Plant phenolic extracts for the quality protection of frying oil during deep frying: Sources,effects,and mechanisms
F Wng ,Yixi Sun ,Shnshn Li,b ,Jing Yn,b ,Wen Qin,b ,Ahmed S.M.Sleh ,Qing Zhng,b,*
a College of Food Science, Sichuan Agricultural University, Ya’an, Sichuan, China
b Key Laboratory of Agricultural Product Processing and Nutrition and Health of the Ministry of Agriculture and Rural Affairs (Jointly Built by the Ministry and the Province), Ya’an, Sichuan, China.
c Department of Food Science and Technology, Faculty of Agriculture, Assiut University, Egypt.
Keywords: Frying oil Phenolic antioxidants Plant extract Physicochemical properties Antioxidant mechanisms
ABSTRACT Protection of frying oil from deterioration by adding plant phenolic extracts to guarantee the quality of fried foods becomes the primary approach to promote the sustainable development of deep frying.Therefore,sources,antioxidant effects,and mechanisms of plant phenolic extracts recently applied in the quality protection of frying oil as well as challenges for the actual use of these extracts are comprehensively reviewed in this study.Spices,herbs,berries,tea leaves,and fruit and vegetable wastes are common sources for preparing phenolic extracts showing comparative antioxidant capacity referring to the synthetic antioxidants.The general effect of using these natural antioxidants is the improvement of thermal stability to extend the shelf life of frying oil and thus the modification of edible quality of fried foods.Specifically,the increases in common quality attributes and amount of hazardous products and the oxidative reduction of unsaturated triacylglycerols without negatively influencing the sensory quality are inhibited when suitable plant extracts are applied.The incorporation of plant phenolic extracts other than synthetic counterparts in frying oil has been demonstrated as a potential method to improve the frying performance of oils.However,challenges for the scale application of plant phenolic extracts,such as the purity,thermal stability,and antioxidant timing,are still needed to be further investigated.
1.Introduction
Frying is an ancient food cooking method that can be traced back to 600 BCE.Due to its simple process and economic benefits,frying has become one of the most popular food cooking methods in family kitchens,fast food restaurants,and instant noodle or potato chip industries [1].Three frying methods,pan,shallow,and deep frying,are commonly classified according to the different amounts of used oil[2].Deep frying is an extensively used method of soaking foods in oils at 150–200◦C,which can endow products with palatable and delicious eating quality in a short time,such as crisp taste,golden color,and unique fragrance[3].
When frying oil is exposed to air and high temperature for a long time,a series of chemical reactions such as thermal oxidation,hydrolysis,isomerization,and polymerization occurs in oil components to make frying oil deteriorate [4].Consequently,the organoleptic and nutritional qualities of finished products declined,and meanwhile,potential toxic compounds,such as trans fatty acids (TFAs) and cyclic fatty acid monomers,are also inevitably formed [5].Moreover,the Maillard reaction also occurs between the amino group-containing compounds of fried food and the components of frying oil or its oxidative products,which significantly affects the formation of eating quality of fried foods and physicochemical properties and thus the safety of frying oil.Therefore,the control of appropriate frying conditions is vital for obtaining desirable eating quality and inhibiting the formation of hazardous products in fried foods.
When the type of frying oil,frying temperature,time,and frying method(pan drying or deep frying,conventional or vacuum frying)are established,antioxidants are generally added to delay the oxidation of frying oil.Antioxidants can be classified into synthetic,semi-synthetic,and natural antioxidants based on their origin (shown in Fig.1).Synthetic antioxidants,such as butylated hydroxytoluene(BHT),butylated hydroxy anisole (BHA),and tert-butyl hydroquinone (TBHQ) are often added to oils to prohibit oxidation degradation during food storage and frying.However,due to the relatively high volatility,thermal instability,and potential adverse effects on human health under frying conditions,the actual utilization of synthetic antioxidants in foods is controversial[6].On the other hand,many antioxidants naturally exist in foods exhibiting antioxidant activity against the oxidative damage;however,their protective effects are limited because of thermal instability [7,8].Therefore,food industry scientists have put a great effort into exploring suitable substituted antioxidants to add to vegetable oil for strengthening its frying performance.Semi-synthesized antioxidants mainly refer to the plant phenolic extracts derivatives with one or two substituent groups(such as methyl,acetyl,benzyl,benzoyl,and amino group).This type of antioxidant has been already reported due to the stability issues of natural antioxidants,such as 4-nerolidylcatechol extracted from cultivatedPiper peltatumorP.umbellatumroots[9]and rosmarinic acid extracted fromRosmarinus officinalis[10].However,the practical application of semi-synthesized natural antioxidants in the protection of frying oil from thermal decomposition has been seldom reported[3,8].
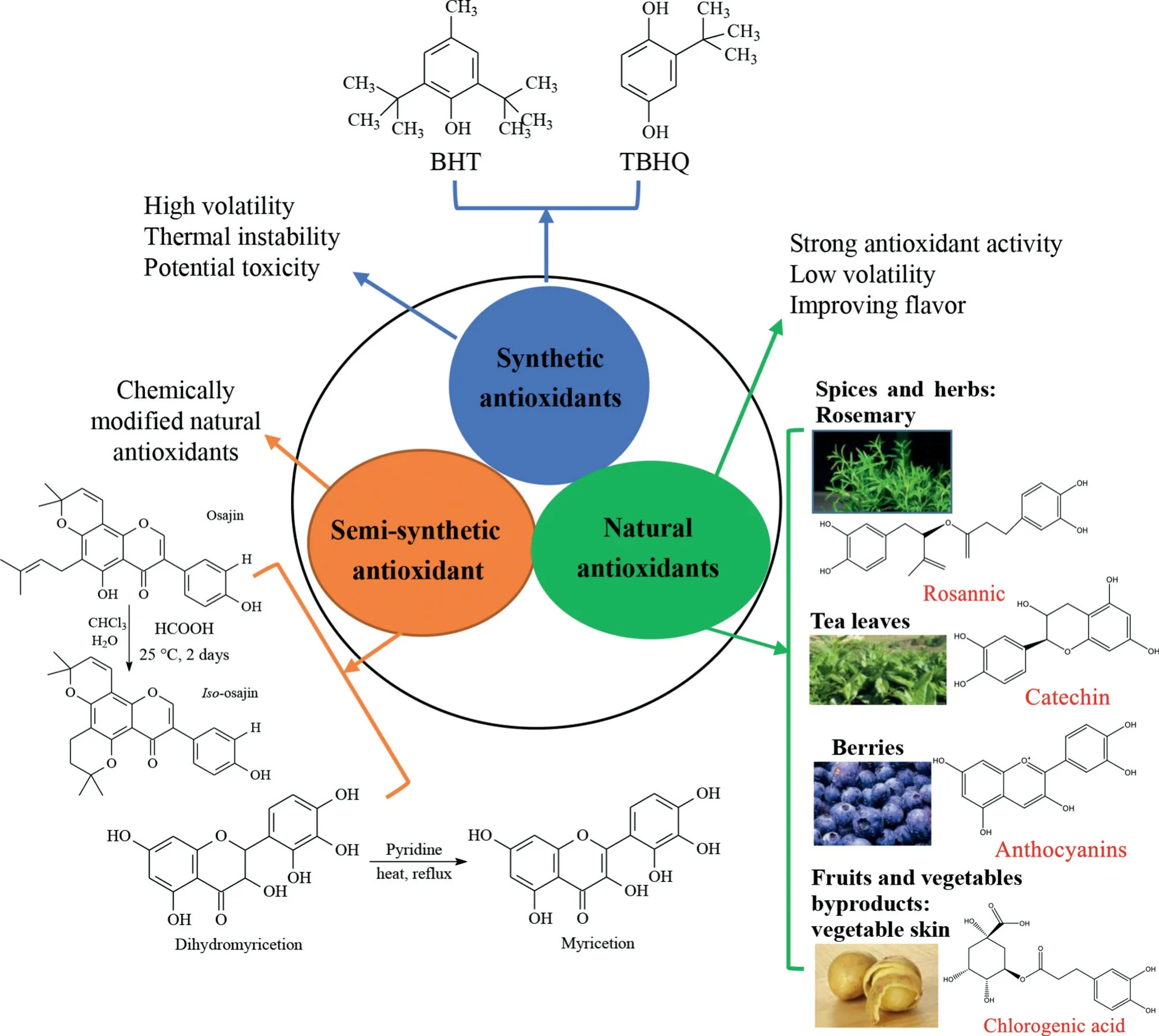
Fig.1. Common types of phenolic antioxidants used to protect frying oils from thermal oxidative decomposition.
