Resolving a nearly 90-year-old enigma: The rare Fagus chienii is conspecific with F.hayatae based on molecular and morphological evidence
Dan-Qi Li ,Lu Jiang ,Hua Liang ,Da-Hai Zhu ,Deng-Mei Fan ,Yi-Xuan Kou ,e,f ,Yi Yang ,**,Zhi-Yong Zhang ,e,f,*
a College of Forestry, Jiangxi Agricultural University, Nanchang 330045, China
b Laboratory of Subtropical Biodiversity, Jiangxi Agricultural University, Nanchang 330045, China
c Lushan Botanical Garden, Jiangxi Province and Chinese Academy of Sciences, Jiujiang 332900, China
d Longxi-Hongkou National Reserve, Chengdu 611830, China
e Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Guangxi Normal University), Ministry of Education, Guilin 541004, China
f Guangxi Key Laboratory of Landscape Resources Conservation and Sustainable Utilization in Lijiang River Basin, Guilin 541006, China
Keywords:Conspecific Fagus chienii F.hayatae Phylogenetics Morphology Conservation
ABSTRACT Taxonomic uncertainties of rare species often hinder effective prioritization for conservation.One such taxonomic uncertainty is the 90-year-old enigma of Fagus chienii.F.chienii was previously only known from the type specimens collected in 1935 in Pingwu County of Sichuan Province,China,and has long been thought to be on the verge of extinction.However,morphological similarities to closely related Fagus species have led many to question the taxonomic status of F.chienii.To clarify this taxonomic uncertainty,we used the newly collected samples to reconstruct a molecular phylogeny of Chinese Fagus species against the phylogenetic backbone of the whole genus using seven nuclear genes.In addition,we examined nine morphological characters to determine whether F.chienii is morphologically distinct from its putatively closest relatives (F.hayatae, F.longipetiolata,and F.lucida).Both morphological and phylogenetic analyses indicated that F.chienii is conspecific with F.hayatae.We recommended that F.chienii should not be treated as a separate species in conservation management.However,conservation strategies such as in situ protection and ex situ germplasm preservation should be adopted to prevent the peculiar “F.chienii” population from extinction.
1.Introduction
Accurate species delimitation and identification are essential for biodiversity conservation and sustainable use of economically important plants,especially in rare taxa that are taxonomically uncertain due to a lack of rigorous research (Thomson et al.,2018;Ding et al.,2019;Nic Lughadha et al.,2019;Cheng et al.,2021).Erroneous classification and misidentification can leave out endangered species that should otherwise be protected(Gibson et al.,2019).Conversely,management action that results from incorrect identification of species wastes resources and funding(Solow et al.,2011).For instance,Torreya grandisvar.jiulongshanensisZ.Y.Li,Z.C.Tang &N.Kang (Kang and Tang,1995) is listed as a Category II protected plant in the Chinese List of Wild Plants (http://www.forestry.gov.cn/main/5461/20210908/162515850572900.html).However,a recent phylogenetic study clearly demonstrated that this endangered plant is a natural hybrid betweenTorreya jackiiChun andT.grandisFort.ex Lindl.,raising doubts about its validity as a key protected plant species (Kou et al.,2017).
FagusL.is an economically important genus that consists of 12 members of deciduous trees(Shen,1992;Peters,1997;Jiang et al.,2022).China accommodates five beech species in subtropical regions between the Qinling Mountains and the Nanling Mountains:F.englerianaSeem.ex Diels (1900),F.longipetiolataSeem.(von Seemen,1897),F.lucidaRehd.&Wils.(Rehder and Wilson,1916),F.hayataePalib.ex Hayata (1911) andFagus chieniiCheng (Cheng,1935;Zhang and Huang,1998;Fang et al.,1999;Denk,2003;Jiang et al.,2022).Although four of these species are widespread in subtropical China,F.chieniihas an exceptionally narrow distribution and is only found in Pingwu County,northern Sichuan(Cheng,1935).Because no specimens have been collected since its establishment based on a single collection in 1935,F.chieniiis believed to be in danger of going extinct(Peters,1997;Guo and Werger,2010).To prevent the extinction of this rare plant,the People's Government of Sichuan Province listedF.chieniias a key protected species in 2016.
