting factors for soybean production worldwide (Boufleuret al.2021; Shiet al.2021a).However, few soybean varieties with high resistance level have been developed up to now.Therefore, the application of fungicide still plays a crucial role in the control of soybean anthracnose.To date, even though resistance to QoI fungicides has been observed in many pathogens,no changes in fungicide sensitivity have been detected in isolates of soybean anthracnose in China.A previous study showed thatC.truncatumandC.gloeosporioideswere the main pathogens of soybean anthracnose in China (Shiet al.2022).Therefore, in order to understand the evolution of fungicide resistance, baseline sensitivities of these two species to picoxystrobin were established.Given that it is unclear whether the isolates collected had previously been exposed to other fungicides with the same mode of action, the mean EC50values obtained in this context cannot be considered as definitive baselines, but rather as relative baselines.Furthermore, the low EC50values and unimodal distribution provide strong evidence that no picoxystrobin-resistant subpopulations ofC.truncatumandC.gloeosporioidesexist in fields.Collectively, the current study can offer a reference for monitoring sensitivity change ofC.truncatumandC.gloeosporioidespopulations to picoxystrobin in the future.
According to the Fungicide Resistance Action Committee (FRAC), QoI fungicides are high resistancerisk molecules, and require resistance management in advance (FRAC 2021).Indeed, QoI resistance has been found in many pathogens, such asB.cinerea(Bannoet al.2009),Magnaporthegrisea(Miaoet al.2020), andC.acutatum(Forceliniet al.2018).In the current study,four picoxystrobin-resistant mutants with RF values of 27.81-37.49, and seven mutants with RF values>100 were produced from four of seven wild-type isolates inC.truncatum.In addition, six mutants with RF values of 8.55-25.85 were derived from three of six sensitive isolates inC.gloeosporioides.The mutation frequencies ofC.truncatumandC.gloeosporioidesto picoxystrobin was 6.50×10-3and 7.20×10-3, respectively.A comparison of the biological characteristics indicated that half of the mutants had a competitive or good fitness compared to the parental isolates.This is consistent with previous studies that showed that a fitness cost is not associated with QoI resistance in plant fungal pathogens (Veloukaset al.2014; Forceliniet al.2018; Renet al.2020).In addition, picoxystrobin did not control HR mutants carrying the G143S mutation, while those with the G137R alternation or no Cytbmutation were controlled to some extent, however, at a lower level than parental isolates under greenhouse trials.Thus, these features suggest that these kinds of picoxystrobin-resistant isolates may survive and develop in the field under the certain selective pressure of picoxystrobin in the future.Altogether, our findings speculated that the resistance risk ofC.truncatumandC.gloeosporioidesto picoxystrobin would be moderate to high.
As previously mentioned, cross-resistance is a typical feature of qualitative resistance by target-site modification(Fernández-Ortuñoet al.2008).The cross-resistance to diverse QoI fungicides has also been reported in numerous other fungi (Miaoet al.2020; Penget al.2022).As expected, picoxystrobin had positive cross-resistance with pyraclostrobin, but no associated cross-resistance with difenoconazole, fluazinam, or propiconazole.
The most widespread resistance mechanism of QoIs is based on point mutations of thecytbgene encoded by mitochondrial DNA, which has a higher mutation frequency when compared to nuclear DNA, a frequency that is increased by the accumulation of reactive oxygen species because of the inhibition of the electron transport system by QoI fungicides (Veloukaset al.2014).Mutations have been found at positions 127-147 and 275-296 of Cytbprotein; these domains are close to each other in the folded protein and are vital for ligand binding (Sierotzkiet al.2000;Fisheret al.2004; Fernández-Ortuñoet al.2010).In the current study, two separate point mutations, G137R and G143S, were detected in picoxystrobin-resistant mutants ofC.truncatum.Moreover, G143S mutation was observed in seven of eleven mutants with RFs higher than 100.The G143S mutation has also been reported in highly resistantM.oryzae(Miaoet al.2020; Penget al.2022),and the homologous G143A alteration has been found in many other fungi (Owatiet al.2017).However, due to the lethal effect of proximal exonic flanking sequences on the 5´-splice, G143A mutation has not been detected in fungal species with an intron downstream of codon 143 (Zenget al.2015).The G137R mutation was identified in four of eleven mutants with RFs of 16.99-37.49.This is thought to be the first report of the substitution from glycine to arginine inC.truncatum.In addition, the G137R mutation has been reported in a small number of pathogenic fungi likePyrenophoratritici-repentis(Sierotzki and Frey 2007;Miaoet al.2020).These results demonstrate that target site mutations play an important role in the resistance ofC.truncatumto picoxystrobin.