Natural phenolic compounds are usually biosynthesized by phenylalanine or tyrosine through the shikimic acid pathway [11].They naturally exist ranging from simple to conjugated or complex compounds,and hydroxyl groups on the benzene ring are responsible for the antioxidant properties of phenolic compounds.Phenolic compounds in plants can effectively delay the thermal oxidative degradation of oils,and meanwhile,reduce the formation of polymerized TAGs and potentially hazardous products such as heterocyclic amines and acrylamide[12,13].Therefore,considering the importance of the application of phenolic antioxidants extracted from plants in frying oil,we shed light on the utilization of plant phenolic extracts for the quality protection of frying oil in this review.
2.Natural phenolic antioxidant and its common types used in oils
Among various synthetic phenolic antioxidants,BHA,BHT,and TBHQ are the most commercially used ones in foodstuffs with a maximum allowable level of 200 mg/kg in food,beverages,or oil products regulated by the U.S.Food and Drug Administration(FDA),the European Union(EU) and Codex Standard[14].Synthetic antioxidants are extensively applied for the quality protection of refined commercial edible oils from oxidative decomposition;however,the safety concern of these antioxidants promotes the development of alternatives to prevent the oxidation of foods [15].In view of the toxicity and volatility of synthetic antioxidants,increasing attention has been paid to the use of natural antioxidants which are widely found in spices,herbs,berries,tea leaves,and raw material wastes.
2.1. Spices and herbs
The effective antioxidant sources in spices and herbs mainly include rosemary (Rosmarinus offificinalisL.),turmeric (Curcuma longaL.),oregano (Origanum vulgareL.),sage (Salvia officinalisL.),and thyme(Thymus vulgarisL.).Antioxidant capacity is not only related to the content of phenolic compounds,but also depends on the number of reactive groups such as the hydroxyl groups(-OH)[16].Exarchou et al.[17]studied the antioxidant activities of phenolic compounds extracted from rosemary,Greek oregano,sage,and summer savory.The results demonstrated that all of them had strong free radical scavenging activity,and the rosemary extract played the highest antioxidative activity,which might be related to the relatively high concentration of phenolic compounds and -OH contained in rosemary.
Rosemary is one of the most commonly used spices in food processing.It is considered a strong lipid antioxidant and metal chelating agent,which can inhibit the formation of polar compounds and polymers and the decomposition of polyunsaturated TAGs.Rosemary extract can also scavenge superoxide radicals [18].These outstanding antioxidant activity benefits from the abundant rosmarinic acid which is a phenolic acid consisting of four phenolic -OH.Specifically,the antioxidant properties of rosemary extract are mainly due to the presence of phenolic diterpenoids such as coumarin and myristic acid.During extraction,myristic acid can be converted to other lipophilic diterpenes[19].Chammem et al.[20] studied the quality of vegetable oils added with rosemary extract under frying conditions and reported that rosemary extract could significantly inhibit the formation of hydroperoxide and polymer,and improve the quality of vegetable oils.They revealed that the rosemary extract had a strong ability to scavenge DPPH radicals.When the concentration of rosemary extract is 0.08%,it has a relatively high free radical scavenging ability of about 81%,which is better than that of BHT with a concentration of 0.1%.Meanwhile,the advantage over synthetic antioxidants is that rosemary extract can produce a desirable taste in fried products,and thus possess a positive effect on improving sensory properties.Redondo-Cuevas et al.[21] comparatively assessed the antioxidant capacity,phenolic content,and ability of natural ground herbs,including black pepper,ginger,turmeric,rosemary,and oregano to improve the oxidation stability of vegetable oils during deep frying.Results exhibited that rosemary powder (0.5%,W/V) was the most effective antioxidant and effectively improved the oxidative stability of sunflower oil (128.91%),olive oil (55.61%),and rapeseed oil(73.20%)compared with the oils without additives during deep frying.The reason is that rosemary powder exhibited the highest antioxidant capacity (573.35 μmol/L Fe2+/g) and total phenolic compounds of 410.16 mg garlic acid equivalents(GAE)/g among the studied herbs,which is attributed to the presence of phenolic diterpenes,such as carnosic acid and carnosol.Based on its outstanding antioxidant activities and protection performance,rosemary has been adopted formally into European regulations as a new food additive for use in foodstuffs(Commission Directive 2010/67/EU and 2020/69/EU).Rosemary extract has also been adopted into Chinese standards for the use of food additives as an antioxidant in vegetable oils with a maximum dosage of 0.7 g/kg and other foods(such as animal fats,meat products,and fried dough products) with a maximum dosage of 0.3 g/kg (GB 2760–2014)[21].
Oregano extract has been shown to be effective in preventing lipid oxidation of fats and vegetable oils.The antioxidant capacity of oregano extract is mainly due to the antioxidant capacity of several phenolic compounds and flavonoids which refer to a series of compounds with two phenolic hydroxyl groups of benzene rings (A-and B-rings) interlinked by a central three-carbon atom,such as apigenin,dihydrocamphorin,and dihydroquercetin [22].Houhoula et al.[23]analyzed the chemical composition of cottonseed oil added with oregano powder (2 g/L) and its ethanolic extract (2 g/L) during deep frying and found that both the oregano powder and extract had inhibitory effects on the formation of polar compounds,conjugated dienes,and TAGs polymers.The peroxide value (POV) and the formation of polar compounds increased linearly during frying;however,the accumulation of conjugated dienes was reduced obviously in oils added with oregano powder or extract.The total polar compound(TPC)content of fresh oil was 5.2%,and it reached 24.2%after frying for 12 h;however,in the oil added with oregano ethanolic extract,the TPC content only reached 19% after the same frying time.Thus,the oregano ethanolic extract exhibited a better inhibitory effect than oregano powder.However,oregano extract has a little positive effect on the formation of free fatty acids(FFAs)during frying due to the volatility of FFAs.
