Twice-split phosphorus application alleviates low-temperature impacts on wheat by improved spikelet development and setting
XU Hui, HOU Kuo-yang, FANG Hao, LIU Qian-qian, WU Qiu, LIN Fei-fei, DENG Rui, ZHANG Lin-jie,CHEN Xiang, LI Jin-cai,
1 School of Agronomy, Anhui Agricultural University, Hefei 230036, P.R.China
2 Jiangsu Collaborative Innovation Centre for Modern Crop Production, Nanjing 210095, P.R.China
Abstract Extreme low-temperature incidents have become more frequent and severe as climate change intensifies.In Huang-Huai-Hai wheat growing area of China, the late spring coldness occurring at the jointing-booting stage (the anther interval stage) has resulted in significant yield losses of winter wheat.This study attempts to develop an economical,feasible, and efficient cultivation technique for improving the low-temperature (LT) resistance of wheat by exploring the effects of twice-split phosphorus application (TSPA) on wheat antioxidant characteristics and carbon and nitrogen metabolism physiology under LT treatment at the anther interval stage using Yannong 19 as the experimental material.The treatments consisted of traditional phosphorus application and TSPA, followed by a -4°C LT treatment and natural temperature (NT) control at the anther interval stage.Our analyses showed that, compared with the traditional application, the TSPA increased the net photosynthetic rate (Pn), stomatal conductance (Gs), and transpiration rate (Tr) of leaves and reduced the intercellular carbon dioxide concentration (Ci).The activity of carbon and nitrogen metabolism enzymes in the young wheat spikes was also increased by the TSPA, which promoted the accumulation of soluble sugar (SS), sucrose (SUC), soluble protein (SP), and proline (Pro) in young wheat spike and reduced the toxicity of malondialdehyde (MDA).Due to the improved organic nutrition for reproductive development, the young wheat spikes exhibited enhanced LT resistance, which reduced the sterile spikelet number (SSN) per spike by 11.8% and increased the spikelet setting rate (SSR) and final yield by 6.0 and 8.4%, respectively, compared to the traditional application.The positive effects of split phosphorus application became more pronounced when the LT treatment was prolonged.
Keywords: optimizing phosphorus application, low-temperature stress, carbon and nitrogen metabolism, young spike development, wheat
1.Introduction
Wheat is one of the most widely planted crops in the world, and yield stability is of great significance for ensuring food security and reducing hunger (Erensteinet al.2021).With the increasing global warming, the frequency and intensity of low-temperature (LT) stress are also increasing, aggravating wheat production instability(Jiet al.2017).LT affects a range of physiological and metabolic processes in wheat, inhibiting their growth and development (Zhaoet al.2019; Hassanet al.2021).The external symptoms of wheat under LT are not obvious,but the long-term freezing injury (<0°C) has caused the withering and mild drying of wheat leaves, the dehydration of stems, leaves, and spikes has negative impacts on yield and quality of wheat (Hanet al.2013; Liuet al.2019).
In the Huang-Huai-Hai wheat growing area of China,late spring coldness, i.e., LT stress during the anther interval stage, frequently occurs in wheat production areas (Jianget al.2022).The anther interval stage is between the jointing and booting stages of wheat, which is the critical vegetative to reproductive transition stage(Xuet al.2022a).At this crucial stage of the pistil and stamen differentiation to tetrad formation, the wheat spike is tender and has a high water content.It is sensitive to environmental factors such as temperature,fertilizer, and soil moisture (Lianget al.2021).LT causes pollen inactivation and spikelet abortion, resulting in the reduction of wheat grain number and grain weight per spike at the mature stage (Yuet al.2022).Jiet al.(2017)showed that the yield per plant of wheat decreased by 4.6-56.4% and 13.9-85.2% after LT at the jointing and booting stages, respectively.
Wheat plants respond to LT through a series of physiological processes to maintain reactive oxygen species (ROS) balance (Hassanet al.2021).Under LT, the antioxidant system of wheat was activated, and antioxidant enzyme activity increased.Among them,superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) are the three most important antioxidant enzymes, and their synergy can effectively reduce the oxidative damage of wheat under LT (Xuet al.2022a).Photosynthesis is one of the most sensitive physiological processes of crops to LT, and its synthetic organic matter is the basis of wheat growth and yield formation (Allen and Ort 2001; Xuet al.2022b).For example, LT can reduce photosynthetic area and pigment content.In addition, the net photosynthetic rate (Pn) and photosynthetic capacity of wheat leaves are damaged by LT (Hanet al.2013).
Carbon and nitrogen metabolisms are two of the most important metabolic processes in crops, which play an important role in nutrient supply, growth, development,and stress resistance of plants (Liet al.2018; Baslamet al.2020).Carbon and nitrogen metabolisms are closely linked.Carbon metabolism provides energy and carbon sources for nitrogen metabolism, and nitrogen metabolism provides organic substances such as enzymes and proteins necessary for carbon metabolism(Erdal 2019).Previous studies have shown that LT can delay young wheat spike development by inducing abscisic acid synthesis and changing the activity of sucrose (SUC) metabolism-related enzymes (Zhanget al.2019; Yuet al.2022).Therefore, it is important to understand the physiological mechanism by which LT affects wheat young spike development during the anther interval stage and determine how to reduce the adverse effects of LT on young spike development to maintain sustainable wheat production.
