Pharmacokinetics of fucoidan and low molecular weight fucoidan from Saccharina japonica after oral administration to mice*
Jiaojiao TAN, Yimin SONG, Jing WANG, Ning WU,5,6,**, Yang YUE, Quanbin ZHANG,2,**
1 Key Laboratory of Experimental Marine Biology, Center for Ocean Mega-Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Biology and Biotechnology, National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Department of Pharmaceutical Engineering, Qingdao University of Science and Technology, Qingdao 266000, China
5 Laboratory for Marine Drugs and Biological Products, National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
6 Nantong Zhongke Marine Science and Technology Research and Development Center, Nantong 226000, China
Abstract The brown seaweed, Sacchairna japonica, has been used in traditional Chinese medicine for over one thousand years.Oral administration of fucoidan or low molecular weight fucoidan (LMWF)from S.japonica could ameliorate kidney dysfunction in chronic kidney diseases and inhibit diabetic vascular complications.In many studies, LMWF was found to be more potent than fucoidan with high molecular weight.However, the pharmacokinetics of LMWF still remains unclear.The purpose of the research is to compare the pharmacokinetics of fucoidan with high molecular weight (136 kDa) with that low molecular weight (9.5 kDa) after oral administration to ICR mice.Since fucose is the main and representative monosaccharide of fucoidans, we evaluate the pharmacokinetics of fucoidan and LMWF by determining the fucose concentration in mice serum.Both fucoidan and LMWF were absorbed following oral administration.Fucoidan and LMWF were provided to mice by oral administration with 60 mg/kg and the maximum Concentration (Cmax) was found at 2.5 h (0.66±0.32 mg/L) for Fucoidan and 1.5 h(1.01±0.56 mg/L) for LMWF, respectively.It seems that LMWF had a higher area under the curve(AUC0–t) and was absorbed more quickly than fucoidan.The estimated bioavailability of LMWF was 28.3% in the mice treated with a single dose of 30 mg/kg.In addition, LMWF was found widely spreaded into different tissues following oral administration and the highest concentration was found in kidney at 19.93±7.02 μg/g.In this study, we first studied the pharmacokinetics of LMWF, in order to help to understand the function of LMWF.And our results shed light on the potential of development of drugs based on LMWF.
Keyword: fucoidan; low molecular weight fucoidan; pharmacokinetics; bioavailability; tissue distribution
1 INTRODUCTION
Algal fucoidans represent a rather heterogeneous group of fucose-rich sulfated polysaccharides with complex and heterogeneous structures (Berteau and Mulloy, 2003).Fucoidans have been reported to have diverse bioactivities, including anticoagulant (Irhimeh et al., 2009; Wang et al., 2011), anti-inflammatory(Ahmad et al., 2021), antioxidant (Wang et al.,2008; Koh et al., 2019), antitumor (Choi and Kim,2013), antihyperglycemic (Pozharitskaya et al., 2020),and antiviral activities (Luthuli et al., 2019; Chaisuwan et al., 2021).These studies confirmed a systemic effect of fucoidans following its oral administration.However, there is still few explanations of how these systemic effects are achieved, and that is mainly due to the imperfect understanding of its pharmacokinetics.
Over the past decade, several studies have been conducted on the quantification of fucoidan in plasma or biological samples.These assays include HPLC with post-column fluorescence derivatization(Zhang et al., 2016), pre-labeled fluorometric assay(Yamazaki et al., 2016), anti-Xa activity, competitive ELISA assay (Tokita et al., 2010a), and a more sensitive sandwich ELISA assay (Tokita et al.,2010b).Based on sandwich ELISA assay, after oral administration of fucoidan fromCladosiphon okamuranus, fucoidan levels in plasma and urine of healthy volunteers increased significantly after 6 and 9 h respectively (Tokita et al., 2010a).Former study was investigated of fucoidan, which was isolated fromFucusvesiculosuswith molecular weight of 735 kDa, by measuring anti-activated factorX(anti-Xa) activity.They found that fucoidan preferentially accumulated in the kidneys, spleen, and liver and showed a relatively long absorption time and extended circulation in the blood after oral administration in rats (Pozharitskaya et al., 2018).All these studies demonstrated that high molecular weight fucoidan could be absorbed in the body after oral ingestion.However, these results are based on indirect measurement of fucoidan.Further investigation of fucoidan pharmacokinetics using more precise assay is needed to validate these results.
In our previous studies, we demonstrated that fucoidan extracted fromSaccharinajaponica(and also calledLaminariajaponica) has very complex structure and diverse bioactivities (Wang et al.,2010).Besides fucose, minor galactose, mannose,glucose and rhamnose were also found in fucoidan fromS.japonica.Fucoidans or low molecular weight fucoidan (LMWF) fromS.japonicahave been proved to be an effective protective agent for chronic kidney diseases and diabetic vascular complications (Zhang et al., 2003, 2005; Yang et al.,2013; Cui et al., 2014; Wang et al., 2014; Li et al.,2017).Oral administration of fucoidan or LMWF could ameliorate kidney damage in chronic renal failure, acute renal failure, and diabetic nephropathy(Wang et al., 2019).