A drug molecule will be most potent when its bioactive conformation matches that of its target’s binding pocket(Zhaoet al.2010).However, in the case of conformational change of the binding pocket, pathogens may develop resistance to the fungicide (Zhouet al.2015).Despite the detected mutation has been reported before (Liet al.2022), molecular docking analysis was carried to better understand the interaction of picoxystrobin and the Cytbprotein.As previous studies reported, the G143S abolished carbon hydrogen bond interactions and other interactions, resulting in docking score from 6.59 to 5.37.The G137R mutation reduced the number of carbon hydrogen bond to one, which could explain why G137R mutation resulted in reduced sensitivity to picoxystrobin.Taken together, these results increase understanding of QoI fungicide-resistance mechanisms.
Interestingly, the typical amino acid alternation was not detected in any of the six picoxystrobin-resistant mutants ofC.gloeosporioides.Similar results have been reported forPodosphaerafusca(Fernández-Ortuñoet al.2008),C.siamense(Chechiet al.2019), andC.gloeosporioides(Renet al.2020), in which no point mutation was found in the low and moderate resistant isolates.Although the activation of AOX-mediated alternative respiratory pathway is another QoI fungicide resistance mechanism(Fontaineet al.2019), the moderate RFs exhibited by theC.gloeosporioidesmutants do not seem to support it because of the similar sensitivity with or without SHAM (data not shown).In some basidiomycetes, the associated stability of the Rieske-FeS protein with Cytbhas been proposed as a mechanism of resistance to QoI fungicides (Brandet al.1993).It is unknown whether a structural change in the Rieske protein contributes to resistance to picoxystrobin inC.gloeosporioides, but its nature as a nuclear-encoded target makes it an ideal candidate.Therefore, further studies remains to be investigated the role of this protein in conferring fungicide resistance toC.gloeosporioides.
Acquired resistance is a phenotypic adaptation in pathogens in response to the selection pressure of fungicide (Hawkinset al.2019), and is a growing concern for sustainable crop production.Therefore, the prediction of resistance in advance of its emergence in the field can enable reasonable and effective management of fungicide.Additionally, integrated management practices,such as crop rotation, use of resistant plant, rotating fungicide of different target site of action, and using QoI preventatively instead of after disease being developed,will help to reduce the risk of fungicide resistance and maintain the high efficacy of the fungicides as long as possible (Zenget al.2015).
5.Conclusion
The baseline sensitivities established will provide the basis for sensitivity shift in twoColletotrichumspecies to picoxystrobin in the field.The resistance risk to picoxystrobin inColletotrichumspecies was ascertained to be medium to high.Two separate point mutations,G137R and G143S, were likely to be the underlying mechanisms of picoxystrobin resistance inC.truncatum.The above results suggest that effective strategies for QoI fungicide resistance management should be implemented to reduce selection pressure on pathogens and then delay the development of resistance.
Acknowledgements
This work was funded by the Natural Science Foundation of Fujian Province, China (2021J01476),East and West Cooperation Project of the Fujian Academy of Agricultural Sciences, China (DKBF-2022-01), the Project of Department of Agriculture and Rural Affairs in Fujian Province (2021PZQS006), the“5511” Collaborative Innovation Project of High-quality Agricultural Development and Surpassment in Fujian Province (XTCXGC2021011), and the Team Project Funding of Scientific Research Innovation of FAAS, China(CXTD2021002-1).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on https://doi.org/10.1016/j.jia.2023.07.037
 Journal of Integrative Agriculture2023年12期
Journal of Integrative Agriculture2023年12期
- Journal of Integrative Agriculture的其它文章
- Biotechnology of α-linolenic acid in oilseed rape (Brassica napus)using FAD2 and FAD3 from chia (Salvia hispanica)
- Analyzing architectural diversity in maize plants using the skeletonimage-based method
- Derivation and validation of soil total and extractable cadmium criteria for safe vegetable production
- Effects of residual plastic film on crop yield and soil fertility in a dryland farming system
- Identifying the critical phosphorus balance for optimizing phosphorus input and regulating soil phosphorus effectiveness in a typical winter wheat-summer maize rotation system in North China
- Characteristics of Mycobacterium tuberculosis serine protease Rv1043c in enzymology and pathogenicity in mice