In modern crop cultivation, phosphorus is an important component of plant metabolism and the second essential macronutrient after nitrogen (Nedelciuet al.2020).Meanwhile, as a non-renewable resource, global demand for phosphorus is expected to exceed supply in 2045(Jianget al.2019).Phosphorus plays a vital role in biological processes, and it also participates in signal transduction, energy dynamics, and enzyme catalysis to enhance the adaptability of crops to the external environment (Soetanet al.2010).Phosphorus application can increase water and nutrient uptake by roots (Shenet al.2011).In addition, phosphorus application can also increase antioxidant enzyme activities, soluble sugar (SS) and proline (Pro) contents in leaves, reduce electrolyte leakage, alleviate the premature senescence of crop plants, and prolong the grain-filling time under LT stress (Nieet al.2015; Lvet al.2017).The storage,redistribution, and utilization of phosphorus among different organs can also provide a nutrition source for wheat reproductive growth (Wanget al.2021).Therefore,increasing phosphorus application is important to improve wheat yield and stress tolerance.However, it is increasingly recognized that the global phosphorus shortage is due to excessive phosphorus fertilization and that unused phosphorus leads to surface water eutrophication through runoff, leaching, and water erosion(Macdonaldet al.2011).Unused phosphorus also reduces the stress resistance of wheat, and optimizing phosphorus application can increase wheat’s absorption and utilization of phosphorus, thereby improving the cold resistance of wheat during the jointing and booting stages (Conget al.2020; Gonget al.2022; Xuet al.2022a).However, it remains unclear whether optimizing phosphorus application (i.e., twice-split phosphorus application, TSPA) can alleviate the adverse effects of LT stress on young wheat spike development at the anther interval stage.
This study aimed to explore the effects of optimizing phosphorus application on wheat growth and development under LT stress at the anther interval stage,focusing on leaf photosynthesis, young spike antioxidant characteristics, carbon and nitrogen metabolism, spikelet phenotype, yield, and its composition.We hope the research will provide a theoretical basis for sustainable phosphorus management and improving wheat stress resistance.
2.Materials and methods
2.1.Plant growth conditions
Experiments were conducted in 2020-2021 and 2021-2022 at the Nongcui Garden of Anhui Agricultural University (31°8´N, 117°2´E) in Hefei, Anhui Province,China.This location has a humid monsoon climate in the northern subtropics.The region is located in the subtropical monsoon climate zone, and late spring coldness is prone to occur every year in the middle of March (Xuet al.2022b).Wheat seeds were sown in pots with a diameter of 26 cm and a height of 35 cm.Each pot was filled with 10 kg of soil before sowing, and then the wheat seeds were covered with 3 cm of soil after sowing.The content of organic matter, available nitrogen,available phosphorus, and available potassium in the soil was 16.3 g kg-1, 112.2 mg kg-1, 23.0 mg kg-1, and 161.6 mg kg-1, respectively.
2.2.Plant material, phosphorus application, and LT treatment
The high-yield wheat cultivar Yannong 19, which is widely planted in the Huang-Huai-Hai wheat growing area of China, was used in this study.Wheat seeds were sown on November 1, 2020 and November 11, 2021, and harvest time for the two years was May 21 of the following year.A two-factor experiment with a randomized block was designed, involving phosphorus application methods and different temperatures.
Referring to the practice of field fertilization in the region, phosphorus, and potassium fertilizers were all used as base fertilizers before sowing, and nitrogen was applied to the soil twice before sowing and at the jointing stage (Heet al.2019; Yeet al.2022).In this experiment, 1.8 g urea (base application of 1.2 g before sowing+top application of 0.6 g at the jointing stage) and 1.7 g potassium sulfate (one-off base application) were applied to each pot during the whole growth period.The traditional phosphorus application (R1) treatment was to apply all 5.0 g superphosphate before sowing.The TSPA(R2) treatment was to apply 2.5 g superphosphate before sowing separately and at the jointing stage.The top application during the jointing stage was on March 1 and March 10 in 2021 and 2022, respectively.Eighteen seeds were sown in each pot, and 9 wheat plants were kept in each pot at the three-leaf stage.
During the anther interval stage (about 125-d-old seedlings), half of the pots were transferred to artificial climate chambers (DGXM-1008; Ningbo Jiangnan Instrument Manufacturing Factory, Ningbo, China;1 300 mm length×630 mm width×1 305 mm height).The LT treatment of the artificial climate chambers was set at-4°C from 1:00-5:00 a.m.The anther interval stage of wheat in 2021 and 2022 was March 15 and March 26,respectively.The other half of the potted plants were placed in the natural temperature (NT) of the field.The average temperature during 1:00-5:00 a.m.was 10°C in 2021 and 2022 (Appendix A).The average moisture content of potting soil on the day of LT treatment in 2021 and 2022 was 18.7 and 19.5%, respectively.After LT treatment, the wheat was moved to the field to grow until mature.Other management measures were the same as the local general high-yield fields.