The scarcity of field collections,however,has led to the taxonomic uncertainty ofF.chienii(Huang et al.,1999).Cheng (1935)provided a brief description ofF.chieniiand noted that it might be of hybrid origin because it shares similar involucres and scales withF.longipetiolatawhile resemblingF.lucidain leaf blade shape and size.Chang and Huang(1988)suspected thatF.chieniimight be an ecotype ofF.lucida,but still considered it as a mysterious finding with a disputed taxonomic rank.Furthermore,F.chieniiwas not recorded by the authors ofFlora of China(Huang et al.,1999),possibly owing to limited information and the resultant taxonomic uncertainty.To date,the species status ofF.chieniiremains an enigma that needs to be resolved using newly available experimental materials.
To resolve the taxonomic status ofF.chienii,we reexamined the type specimens and conducted extensive field investigations in the type locality from 2019 to 2021.A local news report from Sichuan Province led us to a rediscovered population ofF.chieniiin Laohegou Nature Reserve in Pingwu County in 2021 (Li,2016).We used the new collections and samples to reconstruct the molecular phylogeny of ChineseFagusmembers against the phylogenetic backbone of the whole genus(Jiang et al.,2022)based on sequences of seven nuclear DNA markers.We also determined whetherF.chieniiis morphologically distinct from its closely relatives(F.hayatae, F.longipetiolata,andF.lucida) through statistical analyses of nine morphological characters.
2.Materials and methods
2.1.Phylogenetic analyses
Twelve species ofFaguswere examined in this study,withCastanea seguiniiDode used as outgroup.To achieve good coverage,no less than four individuals from separate locations for each Chinese species were included(Fig.1).Samples from 10 individuals ofF.chieniiwere collected.To enlarge the intraspecific representation ofF.hayatae,which was assumed to be conspecific withF.chienii,an additional 10F.hayataeindividuals were included.Seven representative loci from Jiang et al.(2022)were amplified and sequenced forF.chieniiand the newF.hayataesamples,i.e.,F128,F138,P4,P14,P37,P52,and P72 (Table S1).We did not use chloroplast DNA sequences in this study because chloroplast haplotypes are shared frequently withinFagusand are considered ineffective in phylogenetic reconstructions of the genus (Zhang et al.,2013).DNA extraction,PCR amplification,and DNA sequencing followed Jiang et al.(2022).The DNA sequences of additional accessions were from Jiang et al.(2022) (Table S2).Voucher information of the additional samples are shown in Table S2 and the new sequence data of each locus were deposited in GenBank under accession numbers: ON584566-ON584685.

Fig.1. The distribution map of DNA and herbarium samples of Fagus hayatae and F.chienii in this study.Note that F.hayatae has a fragmented distribution in mainland China and Taiwan.1,Pingwu;2,Qinchuan;3,Micangshan;4,Guangwushan;5,Xipingcun;6,Puyuancun;7,Tianshuxia;8,Shennongjia;9,Dalaoling;10,Houhecun;11,Hupingshan;12,Qinliangfeng;13,Sihaishan;14,Lalashan;15,Beichatianshan;16,Tongshan.Relevant citations on the specimens are shown in Tables S2 and S3.
Raw chromatograms were manually checked and aligned using Sequencher v.5.4.6(Gene-Codes Corporation,Ann Arbor,MI,USA),and then refined manually in MEGA 5.05.Phylogenetic analyses were performed based on the concatenated data set using maximum likelihood(ML)and Bayesian inference(BI)methods.ML analysis was carried out using RaxML v.8.2.12 (Stamatakis,2014)software in Linux system with 1000 bootstrap replicates.BI analysis was conducted in MrBayes v.3.2.6(Ronquist et al.,2012).The bestfitting nucleotide substitution models (HKY+I) were decided by jModeltest 2.1.7(Darriba et al.,2012)with the Bayesian information criterion (BIC).Posterior probabilities were approximated by sampling trees using a variant of the Markov Chain Monte Carlo(MCMC) method.A total of 20,000 trees were sampled every 1000th generation.The first 5000 generations (about 25%) were eliminated as “burn-in”.Average standard deviation of split frequencies was less than 0.01.All the phylogenetic trees were visualized in FigTree v.1.4.4 and edited in Adobe Illustrator 2020(Fig.2).To account for incomplete lineage sorting,which can have a major influence on phylogenetic reconstruction,we used a multispecies coalescent approach in StarBEAST2 0.15.5 (Ogilvie et al.,2017,see details in Jiang et al.,2022)to infer the species tree using the seven nuclear loci.

