Application of heat-activated peroxydisulfate process for the chemical cleaning of fouled ultrafiltration membranes
Jiqi Ding, Holing Xio, Xiolong Hung, Yuji Zou, Zhimin Y,Songlin Wng,*, Pngcho Xi,*, Yongshng Chn, Jun M
a Yangtze River Basin Ecological Environment Monitoring and Scientific Research Center, Yangtze River Basin Ecological Environment Supervision and Administration Bureau, Ministry of Ecological Environment, Wuhan 430010, China
b School of Environmental Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, China
c Key Laboratory of Water & Wastewater Treatment (HUST), MOHURD, Wuhan 430074, China
d School of Civil and Environmental Engineering, Georgia Institute of Technology, Atlanta, GA 30332, United States
e State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150090, China
Keywords:Chemical cleaning Peroxydisulfate Heat Membrane fouling Ultrafiltration
ABSTRACT NaClO has been widely used to restore membrane flux in practical membrane cleaning processes, which would induce the formation of toxic halogenated byproducts.In this study, we proposed a novel heatactivated peroxydisulfate (heat/PDS) process to clean the membrane fouling derived from humic acid(HA).The results show that the combination of heat and PDS can achieve almost 100% recovery of permeate flux after soaking the HA-fouled membrane in 1 mmol/L PDS solution at 50 °C for 2 h, which is attributed to the changes of HA structure and enhanced detachment of foulants from membranes.The properties of different treated membranes are characterized by scanning electron microscopy (SEM),atomic force microscope (AFM), attenuated total reflection Fourier transform infrared spectroscopy (ATRFTIR), and X-ray photoelectron spectroscopy (XPS), demonstrating that the reversible and irreversible foulants could be effectively removed by heat/PDS cleaning.The filtration process and fouling mechanism of the cleaned membrane were close to that of the virgin membrane, illustrating the good reusability of the cleaned membrane.Additionally, heat/PDS which can avoid the generation of halogenated byproducts shows comparable performance to NaClO on membrane cleaning and high performance for the removal of fouling caused by sodium alginate (SA), HA-bovine serum albumin (BSA)-SA mixture and algae, further suggesting that heat/PDS would be a potential alternative for membrane cleaning in practical application.
Ultrafiltration (UF) technology has attracted extensive attention in the fields of chemical recovery, cell harvesting, drinking water production and wastewater treatment because of its high performance on the retention of particulates, colloids and microbiological organisms without the addition of chemicals [1,2].However, membrane fouling during the filtration process could result in increased energy consumption and declined membrane flux, which is still the main obstacle for the application of UF technology [3].
Nowadays, many strategies have been developed to prevent or mitigate membrane fouling, such as the pretreatment of feed water, development of anti-fouling membranes and optimization of operating conditions [4–6].In practical membrane process, membrane fouling is still inevitable during the long-term filtration process, and periodical membrane cleaning needs to be conducted for the removal of foulants and recovery of membrane flux [1].Acids,bases, oxidants, chelating agents, surfactants and enzymatic components are the common chemicals applied in membrane cleaning for the removal of different foulants [7].Among these agents,sodium hypochlorite (NaClO) is a widely adopted oxidant for removing organic and biological foulants because of its strong oxidizing ability and low cost [8].However, exposure to NaClO cloud also change some intrinsic membrane properties such as hydrophilicity, pore size distribution and zeta potential, which may reduce the quality of permeate and the lifespan of membrane [9].Furthermore, as a commonly used disinfectant in water/wastewater treatment processes, NaClO can react with organic and micro-biological substances to generate halogenated byproducts with high toxicity.Our recent studies show that NaClO cleaning of UF membranes fouled by humic acid or algae can form a variety of chlorinated byproducts [10,11].Liuet al.investigated the generation of halogenated organics during the online-cleaning of fouled membranes by NaClO, finding that the amounts of generated total organic halogen during this process was estimated to be 648.45 kg per year in China [12,13].Due to the inefficient treatment of the cleaning solution, a substantial portion of these halogenated byproducts would be inevitably discharged into natural water bodies to increase the potential risks for both environment and human health.Therefore,the modification of chemical cleaning process by NaClO should be considered, and it is urgent to explore other oxidation processes for membrane cleaning.