2.3.Sampling and measurements
Photosynthetic parameters of wheat leavesAt 0, 2,4, 6, and 8 d after LT and NT treatments in 2020-2021 and 2021-2022, thePn, stomatal conductance (Gs),intercellular carbon dioxide concentration (Ci), and transpiration rate (Tr) of wheat leaves were measured at 9:00-12:00 a.m.every day.Photosynthetic parameters of leaves were measured by the Li-6400 (LI-COR,Lincoln, NE, USA) portable photosynthetic measurement system.Three randomly selected uppermost expanded leaves of wheat main stems were used to measure the photosynthetic traits in each treatment.
SOD, POD, CAT, and malondialdehyde (MDA)activities of young wheat spikesAt 0, 2, 4, 6, and 8 d after LT and NT treatments in 2020-2021 and 2021-2022,the young wheat spikes (0.1 g) were taken to measure the antioxidant enzyme activity and MDA content with three biological replicates.The frozen spikes were ground to powder with liquid nitrogen and then a ten-thousandth scale (ME204E/02; Shanghai Mettler Toledo Instrument Co., Ltd., Shanghai, China) was used to weigh frozen spikes into several 0.1 g plant tissues.Moreover, these frozen spikes can also be used to measure carbon and nitrogen metabolites and enzymes.
Young spike tissues were ground in liquid nitrogen, and the 2 mL of 0.05 mol L-1phosphate buffer (pH=7.8) was added and homogenated at 4°C.Homogenates were centrifuged at 4°C in a refrigerated centrifuge at 10 000×g for 10 min.The supernatant was used for enzyme assay determination.The activity of SOD (EC 1.15.1.1), POD(EC 1.11.1.7), and CAT (EC 1.11.1.6) was determined by the nitroblue tetrazole photochemical reduction method,guaiacol method, and ultraviolet absorption method,respectively (Zhanget al.2021).The MDA content of young wheat spikes was measured according to the thiobarbituric acid method (Zhao and Cang 2016).
Pro, soluble protein (SP), SUC, and SS contents of young wheat spikesAt 0, 2, 4, 6, and 8 d after LT and NT treatments in 2020-2021 and 2021-2022, the young wheat spikes (0.1 g) were taken to measure the carbon and nitrogen metabolite contents with three biological replicates.
Pro and SP contents were determined by the sulfosalicylic acid method and G-250 Coomassie brilliant blue colorimetry, respectively (Liuet al.2018).The contents of SS and SUC were determined by anthrone colorimetry and resorcinol method, respectively, with slight modification according to previous studies (Wang Xet al.2022).
Carbon and nitrogen metabolism-related enzymes and acid phosphatase (ACP) activities of young wheat spikesOn the day of completion of LT and NT treatments in 2021-2022, young wheat spike samples were taken immediately for measurement of activities of carbon and nitrogen metabolism enzymes with three randomly selected 0.1 g young spike tissues, respectively.The activities of carbon and nitrogen metabolism enzymes were measured using the kit produced by Solarbio Technology Co., Ltd.After the young spike tissues were ground, the reagent was added, and the enzyme activities were measured by a plate reader(Thermo Scientific Multiskan FC, USA).A total of 1 mL of extraction solution was added to the young spike tissues,which were thoroughly grinded before being centrifuged at 4°C and 8 000×g for 10 min and the supernatant was taken for testing.
The activities of nitrate reductase (NR) (EC 1.7.1.3)and glutamine synthetase (GS) (EC 6.3.1.2) were measured using the methods of Wanget al.(2020).The measurement methods of sucrose synthase (SUS)(EC 2.4.1.13), sucrose phosphate synthase (SPS) (EC 2.4.1.14), and AI (EC 3.2.1.26) activities were according to Schrader and Sauter (2002) and Huanget al.(2013), with some modifications, and determined by the plate reader with 480 and 540 nm absorbance values, respectively.
The reagent from the kit was added to the supernatant,stirred thoroughly, heated for 15 min at 37°C and then measured the ACP (EC 3.1.3.2) activity at 510 nm absorbance values.
Spikelet setting rateAt the maturity stage in 2020-2021 and 2021-2022, 30 wheat spikes were randomly selected from each treatment.The total spikelet number (TSN),fertile spikelet number (FSN), and degenerated spikelet number (DSN) per spike were measured, and the spikelet setting rate (SSR) was calculated.
Yield and its compositionAt the maturity stage in 2020-2021 and 2021-2022, three wheat plants were randomly selected from each pot to investigate the grain number (GN) per spike, spike number (SN) per plant, 1 000-grain weight (TGW) and the yield per plant(YPP=SNPP×GNPS×TGW).
2.4.Statistical analysis and plotting
Statistical analysis was performed with SPSS 18.0 (SPSS Inc., Chicago, IL, USA), and differences were judged by the least significant differences test using a 0.05 level of significance.Graphs were drawn using Microsoft Excel 2016 and Origin 2021.