The relationship between molecular weight and biological activities of fucoidans has been extensively reported.The anticoagulant activity of fucoidans was closely related to their molecular weights.High and low molecular weight fucoidans were reported to have different effects on the severity of collagen-induced arthritis in mice (Park et al.,2010).Our previous study also showed that LMWF had higher renoprotective activity than fucoidan at the same doses (Wang et al., 2019).So, all these findings show that the bioactivity between high and low molecular weight fucoidans are indeed different.Therefore, it is very important to determine the pharmacokinetics differences between fucoidan with high molecular weight and that with low molecular weight.However, as we know, most of the pharmacokinetics studies were related to fucoidans from different algae with high molecular weight, the pharmacokinetics of LMWF after oral administration is still unclear.
The USA Food and Drug Administration (FDA)and European Medicines Agency (EMA) clearly denote that monitoring representative chemical constituent(s) in a botanical drug product can provide information of pharmacokinetic measurements(botanical drug development guidance for industry).Fucose is the main and representative sugar in fucoidan.If fucoidan is absorbed in the body, the hydrolyzed fucose level in blood and tissues are expected to elevate.So, in this study, the hydrolyzed fucose level in serum and tissues measured by ion chromatography was employed to determine the absorption of high and low molecular weight fucoidans fromS.japonicaafter oral administration to ICR mice.The bioavailability and tissue distribution of LMWF were also determined.
2 MATERIAL AND METHOD
2.1 Material
Brown algaeS.Japonicawas obtained from the coast of Rongcheng City, Shandong Province, China,in July 2019.The specimen number is 2019-07-SJ-RC.
Fucoidan was extracted fromS.japonicaAresch as described previously (Tan et al., 2020).Briefly,fucoidan was extracted fromS.japonicain hot water for 2 h, the extraction solution was filtered and soluble alginate was precipitated with the final concentration of 1% CaCl2.The supernatant was purified using dialysis membranes with molecular weight cut-off (MWCO) of 3 500 Da, concentrated and finally precipitated with ethanol.The precipitate was dried to get fucoidan.
LMWF was prepared using free radical degradation with the combination of hydrogen peroxide and ascorbic acid (Tan et al., 2020).Fucoidan was hydrolyzed in ascorbic acid and hydrogen peroxide solution with a final concectration of 30 mmol/L (1∶1)for 2 h.After hydrolysis, the solution was filtered and dialyzed (MWCO: 3 500 Da), and precipitated with ethanol to a final concentration of 75%.
The weight average molecular weights of fucoidan and its depolymerized fragment LMWF were 136 kDa and 9.5 kDa, respectively.Both fucoidan and LMWF had similar chemical constituents.The fucose contents of fucoidan and LMWF were 31.6% and 29.6%, and sulfate contents were 33.58% and 32.66%, respectively.The mole rate of fucoidan was 1.00, 0.30, 0.09, 0.07,0.04, 0.05 for fucose, galactose, mannose, glucose,rhamnose, xylose, respectively.And the mole rate of LMWF was 1.00, 0.30, 0.07, 0.09, 0.04, and 0.05 for fucose, galactose, mannose, glucose, rhamnose,and xylose, respectively.
Monosaccharide standards, including fucose (Fuc),galactose (Gal), mannose (Man), glucose (Glu),rhamnose (Rha), and xylose (Xyl), were obtained from Sigma-Aldrich (USA).Sodium hydroxide(50%, w/w) was purchased from ThermoFisher(Waltham, MA) and TFA from J&P.All other chemicals are guaranteed reagent-grade.Deionized water was generated with a Millipori Mingche QGard system (France).
2.2 Animal
ICR mice (20±0.2 g) were provided by Experimental Animal Center of Shandong Province (SCXK(lu)20190003).The animals were maintained on a 12-h∶12-h dark∶light cycle at about 22 °C and relative humidity (60%–70%), allowed free access to standard mice chows and water during the experiments.The experiments were performed in complete compliance with the National Guide for the Care and Use of Laboratory Animals, and were approved by the Experimental Animal Ethics Committee of Institute of Oceanology, Chinese Academy of Sciences, China.
2.3 Intragastric administration
ICR mice (n=6 per time point) were kept under fasting overnight before the experiment.The doses used in the present manuscript were based on the research work (Liu et al., 2018; Wang et al., 2018).LMWF dissolved by water was administered directly into the stomach with lavage needle at corresponding doses (single dose, 30 mg/kg and 60 mg/kg, respectively).For comparison, high molecular weight fucoidan was administered to mice intragastrically by gavage at the dose of 60 mg/kg.Blood samples were collected by venipuncture of the retro-orbital plexus at different time points(before drug administration, 15 min, 1, 1.5, 2, 2.5, 3,4, 5, 6, and 10 h).The blood was centrifuged at 5 000 r/min for 20 min, and then the serum was collected and stored at -80 °C.
2.4 Intravenous administration
Intravenous (i.v.) injection of LMWF (single dose, 30 mg/kg, dissolved by 0.9% normal saline) was administered through caudal vein for determination absolute bioavailability.Blood samples were collected and processed the same way as described in Section 2.3.