In recent years, peroxides including peroxydisulfate (PDS),peroxomonosulfate (PMS), peracetic acid and hydrogen peroxide(H2O2) have been broadly applied in water/wastewater treatment processes [14–16].These peroxides can be activated by ultraviolet(UV), ultrasound, heat, base and transition metals to produce sulfate radical and hydroxyl radical in advanced oxidation processes(AOPs) for the efficient degradation of organic contaminants [17–19].Additionally, AOPs such as Fe(II)/PDS, Fe(II)/PMS, UV/PDS and heat/PDS have also been utilized to mitigate membrane fouling through the pretreatment of feed water, in which the generated reactive species could greatly change the characteristics of typical foulants and reduce their fouling potential in the following filtration process [20–22].Recently, typical AOPs including H2O2-MnO2system and Fe(II)/PMS process were reported to be useful options for the chemical cleaning of fouled membranes, which achieved efficient recovery of membrane flux [23,24].Due to the absence of halogen atom, the application of these peroxides produces much fewer toxic halogenated byproducts than chlorine.Except for transition metals (MnO2and Fe(II)), heat is another unique method for the activation of PDS without the addition of other chemicals.Additionally, the temperature of waste heat generated in many industrial processes usually ranges from 60 °C to 120 °C [25], which can be considered as the energy source for PDS activation to lower the cost of heat/PDS process [22].Previous studies reported that mass transfer of cleaning agents from the bulk solution to the membrane surface is an important limiting factor during the chemical cleaning process, which could be enhanced by the increase of operating temperature [26,27].Thus, the application of heat/PDS may have a synergetic effect for foulant removal in chemical cleaning process.However, as far as we know, very limited study has been conducted in this field.
The chemical reagents and experimental procedures were present in Texts S1 and S2 (Supporting information).The cleaning efficiency was evaluated in the form of flux recovery ratio and fouling resistance removal (shown in Text S3 in Supporting information).The analytic methods were offered in Text S4 (Supporting information).The schematic diagram of experimental set-up was shown in Fig.S1 (Supporting information).