3.Results
3.1.Photosynthetic parameters of wheat leaves
The effects of the twice-split phosphorus application on thePnandGsafter LT treatment were shown in Fig.1-AD, respectively.From 0 to 8 d after LT treatment, thePnandGsof wheat leaves were significantly lower than those of NT treatment, and R1LT and R2LT treatments reached the lowest value at 2 d after LT treatment.ThePnandGsof leaves were increased by TSPA.ThePnof R2NT and R2LT were 5.4 and 8.1% higher than those of R1NT and R1LT, andGsincreased by 5.0 and 7.1% at 8 d after LT treatment, respectively.In addition, thePnat 6 d in 2021-2022 andGsat 8 d in 2020-2021 of R2LT after LT treatment were significantly higher than those of R1LT.

Fig.1 Twice-split phosphorus application (R2) affecting photosynthetic parameters of wheat leaves after low-temperature (LT)treatment.R1, traditional phosphorus application; NT, natural temperature treatment.Pn, net photosynthetic rate; Gs, stomatal conductance; Ci, intercellilar carbon dioxide concentration; Tr, transpiration rate.Values are mean±standard error (n=3).Different letters indicate significant differences (P<0.05).
The effects of the TSPA onCiandTrafter LT treatment are shown in Fig.1-E-H.From 0 to 8 d after LT treatment,theCiof wheat leaves was significantly higher than that of NT treatment, but theTrshowed the opposite trend,the same as the change ofPnandGs.TSPA reducedCi, andGswas increased.TheCiat 4 and 6 d after LT treatment in 2021-2022 were significantly reduced by 7.1 and 8.6% under the TSPA, respectively.In contrast, theTrat 6 d after LT treatment in 2020-2021 was significantly increased by 6.6% under the TSPA.
Taken together, LT treatment significantly reduced thePn,Gs, andTrof wheat leaves, while thePn,Gs, andTrincreased to varying degrees under the TSPA.The changes ofCiunder LT stress and TSPA were completely opposite (Fig.1).
3.2.Antioxidant enzymes and MDA content of wheat spikes
MDA content in young wheat spikes increased after LT treatment, and the activity of antioxidant enzymes increased in a short time to reduce the toxicity of MDA to young wheat spikes.In the meantime, the TSPA increased the activity of antioxidant enzymes and decreased the content of MDA of young wheat spikes(Figs.2 and 3).
The SOD, POD, and CAT activities of young wheat spikes of R1LT and R2LT on the day of LT treatment were significantly higher than those of NT treatment (Fig.2).Subsequently, the activities of SOD, POD, and CAT showed a sharp decline and then a slow increase trend at 2-8 d after LT treatment and reached the lowest at 4 d after LT treatment.During this period, the activity of antioxidant enzymes showed a slowly increasing trend of R1NT and R2NT.

Fig.2 Twice-split phosphorus application (R2) affecting antioxidant enzymes of young wheat spikes after low-temperatur (LT)treatment.R1, traditional phosphorus application; NT, natural temperature treatment.SOD, superoxide dismutase; POD, peroxidase;CAT, catalase.Values are mean±standard error (n=3).Different letters indicate significant differences (P<0.05).
The activity of antioxidant enzymes was increased by the TSPA (Fig.2).After 0-8 d of LT treatment, the promotion ratio of TSPA to antioxidant enzymes of young wheat spikes also constantly increased, which of LT treatment was higher than that of NT treatment.At 8 d after LT treatment, R2LT accumulated more SOD (6.5%),POD (7.8%), and CAT (6.4%) in spikes than R1LT, but not significant.Furthermore, except for SOD in 2021-2022 and CAT in 2020-2021, antioxidant activity did not show a significant difference between R2LT and R1NT at 8 d after LT treatment.
As shown in Fig.3, the MDA content of R1LT and R2LT increased first and then decreased, and it reached the highest level at 4 d after LT treatment.MDA content of R2LT and R2NT at 8 d after LT treatment was decreased by 6.4 and 7.0% than R1LT and R1NT, respectively.

Fig.3 Twice-split phosphorus application (R2) affecting malondialdehyde (MDA) content of young wheat spikes after low-temperature(LT) treatment.R1, traditional phosphorus application; NT, natural temperature treatment.Values are mean±standard error (n=3).Different letters indicate significant differences (P<0.05).
3.3.Carbon and nitrogen metabolite of wheat spikes
The effects of the TSPA on SS and SUC contents of young wheat spikes after LT treatment are shown in Fig.4-A-D.SS and SUC contents of R1LT and R2LT showed a decreasing trend from 0-8 d, significantly increasing from 0-2 d after LT treatment compared with NT treatment.And 6-8 d after LT treatment, NT treatments were higher than LT treatments, and R2NT and R1NT were significantly higher than R1LT.And SS,SUC content of R2LT and R2NT at 8 d after LT treatment was increased by 7.3 and 8.3%, 6.5 and 4.1% than that of R1LT and R1NT, respectively.There was no significant difference between R2LT and R1NT at 6-8 d after LT treatment between R1NT and R2LT.