Majorana syriacais a labiatae plant rich in flavonoids and phenolic compounds and thus exhibits antioxidant activity.Al-Bandak et al.[24]studied the effects of ethyl acetate-derived extract ofMajorana syriacaon the changes in the physicochemical properties of refined corn oil.They stated that the TPC content of corn oil added with 500 mg/kg extract decreased significantly by 1.2% compared with the oil without extract after frying at 185◦C for 14 h and the conjugated diene content also decreased approximately by 0.5 g/100 g.Therefore,Majorana syriacaextract possesses a certain ability to improve the oxidative stability of corn oil during deep frying.
Strong antioxidant capacity is extensively reported to be found in the phenolic extracts of spices and herbs;moreover,some spices or herbs extracts have been already officially identified as legal food antioxidants.Therefore,extracts from spices or herbs deserve further to be explored to postpone the shelf life of frying oil and thus the eating quality of fried foods.
2.2. Berries
Synthetic antioxidants can effectively delay the oxidative degradation of oil during storage;however,during frying,the free radical scavenging ability of many natural polyphenols that existed in berries is significantly higher than those of synthetic antioxidants.Aladedunye et al.[7]studied the changes in quality attributes of rapeseed oil added with 500 μg GAE/g methanol-derived phenolic extracts of red fruits and Sorbus berries under frying conditions.They reported that after frying for 8 h,the TPC content of rapeseed oil in the control group was 29%,which was 25%higher than that of the samples added with red fruits and Sorbus berries extracts.In addition,the increase inp-anisidine value(p-AV) and the decrease in iodine value (IV) were also significantly inhibited.Recently,blueberry byproduct extract obtained by the hydroalcoholic extraction of blueberry residues after juice manufacturing also exhibited an inhibitory response on sunflower oil oxidation heated at 180◦C up to 12 h [25].Results showed that 500 mg/kg blueberry byproduct extract exhibited a similar inhibition of lipid oxidation to 200 mg/kg BHT.However,when the level of blueberry byproduct extract reached 800 mg/kg,the inhibitory effects of lipid oxidation were more prominent with the least POV andp-AV and highest inhibition of oil oxidation value compared with the 200 mg/kg BHT-added oil or the additives-free oil.Therefore,compared with BHT,berries extract showed a more significant antioxidant effect in a prolonged frying time.
2.3. Tea leaves
Tea is the most important solid beverage worldwide due to its recognized health benefits which are closely related to its antioxidant activities.Tea leaves are rich in tea catechins,such as epicatechin,epigallocatechin,epicatechin gallate,and epigallocatechin gallate;thus,they have a strong ability to scavenge free radicals,such as DPPH radicals,hydroxyl radicals,and superoxide anions.Many studies have demonstrated the inhibitory effects of tea extracts on lipid oxidation and thus quality protection of oils during heating or deep frying.Zandi et al.[26] stated that when the concentration of old tea methanolic extract was 0.02%–0.25%,the antioxidant activity was directly proportional to the concentration,and the formation of TPC was inhibited during repeated frying at 180◦C.Meanwhile,the antioxidant activity of 0.1%old tea extract was similar to that of 0.1% rosemary extract.In a study regarding the influence of natural antioxidants on the polymerization of partially hydrogenated rapeseed oil heated at 170◦C for 40 h,the ethanolic extracts of green tea and yellow tea exhibited a higher inhibition degree of the final content of polymers(including dimers,trimers,and oligomers) in oils than those of blackberry or cranberry or even BHT.Tea polyphenols are a general name for catechins extracted from tea,which include epicatechin,epigallocatechin,epicatechin gallate,and epigallocatechin gallate.Due to the componential complexity of tea extract,the mechanism of the anti-polymerization action of polyphenols is still not fully understood.However,the better anti-polymerization properties of natural phenols than that of synthetic antioxidants are probably related to the less volatility and improved solubility of polyphenols against increasing temperature [9].Therefore,tea leaves are also an effective source of natural antioxidants for the quality protection of frying oil.Tea polyphenol is also one of the few antioxidants formally adopted into the Chinese standards for the use of food additives in oil with a maximum dosage of 0.4 g/kg (GB 2760–2014).
2.4. Fruit and vegetable byproducts
As one of the bulk agricultural products,fruits and vegetables are also rich sources of polyphenols,especially the parts of peels and seeds.However,during processing,peels and seeds are often discarded to cause food waste and possibly pollute the environment.Studies have confirmed that fruit peel contains abundant phenolic compounds,such as hydroxycinnamic acid,hydroxybenzoic acid,non-anthocyanin ketones,and anthocyanins and vegetable skin contains caffeic acid,chlorogenic acid,and ferulic acid [27].Therefore,phenols-enriched fruit and vegetable pomace can be considered an excellent source of natural antioxidants.
As a member of the family Rosaceae,loquat also possesses considerable antioxidant activity.Previous studies have described that the antioxidant activity of loquat fruits is related to the presence of hydroxycinnamic and benzoic acids derivatives and cyanidine glycoside.Delfanian et al.[28] studied the effects of ethanolic loquat peel extract on the oxidative stability of soybean oil heated at 180◦C.Results showed that the ability to scavenge free radicals increased with the increase of extract concentration,which was caused by the increase of phenolic and tocopherol compounds in loquat peel extract.The authors also found that 400 mg/kg loquat peel extract exhibited the highest antioxidant activity.During deep frying of potato pieces in soybean oil added with 400 or 1000 mg/kg loquat skin ethanolic extracts,the increase in TPC content,POV,and FFAs of oils was significantly inhibited compared with the oils without additives.Moreover,the protection of soybean oil quality provided by 400 mg/kg ethanolic loquat skin extract was comparable with or in some cases better than that offered by TBHQ.Thus,when applied appropriately,such as under suitable frying conditions,ethanolic loquat skin extract might be a reliable natural antioxidant to guarantee the stabilization of soybean oil during deep frying and finally the quality of fried foods.
Tocopherol is the most common natural antioxidant existing in food and medicine.It is considered to be an effective free radical scavenger and a metal chelating agent.However,under frying conditions,tocopherol is easily lost;thus the antioxidant capacity is not lasting under frying conditions[27].Bopitiya et al.[29]comparatively studied the effects of pomegranate peel extract (2%,W/W) andα-tocopherol(2%,W/W) on the physicochemical properties of white coconut oil during the frying of potatoes at 180±5◦C.They demonstrated that oil samples containing bothα-tocopherol and pomegranate peel extract exhibited a lower POV and prolonged induction period compared with the control group,indicating that both of them had good antioxidant activity.This result might be due to the fact that a large number of phenolic compounds,such as ellagic acid,are contained in pomegranate peel and meanwhile,a synergistic antioxidant effect exists among them.Ethanolic extract of pomegranate peel (0.14%) was also applied to prohibit the formation of acrylamide during deep frying of French fries in soybean oil[30].Results showed that accompanying the decrease in increased amplitude of acid value (AV),POV,and TPC content,an inhibition rate of 83%for the formation of acrylamide was also observed during deep frying.Therefore,the commonly abandoned pomegranate peel is a potential source of antioxidants to stabilize lipid-containing foods or inhibit the formation of hazardous compounds during deep frying.