As shown in Fig.1a and Fig.S2 (Supporting information), the combination of HA and Ca2+resulted in an apparent flux decline with the final normalized flux (J/J0) reducing to approximately 0.51,and the reversible and irreversible fouling resistance were calculated to be 0.34 and 2.99×1011m–1, respectively.This result indicates that irreversible fouling played the dominant role in the fouling process, suggesting that further chemical cleaning was required for membrane flux recovery in this process.
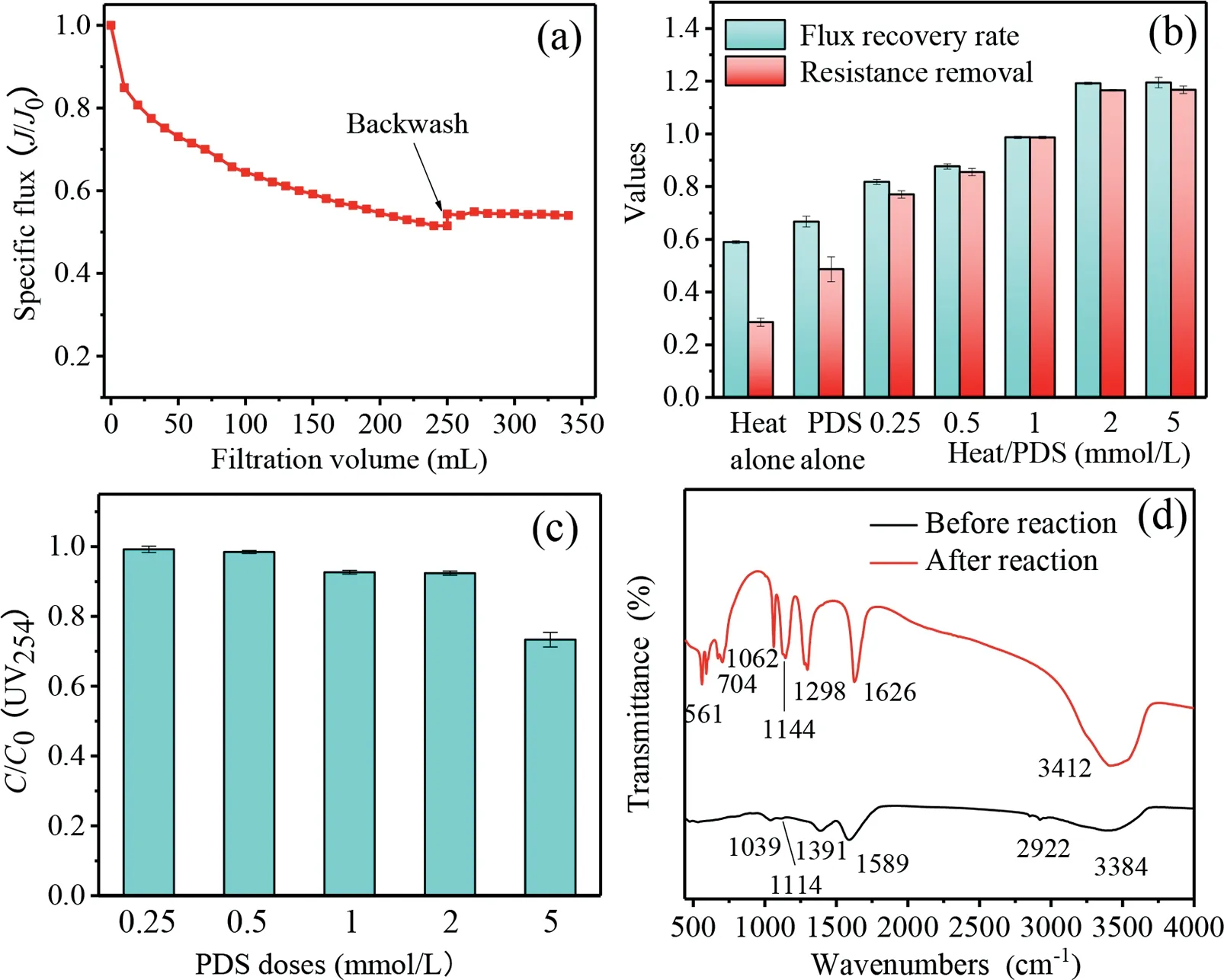
Fig.1.(a) Effect of HA fouling and backwash on membrane flux decline.(b) Performance of different cleaning processes for membrane flux recovery and fouling resistance removal.(c) Variation of UV254 absorbance after directly treating HA by heat/PDS and (d) Variation of FTIR spectra after directly treating HA by heat/PDS.Conditions: feed HA solution 10 mg/L, molecular weight cutoff 50 kDa, cleaning time 2 h, temperature 50 ± 1 °C, initial pH 7.0 ± 0.2, PDS dose 1 mmol/L for PDS alone.
As shown in Fig.1b, the membrane flux recovered to 59% and 67% ofJ0, while 29% and 49% of the fouling resistance were removed after cleaning by heat alone (50 °C) and PDS alone (1 mmol/L), respectively, indicating that foulants deposited on the membrane could be partly removed by these two cleaning processes.The application of heat/PDS process (50 °C) exhibited much better performance for membrane fouling removal.The flux recovery ratio and resistance removal increased from 82% and 77% to almost 100% with the PDS dose ranging from 0.25 mmol/L to 1 mmol/L, suggesting that high temperature can not only improve the cleaning efficiency, but also can reduce the PDS dosages.It should be noted that the recovery ratio of flux further increased to about 119% as the addition of PDS was raised to higher than 2 mmol/L, revealing that the flux of the cleaned membrane was higher than that of the virgin membrane.Correspondingly, about 117% of total fouling resistance was removed after heat/PDS treatment with the PDS dosage higher than 2 mmol/L, showing that the membrane intrinsic resistance was reduced.Similar phenomena could also be found when the fouled membranes were exposed to NaClO or HCl during the chemical cleaning processes, which could be attributed to the enlargement of membrane pore size or the increase of membrane hydrophilicity [28,29].These results indicate that the heat/PDS with PDS dose ranging from 0.25 mmol/L to 1 mmol/L could be applied for the cleaning of HA-fouled membrane.