Fig.2. The maximum likelihood (ML) tree and Bayesian inference (BI) tree based on seven concatenated nuclear genes (4102 bp) for the phylogenetic analysis of Fagus taxa.Numbers above branches are statistical support values for ML and BI.Bold font represents Fagus chienii.Clade I: subgen. Fagus,clade II: subgen. Engleriana.
2.2.Morphological studies
Digital images ofF.chieniiand its putatively closest relatives(F.hayatae, F.longipetiolata,andF.lucida) were derived from the Chinese Virtual Herbarium (https://www.cvh.ac.cn/),the web of Plants of Taiwan (https://tai2.ntu.edu.tw),the Global Biodiversity Information Facility (https://www.gbif.org/) and JSTOR Global Plants (https://plants.jstor.org).ForF.chienii,we examined twelve available specimens in total,seven of which were collected in this study from Pingwu County (the type locality);the remaining five samples were holotype and isotypes with mature fruits deposited in the Royal Botanic Gardens,Kew,South China Botanical Garden,CAS,Chinese National Herbarium,Royal Botanic Garden Edinburgh and New York Botanic Garden,respectively(Table S3).Additionally,we measured 38 specimens ofF.hayatae,and 15 specimens each ofF.longipetiolataandF.lucida.Specimens of the four species were selected to provide a broad geographical representation across their native ranges(Fig.1).
Nine morphological characters,i.e.,leaf length,leaf width,ratio of leaf length/width,leaf petiole length,leaf teeth length,number of secondary veins,cupule peduncle length,bract length,and cupule length,were measured (Fig.3A).According to Shen (1992),these characters are key to distinguishing ChineseFagusspecies.ImageJ software was applied to the examination of characters under investigation.Three well-preserved leaves and/or fruits per specimen were randomly selected for examination.Detailed measurements and voucher information are available in Table S3.

Fig.3. Morphological analyses of Fagus chienii and related species.A,Morphometric measurements in this study.L1: leaf length,L2: leaf petiole length,L3: leaf width,L4: cupule peduncle length,L5:bract length,L6:cupule length,L7:leaf teeth length;B,Scatter plot of the first two principal components(PCs)of PCA based on seven morphological variables of Fagus chienii collected in this study(Fagus chienii),F.chienii types(F.chienii-TYPE),F.hayatae,F.longipetiolata,and F.lucida.Each dot represents a specimen;C and D,Scatter plot of the first two dimensions of PCoA.We treated the specimen of F.hayatae, F.chienii and F.chienii-TYPE as one group (C) and three separate groups (D),respectively. R2 is the proportion of sum of squares from the total in Adonis analysis. P is the significance value of R2.
The values of the nine characters were normalized by mean and standard deviation.To avoid collinearity in subsequent analyses,highly correlated characteristics were then eliminated by Pearson correlation analysis conducted in the IBM SPSS Statistics 26.Principal Component Analysis (PCA) was performed in ORIGIN 2021 to investigate the relationships amongF.chienii, F.hayatae,F.longipetiolata,andF.lucida.The clustering analysis of specimen samples in the scatter plot was tested by discriminant analysis of the first two PCs.
To further test the reliability of the clustering analysis,a Principal Coordinates Analysis (PCoA) using Bray-Curtis dissimilarity index was performed and graphically visualized byVeganpackage andggplot2package in R v.4.1.2 software(Oksanen et al.,2015).We treated the specimen ofF.hayatae, F.chieniicollected in this study(denoted asF.chieniiin Fig.3)andF.chieniitypes(F.chienii-TYPE in Fig.3) as one group and three separate groups,respectively.The significance level of pairwise comparisons between groups was computed based on Adonis test bypairwiseAdonispackage(Martinez Arbizu,2020)according to Bonferroni adjustedP-values.