As a by-product during the processing of rambutan wine or preserved rambutan,Rambutan peel is rich in ellagic acid,quercetin,and rutin.Phuong et al.[31] found that the extracted rambutan peel powder(1000 μg GAE/g) using methanol 80% could delay the oil oxidation during deep frying of soybean oil at 160◦C.Levels of thiobarbituric acid(TBA) reactive substances of potatoes fried in oil fortified with this extract and TBHQ (0.40–0.59 μg/g) are much lower than those of potatoes fried in oil without additives(1.31±0.10 μg/g).Therefore,fruit peels hold an immense potential to be developed as an effective natural antioxidant for the quality protection of frying oil.
Among the total phenolic acids-enriching vegetable sources,potatoes exhibit considerable contents varying from 7.9 mg/100 g(cooked and peeled Rosamunda variety) to 52 mg/100 g (cooked peel of Van Gogh variety) [32].Phenolic compounds in potatoes are mainly concentrated in peel and adhesive tissues,from which the extracts can be used to prevent the oxidation of edible oils or lipid-containing foods[33,34].For instance,Mohdaly et al.[35] comparatively studied the prevention efficiency of potato peel methanolic extract and synthetic antioxidants (TBHQ,BHT,and BHA) against the thermo-oxidation of sunflower or soybean oil at 70◦C for 72 h.Results indicated that the order of oxidative stability for the additive-added oil samples was TBHQ>potato peels>BHT>BHA,which is closely related to the chlorogenic and gallic acids contained in potato peels.
The non-edible parts of fruits and vegetables,the loss during harvesting and processing,and the waste during consumption are strikingly vast worldwide every year.Therefore,the development of fruit and vegetable byproducts based on their antioxidant activities deserves to be explored.It is important to mention that although plant extracts and polyphenols have good potential as natural antioxidants for deep frying applications,a significant disadvantage is their poor solubility in oil.Due to their structural diversity,the solubility of natural phenolic compounds in oil is variable [36].Aladedunye et al.[7] observed that the antioxidant efficiency of polyphenols increased with the increasing temperature,which may be related to the increased solubility or diffusion of polyphenols at high temperatures.In addition,the water vapor and aeration generated during frying process may also improve the solubility and diffusivity of polyphenols.Therefore,it should be indicated that the stability,versatility,and effectiveness of these plant extracts and the developing cost should be further confirmed for extensive application.
3.Effects of phenolic extracts on the physicochemical properties of frying oil
The sensory quality,chemical composition,and nutritional value of oil change during deep frying,which can be reflected by the increase in acidity,formation of secondary oxidation products,decrease in IV,and the increase in levels of hazardous products with low molecular weight and polymers with high molecular weight [37].The effects of plant phenolic extracts on the conventional physicochemical properties and hazardous products (exhibited in Fig.2) during deep frying and the corresponding representative studies (listed in Table 1[7,14,16,19,20,28,38–50]and Table 2[42,49,51–59])are argued in the following text.
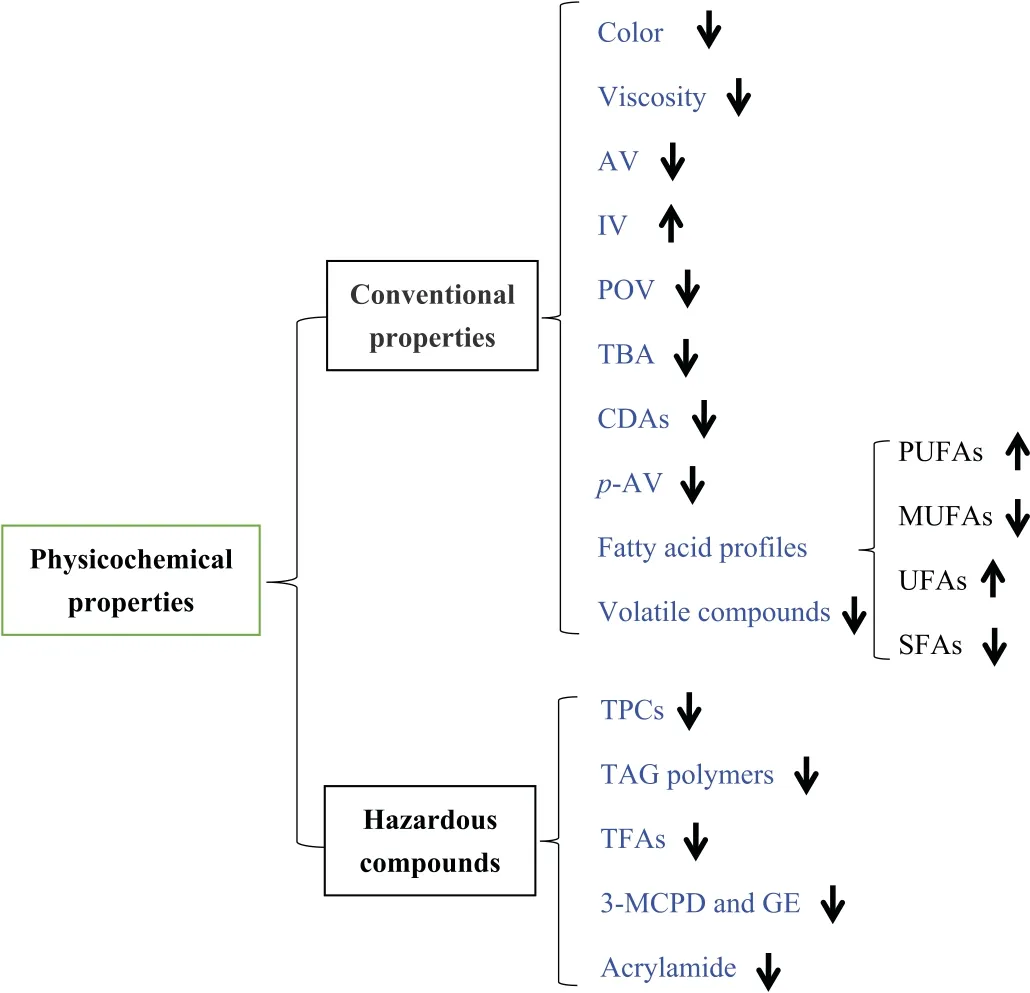
Fig.2. Changes in physicochemical properties of frying oil added with plant phenolic extracts during deep frying.
3.1. Conventional physicochemical properties
3.1.1.Color
Pure TAGs are colorless and each type of oil has its typical color due to the contained natural pigments or the produced colored substances during processing.For instance,the yellow color of fresh frying oil is probably related to the contained lutein and carotenoids.When frying oil is exposed to high temperatures and light for a long time,these pigments are more likely to oxidize to form a darker substance.For instance,the formation and accumulation of oxidative decomposition products of unsaturated TAGs,such as oxidized TAGs and FFAs,contribute to the changes in color of frying oil.Moreover,the Maillard reaction occurs to form considerable colored products to dissolve into and finally darken frying oil during food frying.
Za’atar essential oil rich in 79.5%–86.2% carvacrol and BHT were applied to retard the darkening of palm oil during deep frying of potato slices at 180◦C for 5 consecutive days with a total of 50 frying batches[38].Results showed that both antioxidants slowed the rate of color change during frying and no difference in changes in the color parameters (L*,a*,andb* values) was observed between the natural and synthetic antioxidants.According to the study of Nor et al.[39],French fries were fried in RBD palm oil added with 0.2% ethanolicPandanus amaryllifoliusleaf extracts or 0.02%BHT.After deep frying at 180◦C for 40 h,the oil treated withPandanus amaryllifoliusleaf extracts turned black obviously,but the BHT-added oil exhibits no apparent change.Moreover,it is worth mentioning that during the entire frying process,the color,oiliness,and crispness of French fries have no obvious changes,indicating thatPandanus amaryllifoliusleaf extracts can keep the sensory quality of fried foods during frying.The difference in color change of frying oil caused by different plant extracts might be related to the different composition of polyphenols or the different inhibition degrees of thermal oxidation of frying oil.