2.5 Tissue distribution
LMWF dissolved in water (60 mg/kg dose) was administered directly into stomach as described in Section 2.3.After blood was taken at different time points (0, 1, 1.5, 2, 3, 4, 6, and 10 h), ICR mice were executed by cervical dislocation.The tissues (heart,liver, spleen, kidney, lung, stomach, and intestines)were obtained by surgical resection, and cleaned by ultra-pure water.Before tissue samples were frozen at -20 °C, they were blotted dry with tissue paper.
2.6 Serum and tissue sample preparation
About 50–150 μL serum was pipetted into ampoule bottles, water was added to a final volume of 1 mL,and then 1 mL of 4-mol/L TFA (trifluoroacetic acid)solution was added.The ampoule was sealed and hydrolyzed at 105 °C for 4 h.After that, the reaction mixture was neutralized with 2-mol/L sodium hydroxide to pH 7.0, filtered and diluted to a final volume of 10 mL.10 μL of the obtained filtrate were injected into ion chromatograph for analysis.
The tissue samples were prepared as described previously (Song et al., 2016).Tissue samples were thawed on the ice and weighed accurately.Forxmg of tissue, 4 times volume (μL) of water and 5 times volume (μL) of saturated Borax solution and 10 times volume (μL) of 1% NaClO were added to tissue and were ground together for 2 min at room temperature and quenched with 0.2 times volume (μL)of formic acid, for example, 400 μL of water and 1 000 μL of 1% NaClO and 500 μL of saturated Borax solution were added to 100 mg of tissue and 20 μL of formic acid shut down the reaction.The mixture was centrifuged (4 000 r/min 20 min) to remove insoluble material, and the supernatant was used for the following assay.One milliliter supernatant was transferred into the ampoule bottle, and the next steps was performed similarly to what’s done with the serum.
2.7 HPAEC-PAD analysis
A Thermo Scientific Ion Chromatograph System was used with pulsed amperometric detector (PAD)and Carno PACTMPA10 analytical column (4.0 mm×250 mm; Thermo Scientific Dionex, Sunnyonle,CA, USA) for high performance anion exchange chromatography (HPAEC) analysis (He et al.,2018).The mobile phase was NaOH (18 mmol/L)and NaOH (200 mmol/L), respectively.After 10 μL of hydrolysate was injected, the mobile phases of 18-mmol/L NaOH were used to measure fucose at a flow rate of 1 mL/min, with the column temperature set at 30 °C.The column was washed off by 200-mmol/L NaOH.The procedure for elution was listed in Table 1.PAD with a gold working electrode and an Ag/AgCl reference electrode was accomplished during the test.A standard carbohydrate quadruple potential waveform was used.The date was recorded and calculated using a Chromeleon 7.0 chromatography data system.
2.8 Pharmacokinetic analysis
Pharmacokinetics was analyzed by DAS software through the mean concentrations of fucose obtained from six mice at each time point after they were treated with fucoidan or LMWF.CmaxandTmaxwere taken directly from the concentration-time curves.Bioavailability (F) of LMWF was calculated using the following equation:
2.9 Statistical analysis
The data were presented as means±SD.All statistical analysis was performed using statistical package for social sciences (SPSS, version 11.0).
3 RESULT
3.1 Method validation
The serum concentration and tissue distribution of fucoidans were assessed by measuring the fucose concentration of the hydrolyzed serum or tissue samples.Fucose and other monosaccharides were released by acid hydrolysis and determined by HPAEC combined with PAD analysis.
Although the HPAEC with PAD method is a more accurate way to measure monosaccharides few studies had employed this method to determine fucose concentration in blood and tissue.In present study, we firstly validated the specificity and accuracy of this analytical method.The results showed thatHPAEC with PAD method had highly specificity and good separation (Table 2 & Fig.1) both in serum and tissues.Fucose was well separated from other monosaccharide standards including glucose, galactose,mannose, rhamnose, xylose, and arabinose.Low level of fucose was detected in the hydrolysate of drug-free serum and tissues, suggesting that minor fucose-containing glycans or glycoconjugates were present.In the following experiments, the fucose concentration was calculated as the detected fucose concentration subtracted the baseline values.
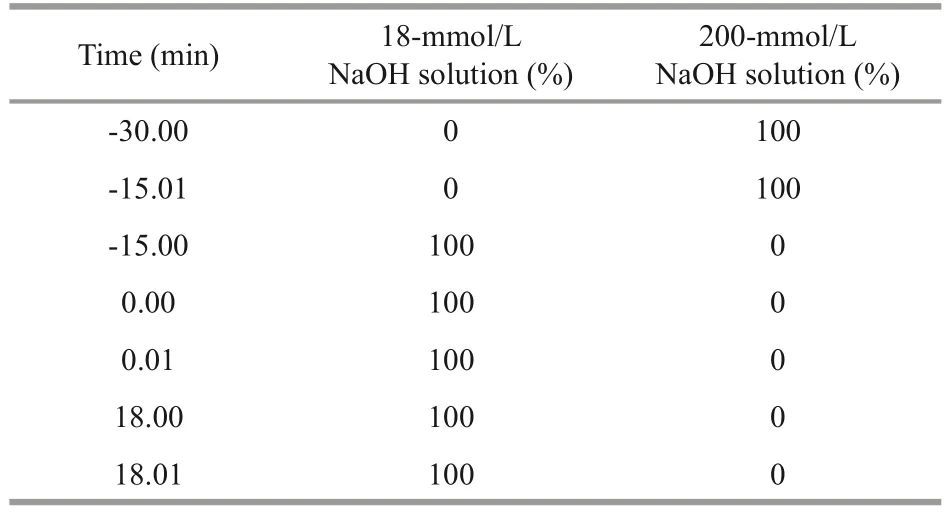
Table 1 The procedure for elution in the measurement time
whereCf: the fucose concentration;Cdf: the fucose concentration after oral administration to mice;C0: the fucose concentration without oral administration to mice.