In addition,comparisons of nine morphological characters between the four beech species were conducted and their significances were evaluated byt-test using theggsignifpackage in R v.4.1.2 software (RcoreTeam,2021).
3.Results
3.1. Molecular phylogenetic relationships of Fagus chienii
The aligned data set consisted of 52 accessions,including twelve taxa ofFagusand one outgroup species,with a concatenated sequence length of 4102 bp.WithinFagus,the total aligned length of concatenated sequences of seven nuclear loci was 4007 bp,and the length of each locus ranged from 291 bp to 657 bp,with an average of 572 bp.The variable sites of each locus ranged from 23 to 48 with an average of 38 and the parsimony informative sites ranged from 22 to 39 with an average of 31 (Table S1).The phylogenetic trees inferred from ML and BI approaches based on concatenated sequences were identical in topology.The species tree (Fig.S1) based on the multi-species coalescent approach was consistent with the ML and BI trees but with relatively low resolution.Below,we only present the ML tree,with posterior probabilities from BI analyses designated (Fig.2).
Our results showed thatFaguswas monophyletic (ML bootstrap=100/BI posterior probability=1.00).The concatenated ML and BI trees both identified two clades,and strongly supported a sister relationship between subgen.Fagus(clade I) and subgen.Engleriana(clade II)(73/0.92 and 100/1.00,Fig.2).All twelveFagusspecies,except forF.chieniiandF.hayatae,were recovered as monophyletic clades.F.lucida,which was assumed to be synonymous or one of the parents ofF.chienii,was sister toF.longipetiolata(74/1.00),both of which were sister to a Japanese beech (Fagus crenataBlume).Notably,F.hayataeintermingled withF.chienii,forming a highly supported clade (86/1.00).In other words,F.chieniiwas distinct from bothF.longipetiolataandF.lucidabut indistinguishable fromF.hayataephylogenetically.In addition,F.chieniidid not form a monophyletic clade as other species of subgen.Fagus,implying thatF.chieniiis not a lineage with an independent evolutionary history.
3.2.Morphological analyses
After Pearson correlation analysis,we excluded leaf length and cupule peduncle length from further morphological analysis.The results of PCA and PCoA were determined by the remaining seven variables (leaf teeth length,ratio of length/width,number of secondary veins,leaf petiole length,leaf width,cupule length,bract length).Both PCA and PCoA clearly showed that three distinct groups,i.e.,F.chieniiplusF.hayatae, F.longipetiolata,andF.lucida,were identified by the scatter plots of the first two axes.In the PCA(Fig.3B),the first two axes explained 74.9% of the variance,with 54.1%for PC1 and 20.8%for PC2,respectively.In PCoA,irrespective of whether the specimen ofF.hayatae, F.chieniiorF.chienii-TYPE were treated as one group or not,the first two principal coordinates accounted for 92.3%of the variance,with 87.2%for PCoA1 and 5.1%PCoA2,respectively.The proportion of sum of squares from the total was significantly high(R2=0.7691,P=0.001 whenF.hayatae,F.chieniiandF.chienii-TYPE treated as one group andR2=0.7996,P=0.001 whenF.hayatae, F.chieniiandF.chienii-TYPE as three separate groups,Fig.3C,D).WhenF.hayatae,F.chieniiandF.chienii-TYPE were pairwise compared,onlyF.hayataeandF.chienii-TYPE showed a significant difference in Adonis analysis (Table 1).

Table 1 Pairwise comparisons of Fagus chienii collected in this study(Fagus chienii),F.chienii types (F.chienii-TYPE), F.hayatae, F.longipetiolata,and F.lucida based on seven morphological characters in Adonis analysis using pairwise Adonis package.