To investigate the released foulants after different cleaning processes, the cleaning of HA-fouled membrane by heat/PDS system were studied, and the variations of UV254absorbance in the cleaning solution were monitored.As shown in Fig.S3a (Supporting information), UV254absorbance in cleaning solutions treated by heat alone and PDS alone were quite low, suggesting limited existence of HA in cleaning solutions.With PDS dose increasing from 0.25 mmol/L to 2 mmol/L, UV254absorbance apparently increased from 0.067 cm-1to 0.175 cm-1.After subtracting UV254absorbance of sole PDS at different dose in Fig.S3b (Supporting information), it can be concluded that the heat/PDS treatment caused easier release of HA from the membrane surface and pores, which is in accordance with the results in a previous study that chemical cleaning of fouled membrane by Fe(II)/PMS could reduce the adhesion force between foulants and membrane [24].

Fig.2.SEM images of different treated-membranes: (a) virgin membrane, (b) HA fouled membrane, (c) membrane cleaned by heat/PDS (0.25 mmol/L), (d) membrane cleaned by heat/PDS (0.5 mmol/L), (e) membrane cleaned by heat/PDS (1 mmol/L), (f) membrane cleaned by heat/PDS (2 mmol/L), (g) membrane cleaned by heat/PDS (5 mmol/L).Conditions: feed HA solution 10 mg/L, molecular weight cutoff 50 kDa, cleaning time 2 h, temperature 50 ± 1 °C, initial pH 7.0 ± 0.2.
To verify the influence of heat/PDS on HA properties, experiments were conducted by applying heat/PDS system to directly treat HA solution.As can be seen in Fig.1c, the decline of UV254absorbance in HA solution was negligible at 0.25 mmol/L PDS,which was 27% when the PDS dose reached 5 mmol/L.UV254represents aromatic chromophores and unsaturated bonds in NOM[30], and the reduction of UV254absorbance indicates the structural damage of HA.FTIR spectra was further performed to analyze the structure change of HA after treatment by heat/PDS.Fig.1d shows that characteristic peaks of HA significantly changed after treatment.Compared with the untreated HA, obvious absorbance peaking at 500-1100 cm-1and around 1298 cm-1in the spectra of HA after reaction could be attributed to the presence of PDS (Fig.S4 in Supporting information).Then the disappearance of characteristic peaks at about 2920 cm-1and 1400 cm-1could be found after reaction, which were related to the typical hydrophobic groups of HA including the C–H, C–H2and C–H3stretching of alkyl structures and the C–H deformation of aliphatic, respectively [31,32].However, the intensities of characteristic absorbance peaks of hydrophilic groups at around 1100 cm-1representing the C–O stretching vibration in esters, ethers and phenols, at about 1600 cm-1corresponding to the C=O stretching vibration of carboxyl and ketones/quinones, and at round 3400 cm-1representing OH groups were significantly enhanced after reaction, respectively [23,32,33].These results suggest that the oxidation of HA by heat/PDS could make HA substances more hydrophilic, which would facilitate the shift and detachment of foulants from the membrane and then increased the cleaning efficiency.
The surface morphologies of virgin, fouled, and cleaned membranes were observed by an SEM.As represented in Fig.2a, a clean and smooth surface of the virgin membrane was found.When the membrane was fed with HA solution, foulants were deposited to form a dense and compact cake layer on the membrane surface(Fig.2b).While the PDS dose was 0.25 mmol/L in the heat/PDS process, it can be seen in Fig.2c that chain flocs of fouling materials appeared on the membrane surface, which indicates that the surface fouling layer was partly destroyed and fragmentized during the cleaning process.With the increase of PDS dose to 0.5 and 1 mmol/L, the foulants were broken into smaller irregular agglomerated fractions and had a more diffused distribution on the membrane surface (Figs.2d and e).More SEM images with high magnification in Fig.S5 (Supporting information) also verified that the size of the agglomerated flocs on the membrane surface decreased with PDS dose increasing from 0.25 mmol/L to 1 mmol/L.When heat/PDS with PDS dosage no less than 2 mmol/L was applied, negligible foulants were found on the membrane surface(Figs.2f and g).These results demonstrate that the performance of heat/PDS for foulants removal from the membrane was improved with the elevation of PDS dose, which is consistent with the increased membrane flux recovery, resistance removal and HA release in the cleaning solution.However, the membranes treated by over-dosed PDS (2 and 5 mmol/L) seemed to have rougher surface compared to the virgin membrane, which is accompanied by the excessive recovered water flux of cleaned membrane.AFM, ATRFTIR and XPS were also applied to analyze the change of membrane surface before and after cleaning, which were detailly discussed in Text S5 (Supporting information), demonstrating the high removal efficiency of foulants after chemical cleaning by heat/PDS.