The standard calibration curve of fucose added to serum exhibited good linearity from the fucose standard concentration of 0.01 to 2 μg/mL, and the correlation coefficient is 0.997,Y=1.0565X–0.0039.This linear relationship between concentration and chromatographic response could be applied to the whole studies.
When the signal-to-noise ratio was 3, 10 ng/mL was considered as the limit of detection (LOD).When the signal-to-noise ratio was 10, the limit of quantification (LOQ) was determined to be 25 ng/mL.
For different ratios of the standard (ranging 50%–150%), the detectable fucose content of standard(i.e., 0.7, 1.75, and 2.8 μg) obtained from serumwere 0.54±0.03, 1.32±0.03, and 2.21±0.15 mg/L,and the average recoveries were 77.47%, with relative standard deviation (RSD%) was 4.83%.The detectable fucose content of standard (i.e., 0.7, 1.75,and 2.8 mg/L) obtained from the lung were 0.54±0.05, 1.29±0.05, and 2.05±0.05 mg/L, and the average recoveries was 75.43%, with relative standard deviation (RSD%) was 5.77%.The data proved that the analysis method achieves the precision and accuracy benchmarks.

Table 2 The specificity of different monosaccharides after hydrolysis
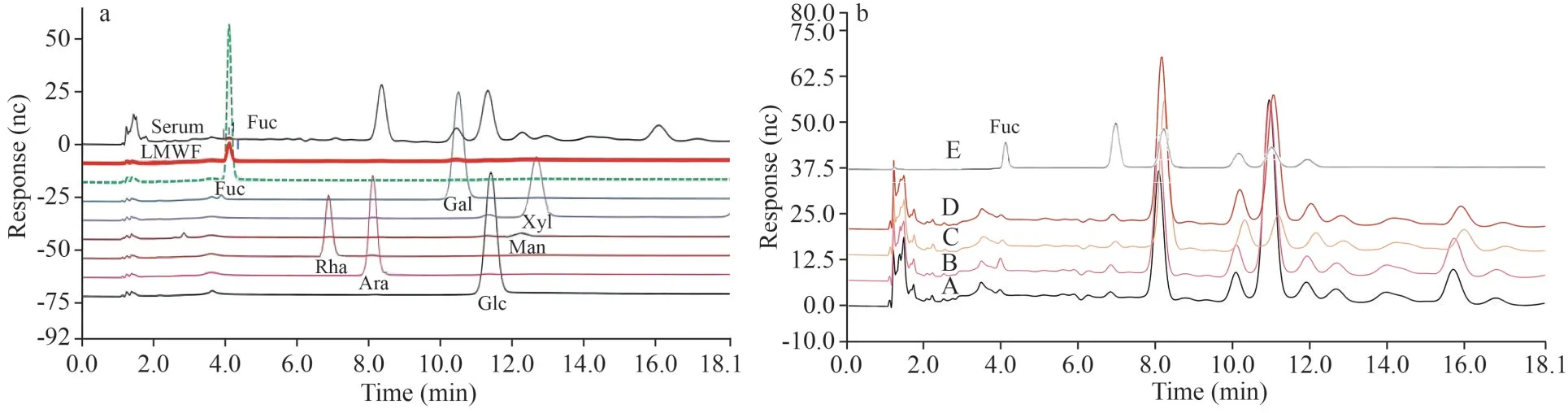
Fig.1 The chromatograph chart of monosaccharide specificity
Samples obtained from intragastric administration in serum and lung tissue were stored at room temperature and their stability was evaluated at 0, 2,4, 6, 8, and 14 h.The detectable concentrations of fucose were 15.17±0.19 and 14.03±0.38 mg/L, with RSD% values of 1.22% and 2.69%.These results indicated that the stability of the sample solution was within the acceptable criteria of 3.0%.
All the results indicated the HPAEC combined with PAD method was appropriate for analysis of fucose in serum and tissues.