In addition,the oxidation of oil absorbed by fried potato chips could indirectly affect consumers’intake of hazardous pro-oxidation products(free radicals).Lalas et al.[40]compared the quality of control oil and mixed oil (80% cottonseed oil,10% sunflower oil,and 10% palm oil)which was added with RoseMax (10% rosemary extract dissolved in ethanol) after frying at 185◦C for 90 s.Through the comparison of induction period measurement,the RoseMax not only improved the oxidation resistance of frying oil,but also strengthened the oxidation resistance of oil absorbed by potato chips,and reduced the darkening and rancidity of oil.Therefore,the retardation of color darkening by natural antioxidants during deep frying is vital for processing fried foods with premium quality and safety.
3.1.2.Viscosity
The increase in viscosity is caused by hydrolysis,polymerization,and isomerization during frying.Among the numerous products,TAG polymers account for the increase in the viscosity of frying oil.With the increase of frying temperature,the viscosity of frying oil generally increases,which can be retarded by adding appropriate natural antioxidants.Bensmira et al.[41] reported that the viscosity of untreated sunflower oil increased from 0.084 to 0.089 Pa⋅s (i.e.,about 6.38% increase),which was significantly higher than that of sunflower oil added with lavender and thyme extracts(i.e.,increased by 1.56%and 1.60%,respectively) during frying from 25 to 200◦C.The synergistic effect between natural antioxidants in lavender and thyme inhibited the loss ofα-tocopherol which is regarded as the most important natural antioxidant in sunflower oil [60],thus delaying the oxidative polymerization.Gao et al.[42]found that 400 mg/kg tea polyphenols could significantly reduce the increase in viscosity of rapeseed oil when subjected to frying potato slices at 180◦C for 8 h per day and a consecutive 3 d.Therefore,tea polyphenols can inhibit the polymerization of oil and thus reduce the increase rate of viscosity.
3.1.3.Acid value(AV)
There are two main reasons for the increase in AV during deep frying.Firstly,most acidity is caused by the increase in FFAs,which are formed by the hydrolysis of triglycerides and the oxidative decomposition of unsaturated TAGs[4].The other part of the increase in acidity is caused by the increase in carboxyl-containing polymer formed by thermal or oxidative polymerization during frying.El-Hamidi et al.[43] revealed that the acidity of sunflower oil added with phenolic acids and flavonoids-containing garlic extract or garlic powder during deep frying at 180◦C was dramatically lower than that of the control group.Therefore,it can be explained that both garlic extract and garlic powder inhibited the decomposition or polymerization of TAG during frying.As discussed in our previous study,different vegetable oils(palm,soybean,and rapeseed oils) added with ethanolic extract ofChuanminshen Violaceum(0.07%) or TBHQ (0.02%) exhibited different increase rates ofAV when applied to fry potato strips at 180◦C for 12 h per day and 3 consecutive days [44].The reason for this phenomenon was explained that the extract or TBHQ exerted its protection by prohibiting the oxidation decomposition of unsaturated TAGs other than by prohibiting the hydrolysis of TAGs.Therefore,similar to pure synthetic antioxidants,natural antioxidants with numerous phenolic compounds possess the similar antioxidant mechanism.
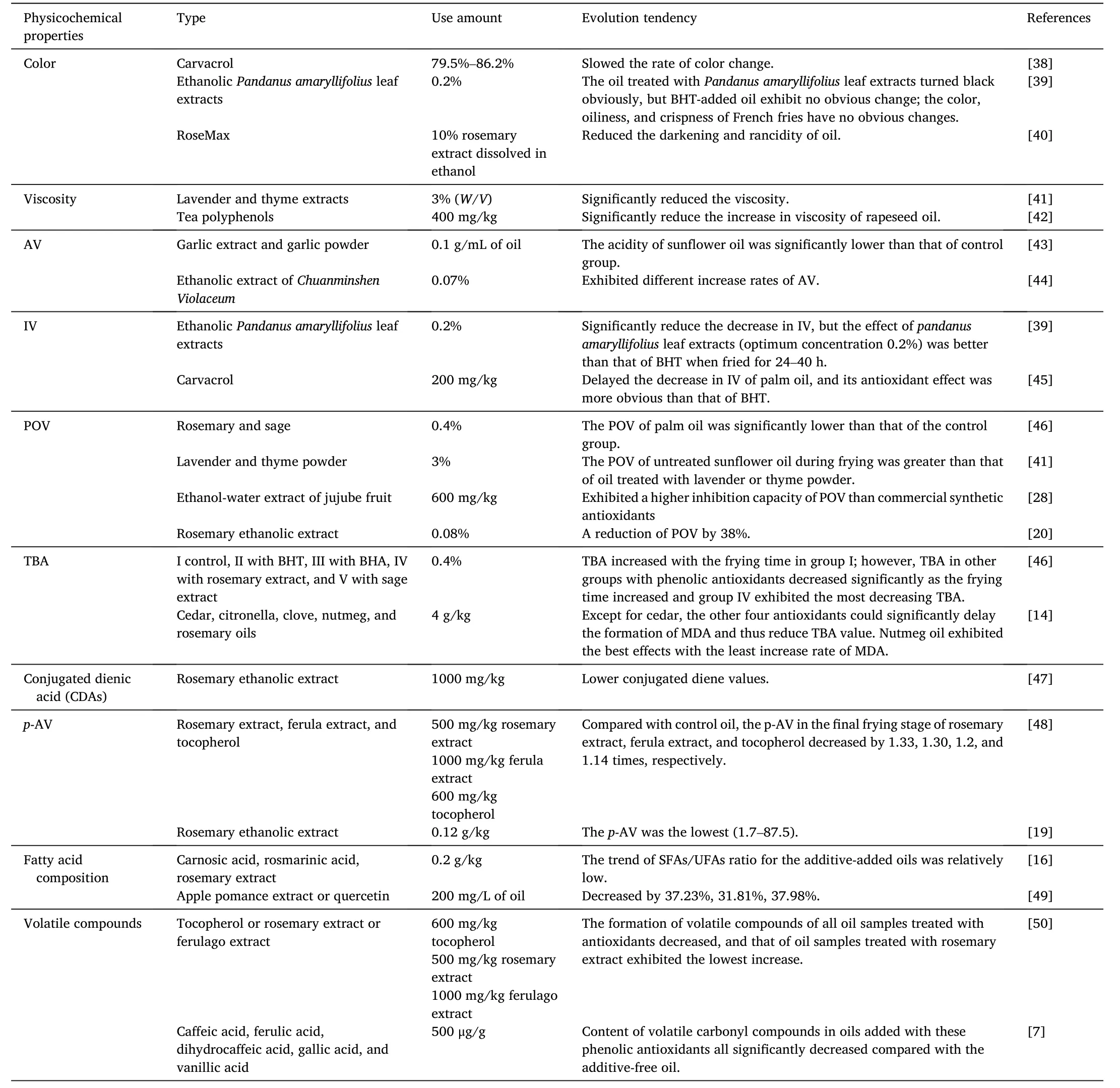
Table 1 Influence of natural phenolic extracts and synthetic antioxidants on the evolution of physicochemical properties of oil during deep frying.