Fig.4 Twice-split phosphorus application (R2) affecting carbon and nitrogen metabolite of young wheat spikes after low-temperature(LT) treatment.R1, traditional phosphorus application; NT, natural temperature treatment.SS, soluble sugar; SUC, sucrose; Pro,proline; SP, soluble protein.Values are mean±standard error (n=3).Different letters indicate significant differences (P<0.05).
Pro content significantly increased, and SP content decreased on the day of LT treatment (Fig.4-E-H).TSPA increased the Pro and SP contents of young wheat spikes from 0-8 d after LT treatment simultaneously.Both Pro and SP contents in young spikes first decreased and then increased at 2-8 d after LT treatment, and the content reached the lowest at 4 d after LT treatment.
Among them, the Pro content of R1LT and R1NT at 8 d after LT treatment in 2021-2022 was significantly increased by 4.9 and 6.9%, respectively.The SP content of young wheat spikes of R1LT and R1NT decreased on the day of LT treatment by 9.4 and 4.2% compared with that of R1NT, and which of R2NT was 5.0% higher than that of R1NT.SP content of R1LT and R1NT at 8 d after LT treatment was significantly increased by 5.9 and 7.9%compared to R1LT and R1NT, respectively (Fig.4-E-H).The above results also showed that the effect of the TSPA under LT stress was better than that under NT treatment(Fig.4).
3.4.Carbon and nitrogen metabolism-related enzymes and ACP activities of wheat spikes
It can be seen from Fig.5 that LT treatment reduced the activities of NR, GS, AI, and ACP, and the activities of SUS and SPS increased in young wheat spikes.After LT treatment, the activities of NR, GS, SUS, and SPS were significantly increased by TSPA, and the activity of ACP was significantly decreased, while AI reduction was not significant.

Fig.5 Twice-split phosphorus application (R2) affecting sucrose synthetase (SUS), sucrose phosphate synthase (SPS), acid invertase (AI), nitrate reductase (NR), glutamine synthetase (GS), and acid phosphatase (ACP) activities of young wheat spikes after low-temperature (LT) treatment.R1, traditional phosphorus application; NT, natural temperature treatment.Values are mean±standard error (n=3).Different letters indicate significant differences (P<0.05).
The activities of NR, GS, SUS, and SPS of R2LT and R2NT were increased by 9.7 and 5.4%, 4.8 and 12.2%, 20.5 and 11.2%, 10.9 and 12.8%, respectively,compared to R1LT and R1NT.Moreover, the activity of AI and ACP of R2LT and R2NT decreased by 7.2 and 8.7%, and 10.9 and 16.3%, respectively, compared to R1LT and R1NT.
3.5.Spikelet setting rate
TSPA affecting spikelet setting rate after LT treatment was shown in Table 1.Overall, LT treatment decreased the TSN of wheat, and FSN and SSR decreased significantly,while SSN increased significantly after LT treatment.At the same time, the TSPA increased TSN, FSN, and SSR and reduced the generation of SSN.Compared with R2LT and R2NT, the SSR of R1LT and R2LT decreased significantly by 23.9 and 21.6%, respectively.The SSR of R2LT was significantly 6.0% higher than that of R1LT.Temperature treatment had an extremely significant effect on TSN, FSN, SSN, and SSR.In contrast, the TSPA had a significant effect on FSN, SSN, and SSR, without a significant effect on SSN.
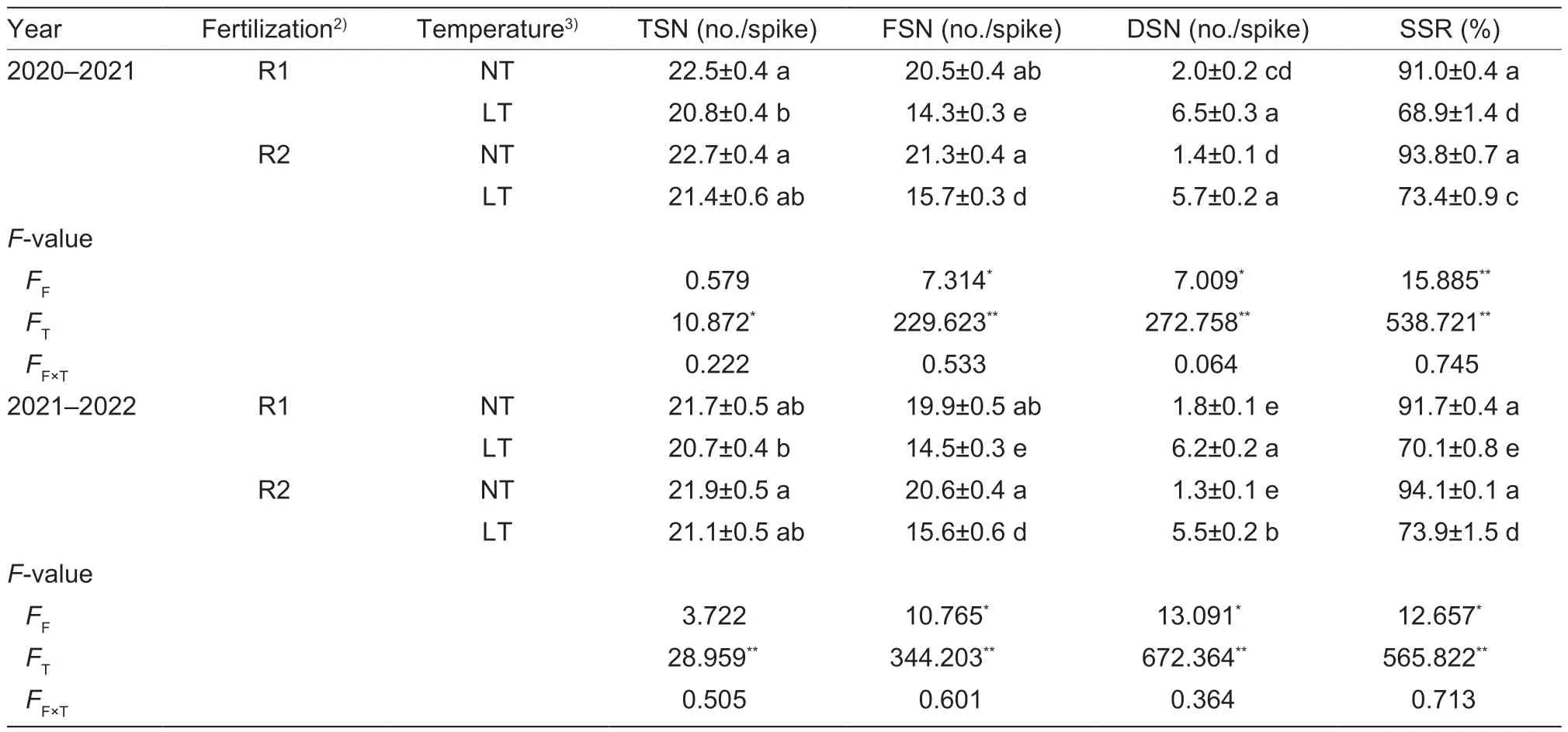
Table 1 Twice-split phosphorus application affecting spikelet setting rate after low-temperature treatment1)
3.6.Yield and yield components