Fig.3.(a) Comparison of membrane fouling between untreated and cleaned membranes.(b) Fouling ratios of untreated and cleaned membranes after HA fouling.Conditions: feed HA solution 10 mg/L, molecular weight cutoff 50 kDa, cleaning time 2 h, temperature 50 ± 1 °C, initial pH 7.0 ± 0.2.
The influencing factors including solution pH and temperature on the cleaning efficiency of HA-fouled membrane by heat/PDS were also evaluated (Fig.S6 in Supporting information) and discussed in Text S6 (Supporting information).Based on the results and discussion, it is achieved that alkaline condition benefit the permeate flux recovery and the resistance removal with damaging the membrane structure.Therefore, neutral condition would be a good choice for the cleaning of HA-fouled membrane by heat/PDS process.As high temperature benefits the PDS activation and the diffusive mass transfer rate, increasing trend of permeate flux recovery and resistance removal was found in the temperature range of 40–70 °C.But the increasing rate gradually decreased and a good cleaning efficiency could be achieved even at 50 °C.
The fouling behaviors of virgin and cleaned membranes were comparatively evaluated through the repeated filtration of HA solution.The filtration of HA solution resulted in a significant flux decline for the virgin membrane, and the normalized flux at the end of the second filtration cycle decreased to around 34% (data not shown).Fig.3a shows that heat/PDS cleaning could improve the permeate flux, and the performance was enhanced with the increase of PDS dose.For instance, the terminal normalized flux during the second filtration cycle increased from 49% to 58% when the initial PDS concentration rose from 0.25 mmol/L to 5 mmol/L.The whole fouling ratios for different treated membranes are illustrated in Fig.3b, showing that the fouling ratio increased from 32%to 46% with PDS dose increasing from 0.25 mmol/L to 1 mmol/L.The fouling ratio and fouling curve of the membrane cleaned by heat/PDS at 1 mmol/L PDS were quite similar to that of the virgin membrane, demonstrating that the cleaned membrane could be reused with little change of membrane fouling behavior.However,the fouling ratio was further elevated to 61% when the heat/PDS process with a PDS dose of 5 mmol/L was applied, which evidences that the membrane had a worse anti-fouling performance.This was in accordance with the result in previous studies that the excess exposure to NaClO could increase the membrane fouling potential [34,35].It should be noted that membranes with higher surface roughness were easier to be fouled because that the particles approaching the membrane surface were more likely to be trapped in the valleys of the rough surface [36].Thus, the slight increase of membrane surface roughness after chemical cleaning with overdosed PDS (Fig.S7d in Supporting information) might partly account for the decreased anti-fouling performance of the cleaned membrane.
To further investigate the influence of chemical cleaning on the fouling mechanisms caused by HA, four classic filtration models were applied to fit the experimental data.The fouling models including complete blocking, standard blocking, intermediate blocking and cake filtration were calculated according to the equations described in Table S1 (Supporting information) [37,38], and the correlation coefficient values (R2) were represented in Table S2(Supporting information).For the virgin membrane, theR2values were 0.8530, 0.9999, 0.9151, and 0.9588 for complete blocking, standard blocking, intermediate blocking and cake filtration,respectively.This illustrates that the fouling mechanism for HA filtration was mainly governed by standard blocking and cake formation, which was followed by the possible participation of intermediate blocking.Similar results could also be found in a previous study [21].When the membranes cleaned by heat/PDS (PDS ≤0.5 mmol/L) were used for the filtration of HA, theR2values for models increased except for standard blocking.This indicates that standard blocking played a decreased role during the fouling formation,which might be due to the presence of residual foulants on the cleaned membrane surface and pores (Figs.2c and d).With the further increase of PDS dose to 1 mmol/L, theR2values for complete blocking, intermediate blocking and cake filtration decreased obviously, and the fouling mechanism was still governed by standard blocking and cake formation.This result proves that the chemical cleaning by heat/PDS at 1 mmol/L PDS did not change the fouling mechanism.