3.2 Pharmacokinetic analysis of fucoidan and LMWF
After oral administration of fucoidan or LMWF to ICR mice, the concentration-time curves of hydrolyzed fucose in serum was test and the results shown in Figs.2 & 3.The results shown that both fucoidan and LMWF were absorbed following intragastric administration.The maximum concentration(Cmax) was found at 1.5 h (0.53±0.13 mg/L, 1.01±0.56 mg/L) after oral treated with LMWF of both the dose of 30 mg/kg and 60 mg/kg.But theCmaxin fucoidan with high molecular weight treated (60 mg/kg)mice serum was found at 2.5 h (0.66±0.32 mg/L).Following the first peak of serum concentration, the serum concentration began to decrease, but the second absorption peak appeared at 6.0 h.For LMWF oral administration,Cmaxincreased proportionally with dosage from 30 to 60 mg/kg.At the same dose concentration (60 mg/kg), theCmaxof LMWF was higher than that of fucoidan in mice serum.

Fig.2 Mean serum concentration of hydrolyzed fucosetime curves after oral administration of low molecular weight fucoidan (30 mg/kg, 60 mg/kg)

Fig.3 Mean serum concentration of hydrolyzed fucosetime curves after oral administration of LMWF(60 mg/kg) and fucoidan (60 mg/kg)
As shown in Table 3, for LMWF, with the increasing dose concentration, AUC(0–t)increased almost proportionately, from 1.45 mg/(L·h) (at 30-mg/kg dose) to 3.13 mg/(L·h) (at 60-mg/kg dose).While AUC(0–t)for fucoidan at the dose of 60 mg/kg was 2.71 mg/(L·h).The distribution half-life (t1/2α) of LMWF was 0.2–0.4 h and terminal elimination halflife (t1/2β) was 15.5–21.2 h.The distribution half-life(t1/2α) of fucoidan was 3.6 h and terminal elimination half-life (t1/2β) was 3.6 h, so the elimination of LMWF and fucoidan follows biphasic pattern for LMWF.The mean residence time (MRT0–t) of LMWF was 3.95 h (30 mg/kg) and 4.13 h (60 mg/kg), respectively,however for fucoidan, the mean residence time MRT0–twas 4.82 h at the dose of 60 mg/L.The MRT0–∞of LMWF was 9.77 h (30 mg/kg) and 10.18 h (60 mg/kg) respectively, but for fucoidan,the MRT0–∞was 5.74 h at the dose of 60 mg/L.The results show that the AUC(0–t)of LMWF at the doseof 60 mg/kg was higher than that of fucoidan and it also had a much longer MRT0–∞than fucoidan (60 mg/kg).
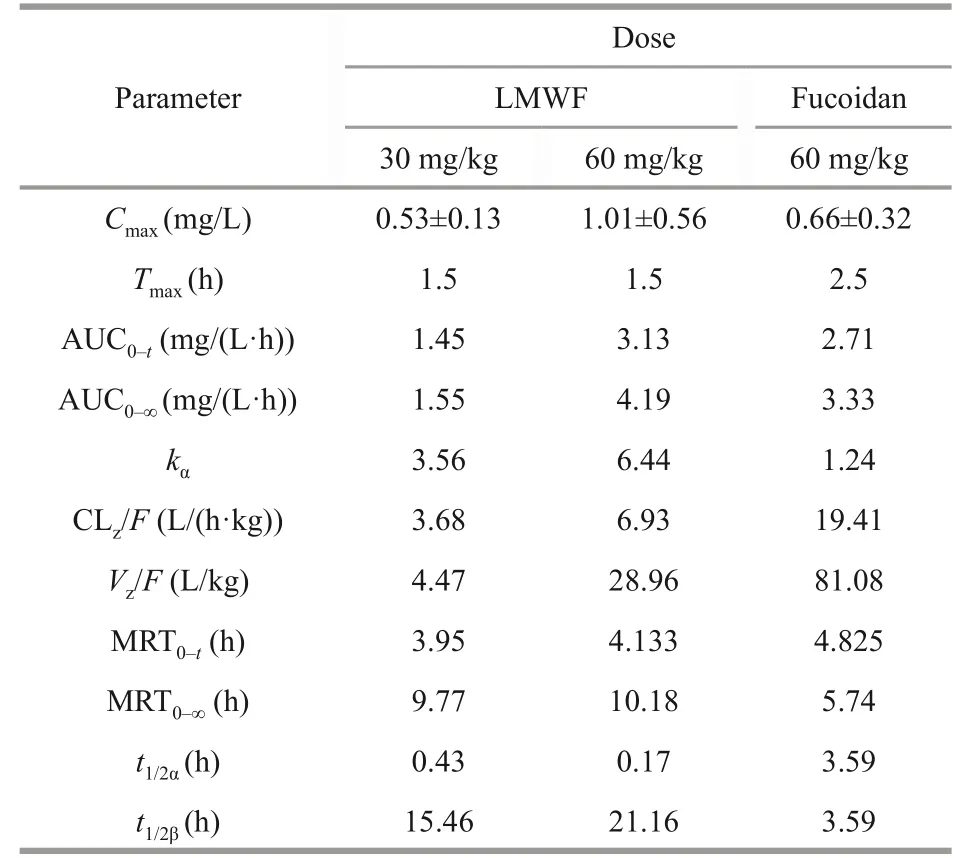
Table 3 Pharmacokinetic parameters of fucoidan and LMWF after intragastric administration
In addition to analysis the pharmacokinetics of fucoidan and LMWF in mice after oral administration,we also studied the pharmacokinetics of LMWF in mice after treated by intravenous administration.Through the data of intravenous administration of LMWF (30 mg/kg), we found that the second peak concentration was found at 4 h after intravenous administration, indicating that hepatointestinal circulation might exist.The bioavailability of LMWF was estimated to be 28.3% after treated with a single dose of 30 mg/kg (Fig.4 & Table 4).