As shown in Table 2, temperature treatment had an extremely significant effect on SN, GN, TGW, and yield,while fertilization, fertilization × temperature had no significant effect on yield and yield components.SN,GN, TGW, and yield were significantly decreased by LT treatment, whereas they were increased by TSPA.Compared with R1NT and R1LT, SN, GN and TGW of R2NT and R2LT increased by 2.8-3.4%, 1.1-7.2%,2.2-2.5%, respectively.Therefore, the TSPA resulted in a 7.4-9.4% increase in the final yield compared to the traditional phosphorus application.

Table 2 Twice-split phosphorus application affecting yield and its composition after low-temperature treatment1)
3.7.Correlation coefficients between spikelet setting characteristics, yield components, and leaf photosynthesis and spike carbon and nitrogen metabolism
The SSR and final yield correlation analysis revealed significant positive correlations withPn,Gs,Tr, SP, AI,and NR (Fig.6).These results indicated that SSR and yield were largely determined by the photosynthetic capacity and spike carbon and nitrogen metabolism.Moreover, there were significant negative correlations between DSN and both SSR and yield.There were also significant positive correlations between NR and GS with SP.Moreover, SS and SUC had positive correlations with SPS and SUS but negative correlations with AI.

Fig.6 Correlation coefficient matrix between photosynthetic rate (Pn), stomatal conductance (Gs), intercellular carbon dioxide concentration (Ci), transpiration rate (Tr), soluble sugar (SS),sucrose (SUC), proline (Pro), soluble protein (SP), sucrose phosphate synthase (SPS), sucrose synthetase (SUS), acid invertase (AI), nitrate reductase (NR), glutamine synthetase(GS), total spikelet number (TSN), fertile spikelet number(FSN), degenerated spikelet number (DSN), spikelet setting rate (SSR), spike number (SN), grain number (GN), 1 000-grain weight (TGW) and yield under different treatments.*, P≤0.05.
4.Discussion
4.1.Optimizing phosphorus application improved photosynthesis, spikelet setting, and yield formation in wheat under LT stress
Photosynthesis determines biomass production, which contributes to the yield formation of the crop, but it is vulnerable to LT stress (Sharmaet al.2020; Yanet al.2021; Xuet al.2022b).Wheat leaves drooped and shriveled after LT stress, especially under freezing stress, and the relative electrical conductivity increased significantly (Hanet al.2013; Liet al.2015; Zhanget al.2022).Following LT stress,Pn,Gs, andTrin leaves usually decreased whileCiincreased.Maet al.(2022)conducted LT treatment on wheat at different stages in spring.They pointed out that compared with the booting and flowering stages, the second leaf’s LT stress at the birth stage decreased leafPnmost seriously by 14.1-16.3%.Moreover, the photosynthetic parameter of leaves decreased continuously for 3 d after LT treatment, before gradual recovery.Venzhiket al.(2011) also pointed out that wheat leaves’ photosynthesis and electron transport tended to be stable about 4 d after LT stress,with chlorophyll content increasing and photosynthesis beginning to recover.Our results were consistent with previous results.As indicated by the findings in this study,thePnof wheat leaves significantly decreased by 29.8-33.5% on the day of LT treatment, andCisignificantly increased by 23.2-30.5%.At the same time, thePnof leaves reached the lowest level at 2 d after LT treatment and then gradually increased.In addition, LT stress inhibited the photosynthesis of wheat leaves by affecting the synthesis of photosynthetic pigments and the activity of photosynthetic enzymes (Yamoriet al.2014).