T-test analyses of all nine morphological characters (P<0.01)showed that there was no significant difference betweenF.chieniiandF.hayatae(Fig.4).Furthermore,significant differences betweenF.chienii/F.hayataeandF.lucidawere detected in terms of leaf petiole length,cupule peduncle length,bracts length,teeth length,and number of secondary veins.Conspicuous divergences betweenF.chienii/F.hayataeandF.longipetiolatawere observed in all the characters except for the ratio of leaf length/width.All the original measurements of morphological traits are presented in Table S4.

Fig.4. Comparisons of nine morphological characters between Fagus chienii, F.hayatae, F.longpetiolata,and F.lucida.The boxes represent the interquartile and the vertical lines represent the range excluding the extreme values (black dots).Different lowercase letters on the top of the vertical lines indicate significant differences (P <0.01) between Fagus species.
4.Discussion
4.1. Taxonomic status of Fagus chienii
Recently,Jiang et al.(2022)reconstructed a robust phylogenetic tree ofFagusin which all eleven species were well recognized except forF.chienii,due to a lack of experimental materials at that time.In this study,although only seven of 28 nuclear single/lowcopy genes of Jiang et al.(2022) were adopted,our phylogenetic trees are essentially congruent with that of Jiang et al.(2022),suggesting the seven nuclear genes are effective in phylogenetic reconstruction and species identification ofFagus.However,F.chieniiandF.hayataeare nested together in our newly reconstructed tree(Fig.2).The PCA,PCoA and thet-test of morphological characters consistently indicate that there is little differentiation between the two species(Figs.3 and 4).Note that although Adonis analysis showed a significant difference betweenF.hayataeandF.chieniitypes,it did not show significant differences betweenF.hayataeandF.chieniiwe collected or betweenF.chieniiwe collected andF.chieniitypes.This pattern is reasonable because the fragmented distribution ofF.hayataemay result in high genetic as well as morphological differentiation within the species (Zhang et al.,2013;Ying et al.,2016;Gao et al.,2020;Li et al.,2021).In this study,we included the holotype and isotype specimens (red dots in Fig.3B,D) specifically,a practice that may avoid the bias introduced by potential misidentification of the newF.chieniicollections.In addition,the location ofF.chieniiis adjacent to the populations ofF.hayataeat the border of Shanxi and Sichuan Provinces (Fig.1);thus,it is possible thatF.chieniirepresents the westernmost population ofF.hayatae.Recently,Liang et al.(2022)have reached a similar conclusion concerning the taxonomic status ofF.chieniibased on genetic differentiation among beech species.However,they recommended thatF.chieniishould be treated as a synonym ofFagus pashanica(i.e.,mainlandF.hayataepopulations),which were synonymized toF.hayataeinFlora Reipublicae Popularis Sinicae(Zhang and Huang,1998).Because intraspecific structure does not warrant a species split (Meikle,1957;Aranda et al.,2014;Feng et al.,2021) and no further information,such as morphological difference and reproductive isolation betweenF.hayatae(sensu stricto)andF.pashanica,is available in Liang et al.(2022),it is premature to treatF.chieniias a synonym ofF.pashanica.Taken together,F.chieniiis highly likely to be conspecific withF.hayataerather than an independent species,resolving a nearly 90-year-old enigma in the taxonomy ofFagus.
The distinction betweenF.chieniiandF.lucida,according toFlora Reipublicae Popularis Sinicae,is that the former has longer cupule bracts that are slightly recurved,and the latter has tuberculate cupule bracts that are closely appressed to the cupule(see Fig.5G,H,I) (Zhang and Huang,1998).However,there are no discernible differences in the two species’ leaf length (6-9 cm inF.chieniivs.6-11 cm inF.lucida) or leaf width (3-4.5 cm vs.3.5-6.5 cm),and the leaf blades of both are ovate to elliptic-ovate.Based on the similarities in leaf blade shape and size,Zhang and Huang (1998)suggested thatF.chieniimight be conspecific withF.lucida.Indeed,our results show that there are no significant differences betweenF.chieniiandF.lucidain leaf length(5.4-8.5 cm inF.chieniivs.6.5-10 cm inF.lucida),leaf width(2.6-4 cm vs.3-5 cm),ratio of leaf length/width(1.9-2.3 vs.1.8-2.3)or cupule length(0.7-1.3 cm vs.0.5-1.3 cm) (Fig.4A,B,C,G and 5).However,we foundsignificant divergences between the two species in the length of leaf petiole (0.3-1 cm inF.chienii/F.hayataevs.0.7-1.9 cm inF.lucida),teeth (0.3-1 mm vs.0.8-1.9 mm),cupule peduncle(0.5-1.8 cm vs.0.3-0.9 cm)and bracts(0.1-4 mm vs.0.5-0.8 mm),and the number of secondary veins(7-11 vs.10-13)(Figs.4 and 5).Therefore,our results disagree with the suggestion of Zhang and Huang (1998) and provide more accurate criteria for discriminatingF.chienii/F.hayataefromF.lucida.