To evaluate the application potential of the heat/PDS process in membrane cleaning, three other cleaning agents including HCl,NaOH and NaClO were selected to treat fouled membranes.As illustrated in Fig.4a, HCl and NaOH played a limited role in the foulants removal with the flux recovery ratios being 60% and 65%,respectively.Poor cleaning efficiencies by HCl and NaOH could also be verified by the low fouling resistance removal of 31% and 45%, respectively.The flux recovery ratio and resistance removal reached 86% and 84%, respectively, when 0.5 mmol/L NaClO was applied to chemically clean the fouled membrane for 2 hours,which was comparable to that achieved by heat/PDS with a PDS dosage of 0.5 mmol/L.Unfortunately, the cleaning of HA-fouled membranes by NaClO can result in the formation of toxic halogenated byproducts.Therefore, the heat/PDS process would be a promising alternative to replace NaClO for membrane cleaning.
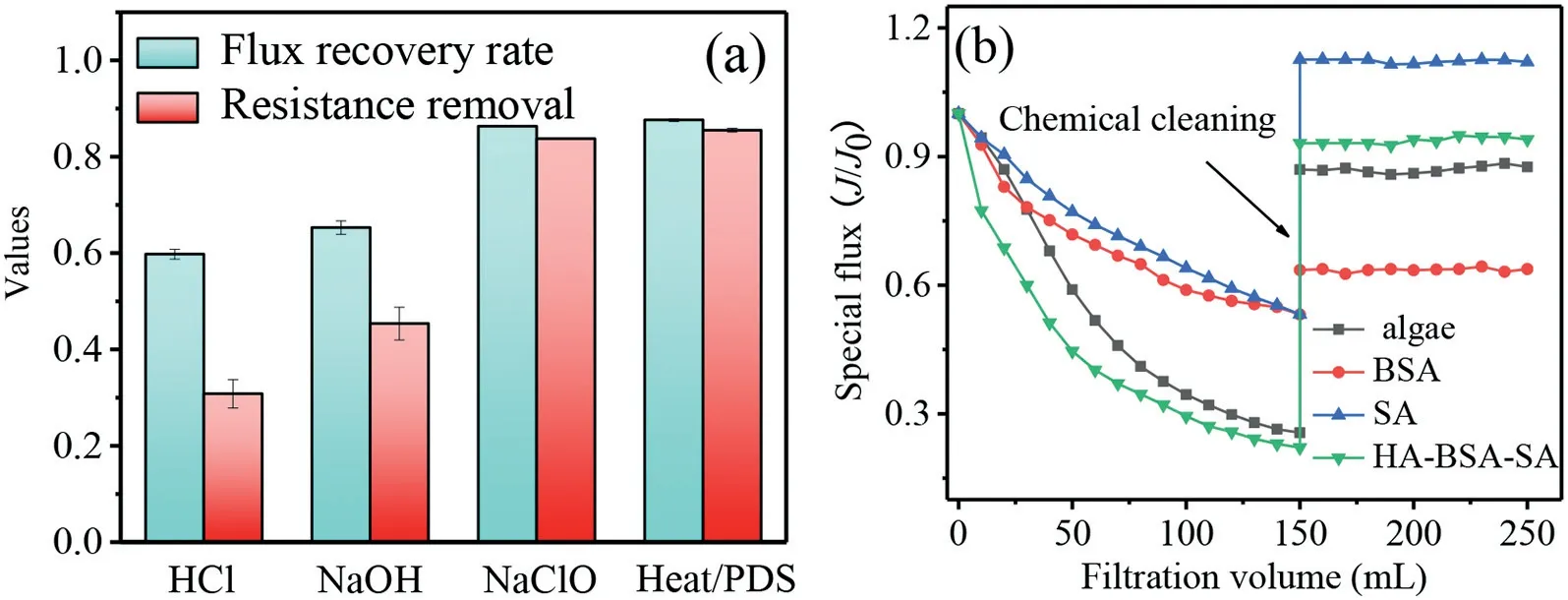
Fig.4.(a) Effect of cleaning agents on membrane flux recovery and fouling resistance removal.Filtration conditions: feed HA solution 10 mg/L, molecular weight cutoff 50 kDa.Cleaning conditions: cleaning time 2 h, temperature 50 ±1 °C, pH 7.0 ± 0.2.HCl=NaOH=NaClO=PDS=0.5 mmol/L.(b) Performance of heat/PDS for the cleaning of membrane fouled by BSA, SA, HA-BSA-SA mixture and algae-laden water.Filtration conditions: feed SA solution 5 mg/L, BSA solution 5 mg/L, HA=BSA=SA=10 mg/L in HA-BSA-SA mixture, cell concentration of 2×106 cells/mL in algae-laden water.Cleaning conditions: cleaning time 2 h, temperature 50 ± 1 °C, pH 7.0 ± 0.2.