3.3 Tissue distribution
The tissue distribution of LMWF in mice heart,liver, spleen, lung, kidney, stomach, and intestines were investigated after oral administration (60 mg/kg)and the fucose concentration in different tissues at different time points was illustrated in Fig.5.It can be seen that LMWF was widely distributed into tissues with the highest concentration of 19.93±7.02 μg/g in the kidney.The least concentration of 0.39±0.08 μg/g was found in heart (Table 5).The concentrations in different tissues apparently varied as expected.The heart withCmaxoccurred at 1 h, followed by a dramatic drop, and a second absorption peak occurred at 6 h.TheCmaxin the kidney remained a high level and the peak concentration occurred at 4 h.TheCmaxin stomach and intestines was 4.15±1.55 μg/g and 9.64±2.09 μg/g.The concentration of LMWF in stomach was quite high, suggesting that it might be primarily absorbed in both stomach and intestines.
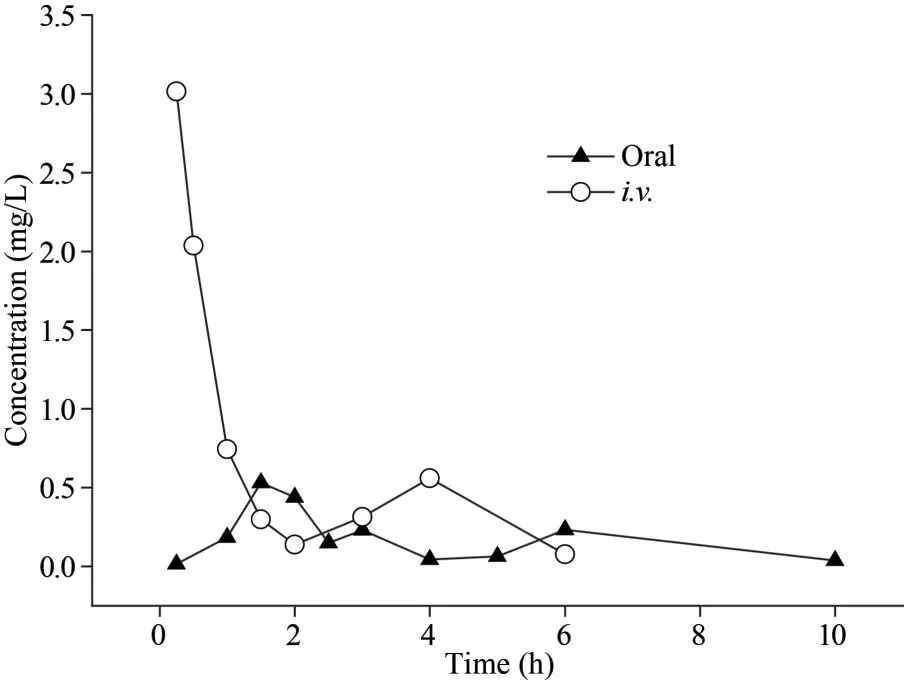
Fig.4 The Mean serum concentration of fucose-time curves after intravenous administration (30 mg/kg)and oral administration (30 mg/kg) of LMWF

Table 4 Pharmacokinetic parameters and bioavailability of LMWF after intravenous or oral administration (30 mg/kg)
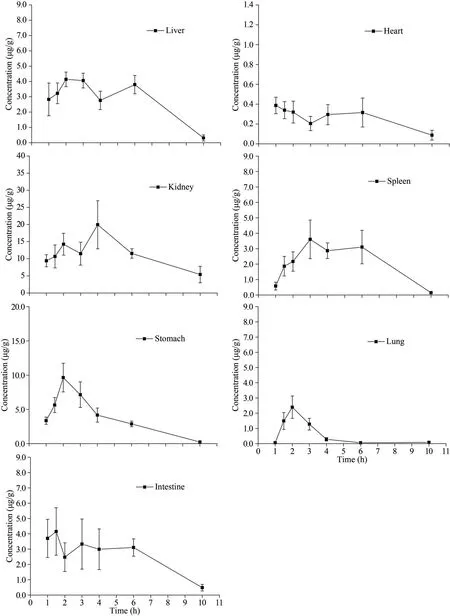
Fig.5 The distribution of LMWF in different tissues after oral administration (60 mg/kg) at different time points
4 DISCUSSION
In the past years, the pharmacokinetic characteristics of fucoidan have drawn more and more attention.Since fucoidan has no chromophores, finding effective microassay methods is one of the key problems in its pharmacokinetic studies.Several assays for the quantification of fucoidan in plasma or biological samples have been reported.These assays include HPLC with post-column fluorescence derivatization(Zhang et al., 2016), pre-labeled fluorometric assay(Yamazaki et al., 2016), plasma fucoidan quantification method based on its anti-Xa activity (Pozharitskayaet al., 2018), competitive ELISA assay (Irhimeh et al.,2005) and a more sensitive sandwich ELISA assay(Tokita et al., 2010b).All these studies demonstrated that fucoidan could be absorbed in the body after oral ingestion.