3.1.4.Iodine value(IV)
The decrease in IV is a sign of the decrease in oil unsaturation or specifically the decomposition of carbon-carbon double bonds of unsaturated TAGs during deep frying.Whether it is the direct interaction of cross bonds to form 1,2-diol or the chain reaction adjacent to double bonds to form volatile degradation products,the final result would lead to a decrease in the IV of frying oil.During frying of French fries in RBD palm olein at 180◦C [39],both BHT andpandanus amaryllifoliusleaf extracts could significantly reduce the reduction of IV,but the effect ofPandanus amaryllifoliusleaf extracts(optimum concentration 0.2%)was better than that of BHT when fried for 24–40 h.Carvacrol mainly existing in oregano,thyme,clove,and other plants is a phenolic compound with strong antioxidant activity.˙Inanç et al.[45] studied the effect of 200 mg/kg carvacrol on the quality of palm oil during deep frying at 150◦C and also demonstrated that carvacrol delayed the reduction of IV of palm oil during frying,and its antioxidant effect was more distinct than that of BHT.The IV of oil samples with carvacrol and BHT and without antioxidants decreased from the initial value of 57.0 to 51.7,49.2,and 46.7,respectively.According to the correlation between changes in IV and the addition of natural antioxidants,polyphenols contained in plant extracts might exert their antioxidant activities by protecting the unsaturated TAGs from decomposition.
3.1.5.Peroxide value(POV)
POV reflects the primary oxidation products formed during the frying process,but with the increasing frying time,POV generally first increases and then decreases.The reason is that hydroperoxides easily tend to be degraded under thermal conditions to form secondary oxidation products,such as hydrocarbons,alcohols,ketones,and aldehydes[46].Therefore,POV can be used as a primary index to judge the degree of oil oxidation,and at the same time,it may be used as an index of lipid peroxidation.
Man et al.[60]studied the physicochemical properties of RBD palm oil after frying at 180◦C for 6 d and found that the POV of palm oil treated with rosemary (0.4%) and sage (0.4%) was significantly lower than that of the control group.As reported by Bensmira et al.[41],the hydroperoxide amount of sunflower oil increased by about 150%when subjected to heating from 25 to 200◦C for 30 min,while that of sunflower oil treated with lavender and thyme powder increased only by about 62% and 65%,respectively.Therefore,the POV of untreated sunflower oil during frying is significantly greater than that of oil treated with lavender or thyme powder.The antioxidant activity of thyme powder was mainly related to its 70% volatile compounds.Thyme essential oil was previously proven to reduce the loss ofα-tocopherol in olive oil when heated at 180◦C for 10 min [61].Jujube fruit extract prepared using supercritical carbon dioxide,ultrasound-assisted,and solvent(ethanol)extraction methods were used to extend the shelf life of soybean oil when used to fry potato pieces at 180◦C for 24 h [28].Results showed that the ultrasound-assisted ethanol-water extract at 600 mg/kg exhibited a higher inhibition capacity of POV than commercial synthetic antioxidants,such as BHA,BHT,and TBHQ.A reduction of POV by 38% after 30-h heating of soybean and sunflower oil mixture (1:1,V/V) at 180◦C was observed after adding rosemary ethanolic extract[20].However,at the first 6-h heating of this mixed oil,the POV increased by about 100%in the control and nearly unchanged in the rosemary ethanolic extract-added oil.These results suggest that the polyphenols contained in plant extracts can inhibit the formation of hydroperoxides or the initial stage of oxidation of unsaturated oil during deep frying.
3.1.6.Thiobarbituric acid(TBA)
TBA is a reliable index widely used to determine the level of secondary oxidation products formed during deep frying,which is related to the content of malondialdehyde(MDA)formed during lipid oxidation.The accumulation of these secondary oxidation products during repeated frying affects the quality of the oil,which is the main reason for the odor of frying oil.Man et al.[60]studied the changes in the quality of RBD palm oil added with different antioxidants(BHT,BHA,rosemary extract,and sage extract)during frying at 180±5◦C for 7 consecutive days.Results showed that the TBA increased with the increasing frying time in the control;however,TBA in other groups with phenolic extracts decreased evidently and oil with rosemary extract exhibited the most decreasing TBA.The reason for this discrepancy is that rosemary extract can efficiently delay lipid oxidation by effectively supplying hydrogen to free radicals and meanwhile keeping highly stable at high temperatures.
Yang et al.[62] comparatively studied the changes in physicochemical properties of palm oil added with five natural antioxidants(cedar,citronella,clove,nutmeg,and rosemary oils)after deep frying at 180◦C for 6 h per day in 5 consecutive days.They reported that except for cedar,the other four antioxidants could significantly delay the formation of MDA and thus reduce TBA value;wherein,the nutmeg oil exhibited the best effects with the least increase rate of MDA.The MDA content of nutmeg oil-added palm oil(2 g/kg)was 44.1%–74.0%lower than that of the control during heat treatment.Meanwhile,with the increase of nutmeg oil concentration (1–5 g/kg),the content of MDA remarkably decreased;therefore,the higher the nutmeg oil concentration was,the better the antioxidant effect obtained.Although a pronounced retardation of MDA formation was observed when nutmeg oil was applied at high concentrations,the cost and flavor of the additive should be also carefully considered for the actual application.
3.1.7.Conjugated dienic acids(CDAs)
According to the study of Shahidi et al.[63],the oxidation of polyunsaturated fatty acids (PUFAs) involves the formation of hydroperoxide and following the CDAs due to the replacement of carbon-carbon double bonds.The CDAs can remain in frying oil to be detected using photo spectroscopy at 232 nm.Choe et al.[64] reviewed that the formation of CDAs in blended soybean and roasted sesame oils were lower than that of pure soybean oil when subjected to heating at 160◦C.The reason is that sesame oil contains thermally stable compounds with antioxidant activities,such as sesamol and sesamin [65].In addition,they also concluded that the higher the content of sesame oil in blended oil was,the less the CDAs formed.Similarly,in a comparative experiment conducted by Ramalho et al.[47],lower conjugated diene values were found for the refined soybean oil added with 1000 mg/kg rosemary ethanolic extract during the frying process at 180◦C.Therefore,polyphenols contained in rosemary ethanolic extract can inhibit the oxidation of frying oil,thus inhibiting the formation of CDAs.
3.1.8.p-Anisidine value(p-AV)
p-AV is a measurement of the level of secondary oxidation products formed via the oxidation of frying oil,especially the content of aldehyde compounds,such as 2,4-dienes and 2-olefins produced by the decomposition of hydroperoxides.Alizadeh et al.[50] comparatively studied the effects of 100 mg/kg TBHQ,500 mg/kg rosemary extract,1000 mg/kg ferula extract,and 600 mg/kg tocopherol on the oxidation of a mixed oil consisting of palm oil and sunflower oil(1:1,W/W)when subjected to frying with potato slices at 180◦C for 5 h with a total of 25 frying batches.Results showed that thep-AV of all oil samples increased with the increasing frying time.However,compared with the control,thepanisidine formed in the final frying stage of TBHQ,rosemary extract,ferula extract,and tocopherol decreased by 1.33,1.30,1.2,and 1.14 times,respectively.TBHQ possesses twopara-hydroxyl groups and thus has superior antioxidant activity in the reproductive stage of oil oxidation because of its high hydrogen donor capacity.