It was reported that phosphorus application could increase the activity of photosynthetic pigments and photosynthetic enzymes in leaves, thus improving the photosynthetic and light energy utilization rate (Radyet al.2018; Sahandiet al.2019).Moreover, optimizing phosphorus application can also maintain cell expansion by maintaining high leaf water potential, thus improving stomatal conductance and photosynthetic rate, alleviating the harm of LT stress on wheat leaf photosynthesis(Waraichet al.2011).Our results showed that the TSPA could improve thePn,Gs, andTrand reduceCiof wheat leaves after LT treatment.At the 8 d after LT, thePn,Gs,andTrof R2NT and R2LT were increased by 5.4 and 8.1%,5.0 and 7.1%, 5.2 and 7.3%, andCiwas decreased by 5.7 and 8.6%, respectively, compared with R1NT and R1LT.The effects of LT stress on the photosynthetic parameters of wheat leaves could be alleviated by the TSPA, and the effects became more evident at the end time of LT stress.
Moreover, the improvement of LT treatment was better than that of NT treatment.Due to the reduction of leaf photosynthesis and production of photosynthetic products,SSN per spike increased significantly after LT stress.This study showed that the TSPA can effectively reduce SSN and improve SSN per spike.This was mainly because photosynthesis can increase the input of photosynthetic products to the spikelet after optimizing phosphorus application to provide sufficient nutrition sources for spikelet development (Radyet al.2018; Asaiet al.2021).
4.2.Optimizing phosphorus application enhanced antioxidant capacity in wheat under LT stress
LT stress induces plant cells to produce a large amount of ROS, and excessive accumulation of ROS reacts with lipid peroxidation to produce membrane lipid peroxidation product, i.e., MDA.MDA content reflects the degree of cell membrane lipid peroxidation (Mahajan and Tuteja 2005; Hassanet al.2021).Under LT stress, plants must enhance their antioxidant capacity to maintain ROS balance, which is also an important manifestation of plant cold resistance (Yaoet al.2021).SOD can remove active oxygen and free radicals, POD can decompose the hydrogen peroxide produced, and CAT can remove excess active oxygen, protecting cells and enhancing stress resistance (Farhangi-Abriz and Torabian 2017).In the previous study, the MDA content of young wheat spikes was 65.0-87.5% higher under NT treatment(16°C) on the first day than that under LT treatment(-4-4°C), and the activities of SOD, POD, and CAT were 31.0-36.0%, 21.5-36.0% and 33.0-59.5% higher than that under NT treatment, respectively (Jianget al.2022).As revealed by our results, the activities of SOD, POD,and CAT of R1LT and R2LT were significantly increased by 45.1-51.8%, 48.0-56.1%, and 39.5-47.2% compared with R1NT on the day of LT treatment, respectively.Subsequently, the activity of antioxidant enzymes of R1LT and R2LT decreased sharply, lower than that of R1NT and R2NT.This may primarily occur when young wheat spikes perceived LT stress, and the antioxidant enzyme system was activated to reduce MDA content.However,the antioxidant system was damaged at 2-4 d after the end of LT stress, resulting in the reduction of SOD, POD and CAT enzyme activities.This may be because wheat underwent a process of cold acclimation after LT stress,increasing the accumulation of ROS and decreasing the activity of antioxidant enzymes (Sunet al.2006).Since then, wheat plants have recovered slowly under LT treatment, and the activity of antioxidant enzymes in young spikes increased, but was always lower than that under NT treatment.
Studies have shown that phosphorus application could improve the activity of antioxidant enzymes and reduce the accumulation of MDA in plants under adversity stress(Nieet al.2015; Tariqet al.2019).Bamagooset al.(2021)further pointed out that biochar coupling with phosphorus fertilization could effectively improve the activity of crop antioxidant enzymes and reduce the oxidation caused by temperature stress.This may be owing to the fact that the phospholipids in plant cell membranes play a crucial role in cold resistance, which can resist dehydration caused by LT stress and maintain the stability of biological membranes.Our research results revealed that the TSPA could reduce the accumulation of MDA in young wheat spikes and improve the activities of SOD, POD, and CAT.More specifically, 8 d after the end of LT treatment,the content of MDA in young wheat spikes decreased by 19.6%, and the activities of SOD, POD, and CAT increased by 6.0, 7.0, and 5.9% by TSPA, respectively.