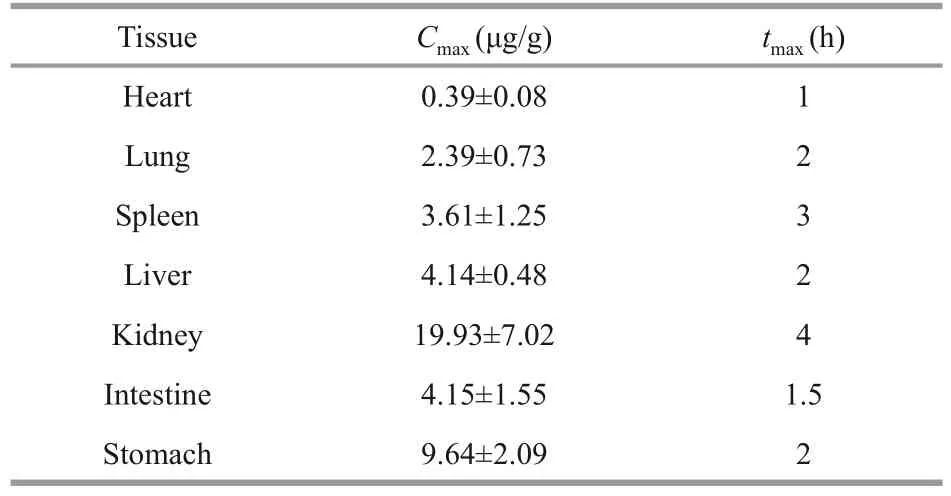
Table 5 The tissue distribution of LMWF after intragastric administration (60 mg/kg)
Fucoidan is a type of fucose-rich sulfated polysaccharide in brown seaweed, and fucose is the main and representative sugar in fucoidan.If fucoidan is absorbed in the body, the hydrolyzed fucose level in blood and tissues are sure to be elevated.Therefore, in this study, the concentration of fucose is quantified to characterize the pharmacokinetic of fucoidan.Comparing to the method of derivation(Jin et al., 2013), the method in this study is used to quantify the serum concentration after oral or intravenous administration, and to measure microscale content in tissues without considering the influence of proteins.In our previous study, we developed a HPLC method with postcolumn fluorescence derivatization to analysis LMWF distribution in rabbits and the limitation of detection and the limitation of quantification were determined to be 0.125 and 0.5 μg/mL respectively (Zhang et al.,2016), the sensitivity was too low for the qualitative analysis of fucoidan after intragastric administration to rabbits.So, in this study we use the HPAEC with PAD method, and the measurement is more straightforward and sensitivity.The LOD of anti-Xa activity assay was 0.01 μg/mL (Pozharitskaya et al.,2018).However, because the anti-Xa activity was markedly related to the molecular weight of fucoidans.The low anticoagulant activity of low molecular weight fucoidan made it difficult to be detected by anti-Xa assay.The LOD of this measurement was 10 ng/mL, suggesting that it could be used for analyses of fucoidan after oral administration.
Our previous studies demonstrated that LMWF fromS.japonicahad excellent effect on diabetic vascular complications and chronic kidney diseases in rats and mice models with doses ranged from 30 to 100 mg/kg (Zhang et al., 2003, 2005; Yang et al.,2013; Cui et al., 2014; Wang et al., 2014, 2018; Liu et al., 2018).So, in this study, the doses of 30 and 60 mg/kg were selected to investigate the pharmacokinetics and tissue distribution of fucoidans.The results in this study demonstrated that both fucoidan and LMWF were detected in ICR mice serum 15 min after intragastric administration.TheCmaxwas observed at 1.5–2.5 h for LMWF and fucoidan at different doses.TheCmaxfor LMWF and fucoidan at the dose of 60 mg/kg were 1.01 and 0.66 mg/L respectively.Considering the fact that fucoidan contained about 30% fucose content, so the exact maximum fucoidan concentration was about three times of the detected fucose concentration.In previously reported study by Pozharitskaya et al.(2018) sing an anti-Xa activity assay, fucoidanF.vesiculosuswith molecular weight of 735 kDa was detected in rat plasma 30 min after the intragastric administration.The observedCmaxwas 0.125 μg/mL at 4 h.In another study, they also found that topical application of fucoidan to rats could penetrated the skin and distributed into the skin, striated muscle, and plasma with AUC0–48=0.94 (μg·h)/g, 2.22 (μg·h)/g,and 1.92 (μg·h)/mL, respectively (Pozharitskaya et al., 2019).In an ELISA competitive-based assay, the plasma fucoidan concentration in samples from volunteers who received 3 g/d.of extracts containing 10% or 75% fucoidan from U.pinnatifida was 4.00 or 12.99 mg/L, respectively, while the highest concentration after 12 days was 5.08 or 15.75 mg/L,respectively (Tokita et al., 2010a).The maximum serum concentrations of fucoidan and LMWF in our study were higher than the levels obtained by Pozharitskaya et al.(2019), but below the levels obtained by Tokita et al.(2010a).This difference may be explained by the difference in fucoidan resources and structure, doses used, different trial,and analytical methods used.As a promising antiinflammatoty formulation, consisting of fucoidan-based cream with 13% Kolliphor P 407, 1% Transcutol P,and 5% PEG 400, about 90% of fucoidan was released and passed into the dissolution medium after 1 h,100% fucoidan was released after 2 h (Obluchinskaya et al., 2021).Since the form of fucoidan is different form ours so we speculate the pharmacokinetic parameters might be a little different.But the trends in all these studies were similar, suggesting that different fucoidans had similar absorption mode.