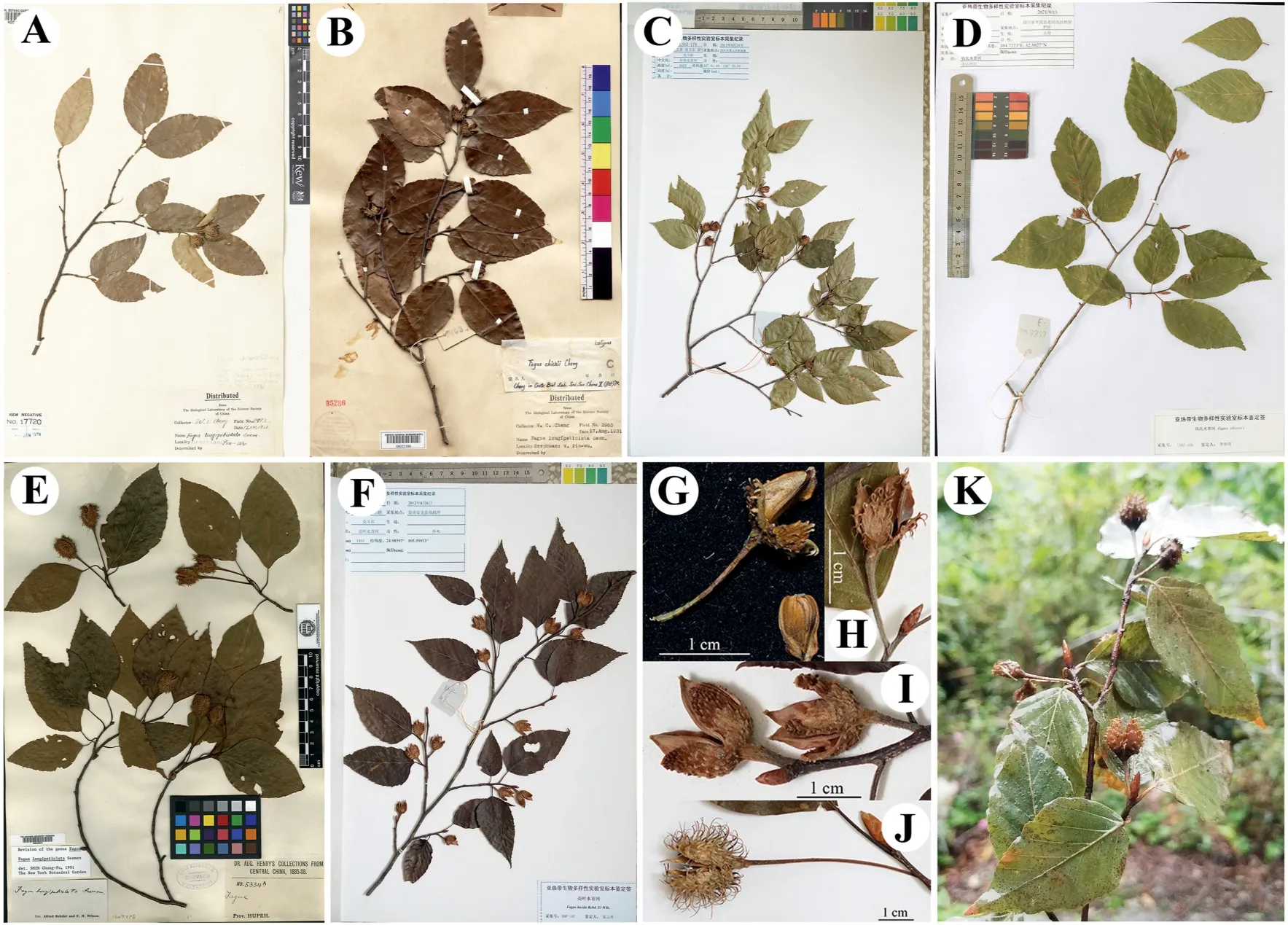
Fig.5. Specimens of Fagus chienii,F.hayatae,F.lucida and F.longipetiolata.A,B,holotype and isotype of F.chienii from Royal Botanic Gardens,Kew and Chinese National Herbarium,respectively;C,Specimen of F.hayatae we collected from Guangwushan,Sichuan;D,Specimen of F.chienii we collected from Pingwu County;E,holotype of F.longipetiolata;F,Specimen of F.lucida we collected from Xianheping,Guizhou;G,H,I,J,Fruit characters of F.chienii, F.hayatae, F.lucida,and F.longipetiolata,respectively;K,Fruiting branch,photographed by D.Li.The holotype specimens of F.hayatae and F.lucida are not shown because there are no fruits or inflorescences on them.
In addition,our results also reject the hypothesis thatF.chieniiis a hybrid betweenF.lucidaandF.longipetiolata(Cheng,1935),becauseF.chieniiis neither clustered with its putative parental progenitors in phylogenetic tree(Fig.2)nor intermediate betweenF.lucidaandF.longipetiolatain PCA,PCoA plots as well as box and whisker plots(Figs.3 and 4).
4.2.Conservation implications
Without a stable taxonomy,it is difficult to implement management strategies that will adequately conserve diversity(Garnett and Christidis,2017;Sun et al.,2017;Thomson et al.,2018).Because no additional individuals of“F.chienii”had been found since its first discovery,some researchers believed that this species was extremely endangered or on the edge of extinction (Peters,1997;Guo and Werger,2010).Accordingly,the People's Government of Sichuan Province listed “F.chienii”as a key protected wild plant in 2016 and great efforts have been invested to protect this rare species.In this study,however,we demonstrated that “F.chienii” is conspecific withF.hayataeand should be synonymized to the latter.Therefore,it is unnecessary to treat“F.chienii”as a separate species in conservation management.