4.3.Optimizing phosphorus application regulated carbon and nitrogen metabolism in wheat under LT stress
Coordination between carbon and nitrogen metabolism is crucial for crop growth, development, and stress response(Baoet al.2015; Liet al.2021).LT stress can negatively affect the carbon-nitrogen metabolism of wheat plants by reducing photosynthesis, reducing nitrogen absorption,increasing respiratory loss, and changing enzyme activities (Tayloret al.2004; Janmohammadiet al.2015;Dubeyet al.2021).Photosynthetic products mainly exist in the form of SUC and are transported to spikes,while the activities of SPS, SUS, and AI coordinates the synthesis and degradation of SUS (Halfordet al.2011;Saddheet al.2021).The activities of SPS and SUS increased significantly under LT treatment at the booting stage, resulting in enhanced accumulation in young spikes.Besides, LT stress also induced the accumulation of SUC, SP, and Pro in crop plants (Songet al.2015).In our study, the activity of synthetase (SPS and SUS)increased, while the activity of catabolic enzymes (e.g.,AI) decreased.And SS and SUC increased significantly on the day of LT treatment.We also found that the TSPA can improve the synthetase activity and carbohydrate content under both NT and LT treatments.Due to the reduction of NR and GS activities, SP decreased after LT stress.In addition, the TSPA on the day of LT treatment increased NR, GS, SP, and Pro by 9.7, 12.2, 5.8, and 4.9%, respectively.
At present, some studies have focused on the mechanism of optimizing phosphorus fertilizer application to improve crop carbon and nitrogen metabolism.Phosphorus is involved in adenosine triphosphate production and provides energy for carbon metabolism and nutrient transport (Amtmann and Armengaud 2009).Cultivation techniques such as optimizing phosphorus application can promote resource transport from source organs to sink organs through the phloem to maintain floret, spikelet development, and young spike growth(Jeonget al.2017; Wanget al.2018).Likewise, Wang Wet al.(2022) pointed out that LT and salicylic acid priming could coordinate carbon and nitrogen metabolism under freezing conditions by promoting the accumulation of Pro and SUC in wheat plants.This is because SS and Pro can reduce the harm of LT stress by scavenging ROS and stabilizing osmotic pressure (Saddheet al.2021).As a result, the TSPA could alleviate the damage of LT stress on the development of young spikes by increasing the activity of carbon and nitrogen metabolism synthesis enzymes and increasing the accumulation of carbohydrates, SP, and Pro in young wheat spikes.
The research on ACP activity is currently mainly focused on activating and utilizing acid phosphatase in phosphorus-deficient soil (Zuccariniet al.2020; Dinget al.2021).With increased ACP activity, plants can more efficiently hydrolyze organic phosphate compounds in the soil, releasing inorganic phosphate for root uptake (Yang and Yang 2021).In this study, ACP activity decreased after LT treatment.Compared with the traditional phosphorus application treatment, the activity of ACP under the TSPA was significantly reduced by 13.6%.This may be because the TSPA ensures the absorption and utilization of phosphorus by wheat plants and does not need to increase the ACP activity to activate more phosphorus.
5.Conclusion
LT stress during the anther interval stage reduced the photosynthetic characteristics of wheat leaves and slowed spike development.After LT stress, young wheat spikes undergo a process from response to damage to recovery.LT induced the accumulation of MDA in the young spikes,and the content of organic nutrients such as SS and SP gradually decreased in the young spikes, reducing SSR per spike.Among the effective strategies to alleviate the adverse effects of LT stress in wheat, optimizing phosphorus application is a meaningful and practical technology.In the current study, we confirmed that the TSPA could improve the photosynthetic characteristics of leaves and young spike development.With improved antioxidant capacity, the enzyme activities in the direction of carbon and nitrogen metabolite synthesis of young wheat spikes increased.TSPA increased the accumulation of SS, SUC, SP, and Pro, which could improve young wheat spike and spikelet development,leading to increased SSR and final yield.This paper provides a theoretical basis for improving crop resistance to stress and breeding stress-resistant and phosphorusefficient cultivars.
Acknowledgements
This work was supported by the Major Science and Technology Projects in Anhui Province, China(202003b06020021), the Natural Science Foundation of Anhui Province, China (2008085QC122), the Postgraduate Quality Engineering Project in Anhui Province, China (2022cxcysj066), and the Special Fund for Anhui Agriculture Research System, China.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendixassociated with this paper is available on https://doi.org/10.1016/j.jia.2023.09.013
 Journal of Integrative Agriculture2023年12期
Journal of Integrative Agriculture2023年12期
- Journal of Integrative Agriculture的其它文章
- Biotechnology of α-linolenic acid in oilseed rape (Brassica napus)using FAD2 and FAD3 from chia (Salvia hispanica)
- Analyzing architectural diversity in maize plants using the skeletonimage-based method
- Derivation and validation of soil total and extractable cadmium criteria for safe vegetable production
- Effects of residual plastic film on crop yield and soil fertility in a dryland farming system
- Identifying the critical phosphorus balance for optimizing phosphorus input and regulating soil phosphorus effectiveness in a typical winter wheat-summer maize rotation system in North China
- Characteristics of Mycobacterium tuberculosis serine protease Rv1043c in enzymology and pathogenicity in mice