After oral administration, there were two absorption peaks found at 0–10 h for all dosages.This phenomenon has never been reported before.It indicated that hepatointestinal circulation might have happened in the process of fucoidan absorption.But this hypothesis needs further investigation in future studies.
The estimated bioavailability of LMWF was 28.3%after a single dose of 30 mg/kg.Pozharitskaya et al.(2019) reported that the bioavailability of fucoidan after topical application were estimated to be 17.7%±7.7%.Since topical administration is not considered very efficiently, the estimated bioavailability of 28.3% for LMWF in our study was particularly high.
We found that LMWF was widely distributed in various tissues following oral administration.From the results, LMWF seems to be absorbed in both stomach and intestines, with a higher absorption peak concentration.LMWF was found in the kidney with high concentration, and this could explain why fucoidan or LMWF were used to treat chronic kidney diseases (Wang et al., 2019).
The pharmacokinetic parameters of fucoidan and LMWF at the dose of 60 mg/kg were compared in this study.Both fucoidan and LMWF were absorbed following intragastric administration.Cmaxwas found at 1.5 h (1.01±0.56 mg/L) for LMWF at the dose of 60 mg/kg, and 2.5 h (0.66±0.32 mg/L) for fucoidan at the dose of 60 mg/kg.AUC0–tof LMWF and fucoidan at the dose of 60 mg/kg were 3.13 and 2.71 mg/(L·h) respectively.These results suggested that LMWF was absorbed quite quickly and had a higher AUC0–tthan fucoidan.MRT0–∞of LMWF and fucoidan at the dose of 60 mg/kg were 10.18 and 5.74 mg/(L·h) respectively, these results suggested that LMWF was had a longer MRT0–∞than fucoidan.Many studies have shown that molecular weight plays an important role in the biological activities of fucoidan.We previously reported that fucoidan fromS.japonicahave protective effect on chronic renal failure, diabetic nephropathy, and acute kidney injury (Zhang et al., 2005; Yang et al., 2013; Wang et al., 2014).LMWF at the same dose had a better protective effect than fucoidan (Zhang et al., 2003;Tan et al., 2020).Since fucoidan and LMWF had similar sugar constituents but different molecular weight, the difference in renoprotection between fucoidan and LMWF might at least partly due to their different pharmacokinetics.
Since the measurement used in this study is based on the hydrolyzed fucose concentration, the present work could not distinguish whether LMWF or fucoidan was degraded or not in the body.Previous study illustrated that fucoidan degradation occurred in the excretory system, possibly in the kidney, but not in the intestine by bacterial flora(Imbs et al., 2020).Tokita et al.(2010a) reported that,in the serum and urine of healthy volunteers, the molecular weight of the serum fucoidan remained unchanged (66 kDa), whereas that of urine fucoidan was significantly reduced (1.8–3.1 kDa).Our previous study using HPLC post-column fluorescence derivatization method also revealed that the molecular weight of the serum LMWF remained unchanged afteri.v.injection (Zhang et al., 2016).Therefore, it is likely that fucoidan or LMWF was not degraded in the serum, but the metabolism of LMWF after oral administration remains to be studied in the future.
5 CONCLUSION
The pharmacokinetics of fucoidan and LMWF were evaluated by measuring the serum fucose concentration after oral administration or intragastric administration.It is found that both fucoidan and LMWF were absorbed following intragastric administration.At the dose of 30 mg/kg, LMWF was absorbed more quickly and had a higher AUC0–tthan fucoidan.The estimated bioavailability of LMWF was 28.3% after a single dose of 30 mg/kg.LMWF was widely distributed in tissues following oral administration and the highest concentration was found in kidney.The results obtained in this study may provide reference for clinical trial design and scheme based fucoidan or LMWF.
6 DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2023年5期
Journal of Oceanology and Limnology2023年5期
- Journal of Oceanology and Limnology的其它文章
- Motion simulation of moorings using optimized LSTM neural network*
- Diel, seasonal, and annual variations of fish assemblages in intertidal creeks of the Changjiang River estuary*
- Spatio-temporal dynamics of cyanobacterial abundance and toxicity in a Mediterranean hypereutrophic lake*
- A new decomposition model of sea level variability for the sea level anomaly time series prediction*