Guo et al.[19]analyzed the effects of rosemary ethanolic extract on the changes inp-AV of palm oil during deep frying of potatoes for 5 h per day and a consecutive 5 d.They revealed that during the entire frying process,thep-AV of RBD palm oil with 0.12 g/kg rosemary ethanolic extract group was the lowest (1.7–87.5),which was significantly different from that in the control (1.7–93.4).In addition,it was found that TBHQ had a better effect on inhibiting lipid oxidation under the same conditions,which was inconsistent with the results of Alizadeh et al.[50].The possible reason is that the antioxidant capacity of TBHQ is fixed by its pure phenolic compounds,while the rosemary ethanolic extract is a mixed system containing diverse phenolic compounds and thus possesses synergistic antioxidant effects [19].
3.1.9.Fatty acid composition
Most reactions (generally thermal oxidation) occurred during deep frying centered on the carbon-carbon double bonds contained in fatty acids,thus leading to a significant decrease in the content of unsaturated fatty acids (UFAs,especially polyunsaturated ones).Therefore,maintaining fatty acid profiles or postponing the oxidation of PUFAs is the main objective for the quality control of frying oil,which has also been achieved by adding suitable plant phenolic extracts.
Li et al.[16] studied the changes in the fatty acid composition of refined soybean oil added with 0.2 g/kg TBHQ,natural antioxidantscarnosic acid,rosmarinic acid,and rosemary extract during frying at 180± 5◦C.Results exhibited that with the increasing frying time,the saturated fatty acids (SFAs)/UFAs ratio increased significantly for the control;however,the trend of SFAs/UFAs ratio for the additive-added oils was relatively low,indicating that both synthetic and natural antioxidants had a better inhibition effect on the oil oxidation during the frying process.Manzoor et al.[49] also demonstrated that apple pomance extract also effectively slowed down the decrease rate of PUFAs.Generally,the content of PUFAs decreases,while the content of monounsaturated fatty acids (MUFAs) and SFAs increases with the oxidation process during deep frying.For instance,after frying at 180◦C for 25 h,the PUFAs content of the oleic and linoleic acid-enriched mustard oil added with apple pomance extract,quercetin,and TBHQ or control oil decreased by 37.23%,31.81%,37.98%,and 51.45%,respectively;however,MUFAs and SFAs contents increased by 4.92%,3.35%,5.41%,and 8.67% and 25.36%,18.71%,24.87%,and 37.43%,respectively.Therefore,compared with synthetic antioxidants,natural antioxidants can better maintain the fatty acid composition of frying oil and delay the oxidation of oil during deep frying.
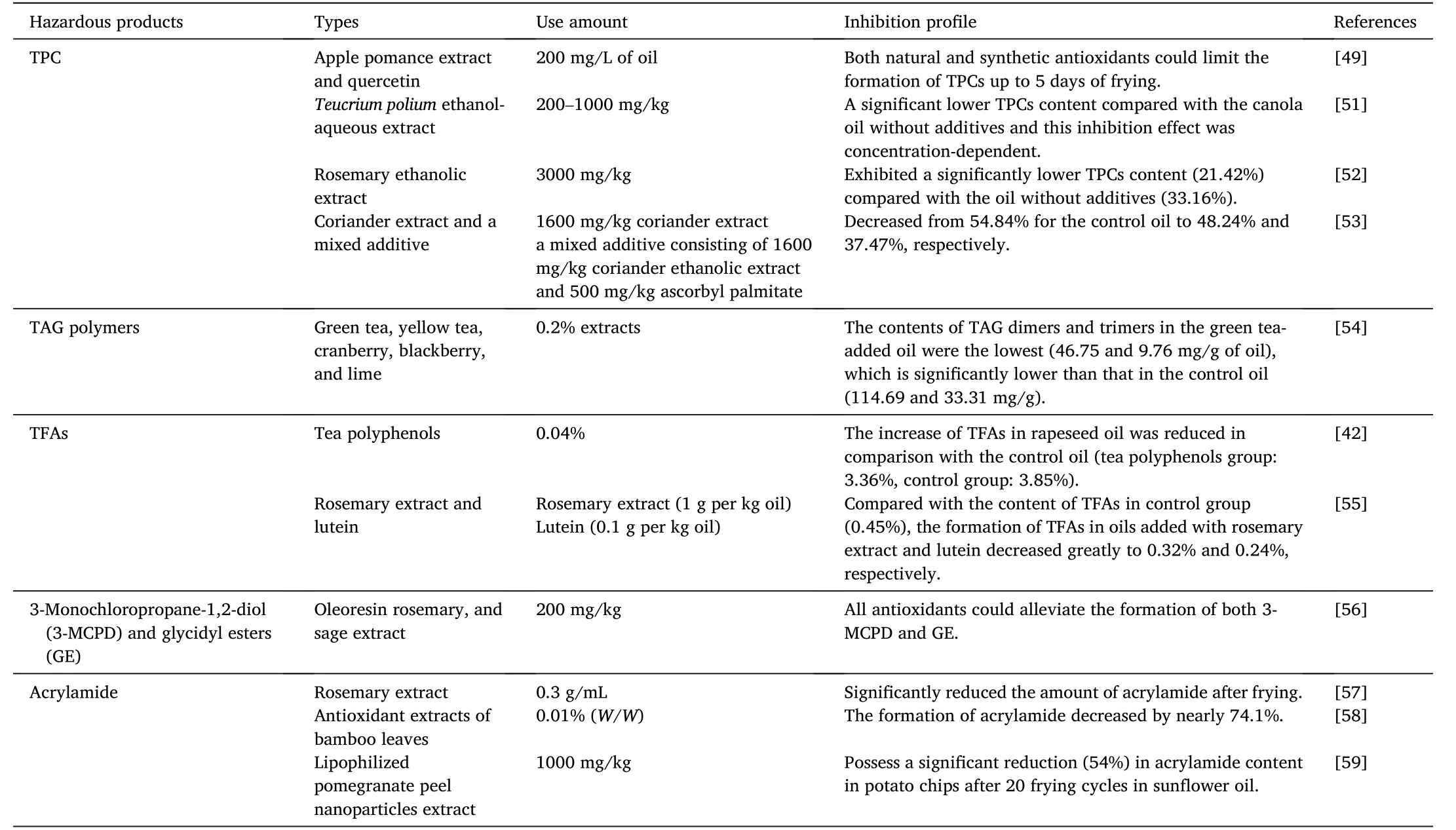
Table 2 Influence of natural phenolic extracts and synthetic antioxidants on the formation of hazardous products during deep frying.
3.1.10.Volatile compounds
Apart from the constituents existing in frying oil or being absorbed by fried foods,numerous volatile compounds,such as aldehyde volatiles[66]and non-aldehyde volatiles[67]formed during deep frying.These volatile compounds are mainly derived from the oxidative decomposition of unsaturated TAGs;thus,most of them can easily volatilize away from frying oil with the increase of heating temperature and time during deep frying.Therefore,effective inhibition of the formation of volatile compounds is also extensively investigated by applying appropriate control methods.