To comprehensively evaluate the practical potential of heat/PDS for membrane cleaning, heat/PDS was employed to clean the membranes fouled by other typical foulants including SA, BSA, HABSA-SA mixture and algae-laden water, and the rejection rate of membrane before and after cleaning was also evaluated.As illustrated in Fig.4b and Fig.S8a (Supporting information), the filtration of SA, BSA, HA-BSA-SA mixture and algae-laden water resulted in serious flux decline with final normalized flux (J/J0) values at 0.52, 0.53, 0.22 and 0.26, respectively.After the chemical cleaning by heat/PDS, the flux of SA-fouled and BSA-fouled membrane was recovered to 1.12 and 0.63, respectively, indicating that SA was easily removed by heat/PDS, which was contrary to BSA.Previous studies have reported that high temperature and oxidation by chemicals could induce the misfolding and aggregation of proteins [4,39].The low cleaning efficiency of BSA-fouled membrane by heat/PDS might be attributed to the enhanced crosslinks in BSA aggregates, which was difficult to be removed from membrane surface and pores [40].The flux recovery ratio and resistance removal of membrane fouled by HA-BSA-SA reached 0.94 and 0.98,respectively, revealing that the organic composite fouling which was commonly existed in practical water was easily removed after cleaning by heat/PDS.In addition, fouling caused by algae-laden water could also be effectively removed by heat/PDS with flux recovery ratio and resistance removal being 0.87 and 0.95, respectively.Thus, it could be concluded that heat/PDS cleaning was a promising method for the removal of SA, HA-BSA-SA and algae fouling.Fig.S8b (Supporting information) shows that rejection rate of virgin and cleaned membranes for BSA and SA had no significant change (P >0.05), while membrane rejection rate for HABSA-SA was slightly decreased from 81.8% to 80% (P <0.05) after cleaning, which was still kept in a high level.These results show that heat/PDS cleaning has limited influence on the membrane rejection performance, and the cleaned membrane can be reused in water treatment.
This study developed a novel heat/PDS process to clean the HAfouled membranes, and the cleaning efficiency was promoted with the increase of PDS dose.Heat/PDS cleaning could improve the hydrophilicity of HA, which was accompanied by the effective removal of reversible and irreversible foulants from the membranes.The heat/PDS process exhibited a comparable cleaning efficiency to NaClO without forming toxic halogenated byproducts, which was more effective than acid and alkaline cleaning.Heat/PDS also showed high cleaning performance for SA, HA-BSA-SA and algae fouling.When heat/PDS is applied in practice for membrane cleaning, waste heat in industrial processes and solar energy will be an economical choice for heating water, which will efficiently reduce the cost of cleaning process.Thus, heat/PDS would be a promising choice to replace NaClO for the chemical cleaning of membranes fouled by organic compounds.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the Natural Science Foundation of China (Nos.52070081, 51578258 and 51878308) and the National Key Research and Development Program of China (No.2022YFC3203500).We also gratefully thank the analysis offered by the Analytical and Testing Center of HUST.The valuable work of the editor and anonymous reviewers is also appreciated.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.108316.
 Chinese Chemical Letters2023年10期
Chinese Chemical Letters2023年10期
- Chinese Chemical Letters的其它文章
- Tribute text in memoriam of James N.Seiber (1940–2023)
- Recent advances in MXenes-based glucose biosensors
- Oxidative cyclopalladation triggers the hydroalkylation of alkynes✩
- An integrated supramolecular fungicide nanoplatform based on pH-sensitive metal–organic frameworks
- Probing the effect of nitrate anion in CAN: An additional opportunity to reduce the catalyst loading for aerobic oxidations✩
- Nickel-catalyzed reductive coupling reaction of monofluoroalkyl triflates with alkyl carboxylic acids toward the synthesis of α-alkyl-α-fluoro-alkylketones✩