However,F.hayataeis a second-class protected plant in the Chinese List of Wild Plants under State Protection (http://www.forestry.gov.cn/main/5461/20210908/162515850572900.html) and is also an endangered species in the IUCN Red List of Threatened Species(International Union for Conservation of Nature and Natural Resources,2022).It is thus essential to protect the “F.chienii” population because it belongs toF.hayatae.Furthermore,given that“F.chienii” is located at the northwestern edge ofF.hayataerange,this population may be of peculiar conservation values because elevated genetic drift and reduced gene flow in association with local adaptation in the periphery are likely to result in the formation of distinct ecotypes (Hampe and Bairlein,2000).Furthermore,peripheral populations are always more prone to extinction and genetically less diverse than those from the center(Hampe and Petit,2005).This situation could be worse for “F.chienii” (F.hayatae) in Pingwu County because it was overexploited from the 1960s-1990s for its high-quality timber.Although the conservation value of the“F.chienii” population needs to be further evaluated by sophistical population genomic approaches (Garner et al.,2016;Zhang et al.,2021) and reciprocal transplant experiments (Ågren and Schemske,2012;Lortie and Hierro,2021),we recommend that effective conservation measures such asin situprotection andex situgermplasm preservation should be prioritized by the local government and the germplasm bank of wild species to prevent this peculiar population from extinction (Frankham,2010;Rawat and Agarwal,2015;Heywood,2017).
4.3.Taxonomic treatment
Fagus hayataePalib.ex Hayata Journ.Coll.Sci.Univ.Tokyo 30:286.1911 -Type: China,Taiwan: Taoyuan,Chatienshan,alt.1700 m,5 Feb.1906,N.Konishi(holotype TAI-T00127!;isotype unseen).
=F.chieniiCheng,Contr.Biol.Lab.Sci.Soc.China 14:70.1935.Syn.Nov.-Type:China,Sichuan:Pingwu County,alt.1300m,17 Aug.1931,W.C.Cheng 2903(holotype K-000832761!;isotypes E-00098603!,HUH (A)-00033870!,IBSC-0001170!,NAS-0007 0338!,NAS-00070339!,NY-00248568!,PE-00022177!,PE-00022178!,PE-00022179!,PE-00022180!,PE-00022181!,PE-00022182!).
=F.hayataevar.zhejiangensisM.C.Liu&M.H.Wu ex Y.T.Chang&C.C.Huang,Acta Phytotax.Sin.26: 115.1988 -Type: China,Zhejiang,Yongjia County,Sihai-shan,7 Oct.1980,M.H.Wu 619(holotype FJSI-015724!;isotype unseen).
=F.pashanicaC.C.Yang,Acta Phytotax.Sin.16:100,pl.1.1978.-Type: China,Sichuan,Nanjiang County,13 Aug.1975,C.C.Yang75011(holotype unseen;isotypes CDBI-0172185!,IBSC-0001171!,IBSC-0001172!,NAS-00070342!,PE-00022195!).
Description.Trees up to 20 m tall.Winter buds to 1.5 cm.Leaf petiole 0.3-1 cm long;leaf blade ovate,3.8-8.4 cm long,2.1-4 cm broad,base broadly cuneate to acuminate;midvein flexuous toward apex;secondary veins (5) 7-11 on each side of midvein,ending in teeth;teeth 0.3-1 mm long.Peduncle 0.5-1.8 (-2) cm,pilose.Cupule 6-13 mm long;bracts linear,recurved,pilose,0.1-4 mm long.Nut as long as cupule,with very small wings near apex.
Phenology.It starts to flower from April to May;its fruits are mature from October to November.
Distribution and habitat.It is currently known in Taiwan(Taipei,Taoyuan,Hsinchu,Yilan),Zhejiang(Yongjia,Lin'an),Hunan(Shimen),Hubei (Yichang,Xingshan,Baokang,Xuan'en),Chongqing (Chengkou),Sichuan (Nanjiang,Qingchuan,Wangcang,Pingwu) and Shaanxi (Pingli,Xixiang) and grows in forests at elevations between 1000 and 2000 m.
Author contributions
Z.Z.,Y.Y.and D.L.conceived and designed the work.D.L.,H.L.and D.Z.collected and identified material.D.L.,L.J.,Y.Y.,D.F.and Y.K.carried out the laboratory work and performed analyses.L.J.designed primers.D.L.drafted the manuscript and figures.Z.Z.and Y.Y revised the manuscript.All authors have read,commented and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to Ming Yang(Laohegou Nature Reserve,Pingwu County,Sichuan,China) for his assistance in the field collection.This study was supported by grants from the National Natural Science Foundation of China (No.31770236),the Guangxi Key Research and Development Projects (Guike AB21220057),and Training Program for Academic and Technical Leaders (leading talents) in Major Disciplines of Jiangxi Province (grant to Zhiyong Zhang,20213BCJ22006).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.01.003.
- 植物多样性的其它文章
- Is intraspecific trait differentiation in Parthenium hysterophorus a consequence of hereditary factors and/or phenotypic plasticity?
- Convergent relationships between flower economics and hydraulic traits across aquatic and terrestrial herbaceous plants
- Integrative analysis of the metabolome and transcriptome reveals the potential mechanism of fruit flavor formation in wild hawthorn(Crataegus chungtienensis)
- Evidence of the oldest extant vascular plant (horsetails)from the Indian Cenozoic
- Climate change impacts the distribution of Quercus section Cyclobalanopsis(Fagaceae),a keystone lineage in East Asian evergreen broadleaved forests
- Drivers of the differentiation between broad-leaved trees and shrubs in the shift from evergreen to deciduous leaf habit in forests of eastern Asian subtropics