Alizadeh et al.[50] evaluated the effects of 600 mg/kg tocopherol,500 mg/kg rosemary extract,and 1000 mg/kg ferulago extract on the oxidation of a mixture of sunflower oil and palm oil(1:1,W/W)during deep frying at 180◦C,respectively.Results showed that after six frying treatments,the formation of volatile compounds in all oil samples treated with antioxidants decreased remarkably,and that of oil samples added with rosemary extract exhibited the lowest increase.Compared with the control oil,the concentrations of hexanal and heptanal in the rosemary extract-added oil exhibited a reduction of 38.3%and 17.65%.Aladedunye et al.[7] studied the effects of five phenolic acids,caffeic acid,ferulic acid,dihydrocaffeic acid,gallic acid,and vanillic acid,on the quality of canola oil(with a concentration of 500 μg/g)during deep frying at 185 ± 5◦C.Results exhibited that the content of volatile carbonyl compounds in oils added with these phenolic extracts all significantly decreased individually by 57%,52%,58%,54%,and 42%compared with the control oil.The results were consistent with previous findings in which derivatives of cinnamic acid have better antioxidant capacity than the corresponding benzoic acid analogs.This also proves that phenolic extracts can improve the quality of frying oil reflected by reducing the production of volatile compounds.
3.2. Potential hazardous compounds
The thermal or oxidative decomposition and polymerization during the frying process mainly produce two types of products: volatile products and nonvolatile products.A portion of volatile compounds evaporates away with steam into the atmosphere during deep frying,and the remaining volatile compounds in oil would undergo further chemical reactions or be absorbed in fried foods.Non-volatile products are mainly formed by thermal oxidation and polymerization of UFAs during deep frying.These products can not only further promote the degradation of frying oil,but also be absorbed by fried foods,and will be ingested by consumers,thus potentially causing health issues [68].For example,lipid peroxide,MDA,TAG polymers,heterocyclic amines,acrylamide,and others formed during the frying process may lead to coronary heart disease,diabetes,and cancers [52].Hosseini et al.[69]reported that the risk of hypertension was positively correlated with the intake of polar compounds in edible oil.The increase in the content of TFAs and the decrease in the content of essential fatty acids in deteriorated oil will lead to an increased risk of atherosclerosis [42].Therefore,prevention or control of the formation of potentially hazardous compounds during deep frying is another vital task for the processing of healthy fried foods.Fortunately,most hazardous compounds form due to the changes in the molecular structure of unsaturated TAGs during deep frying;thus,the levels of these hazardous compounds also can be reduced by adding suitable plant phenolic extracts.
3.2.1.Total polar compounds(TPCs)
The nonpolar part of deteriorated frying oil is generally composed of unchanged TAGs and thermally polymerized TAGs during deep frying.Apart from these major ingredients,TPCs formed mainly via hydrolysis and oxidation are considered to be nonvolatile compounds and generally distribute in frying oil to be possibly absorbed in fried foods [70].Therefore,the higher the content of TPC is,the worse the quality of the oil is.Therefore,the TPCs content is formally adopted by many countries to judge the service life of frying oil or used as a discarding level of used frying oil,such as 27%by China and Austria,25%by France,Italy,and Spain,and 24% by Germany[70].
Generally,no matter how frying oil is treated or the frying process is controlled,the TPCs content increases with the increasing frying time due to the intrinsic reactivity of unsaturated TAGs.Therefore,an effective way to reduce the formation rate of TPCs is a major breakthrough for manufacturers or researchers.In general,the addition of plant phenolic extracts inhibits the formation of TPCs or reduces the increase rate of TPCs in frying oil during deep frying[71].For instance,Manzoor et al.[49] comparatively studied the effects of apple pomace ethanolic extract,quercetin,and TBHQ(200 mg/L)on the formation of TPCs of mustard oil during deep frying with potato strips at 180◦C for 5 consecutive days and each day proceeded 5 h with a total of 10 frying cycles.Results showed that both natural and synthetic antioxidants could limit the formation of TPCs up to 5 days of frying;however,the control oil crossed the regulated limit of TPCs(27%)after 15-h frying.In addition,canola oil added withTeucrium poliumethanol-aqueous extract showed a significantly lower TPC content compared with the canola oil without additives during frying with potato pieces at 180◦C for 30 h and this inhibition effect was concentration-dependent from 200 to 1000 mg/kg [51].Similar result was also reported by Moufakkir et al.who added rosemary extract to preserve soybean oil for frying breaded butterfly shrimp[72].Therefore,incorporation of plant extracts possesses a negative impact on the increase in the content of TPCs,which is conducive to the improvement of safety of fried foods.
As for the influence of synthetic antioxidants on the antioxidant capacity of natural extracts during deep frying,many studies have been conducted to obtain different conclusions.Casarotti et al.[52] found that after heating at 180◦C for 20 h,soybean oil added with 3000 mg/kg rosemary ethanolic extract exhibited a significantly lower TPCs content(21.42%) compared with the oil without additives (33.16%).Meanwhile,the TPCs content of soybean oil added with 50 mg/kg TBHQ(27.91%)or a mixture of 3000 mg/kg rosemary ethanolic extract and 50 mg/kg TBHQ (22.71%) was also studied,and the inhibition effect was not as good as that of rosemary ethanolic extract alone,indicating that there was no synergistic effect between TBHQ and rosemary ethanolic extract.However,Angelo et al.[53]stated that after heating at 180◦C for 30 h,the content of TPCs in sunflower oil added with 1600 mg/kg coriander extract and a mixed additive consisting of 1600 mg/kg coriander ethanolic extract and 500 mg/kg ascorbyl palmitate decreased from 54.84% for the control oil to 48.24% and 37.47%,respectively.Therefore,coriander ethanolic extract can delay the oxidation of lipids,and its antioxidant capacity can be further modified by adding suitable ascorbyl palmitate due to the strong synergistic effect between these two types of antioxidants.The synergistic effect between synthetic and natural plant extract might depend on the types of both antioxidants.
3.2.2.Triacylglycerol polymers(TAG polymers)
Polymers are an inevitable product formed during deep frying which is observed in both polar and nonpolar parts of the oil.TAG polymers refer to the dimers,trimers,and oligomers,which are mainly the thermal oxidation products with molecular weight higher than the parent TAG.Under the effects of oxygen and heating,oxidative and thermal polymerization takes place among oil molecules,resulting in the formation of many complex polymers with C–C,C–O–C,and C–O–O–C bonds among TAG molecules.The accumulation of these complex polymers often leads to the deterioration of oil[4].Due to the potential harm to consumers,the content of TAG polymers has also been officially adopted by many countries to regulate the appropriate use of frying oil,such as the 10% by Belgium,12% by Austria and Germany,14% by France,and 16%by the Netherlands[73].
Kmiecik et al.[54]studied the effects of green tea(0.2%),yellow tea(0.2%),cranberry (0.2%),blackberry (0.2%),lime (0.2%),and BHT(0.02%)on the formation of TAG polymers in hydrogenated rapeseed oil heated at 140◦C for 40 h.Results exhibited that the content of TAG dimers or trimers in oils with antioxidants was lower than that in the control oil and the contents of TAG dimers and trimers in the green teaadded oil were the lowest (46.75 and 9.76 mg/g of oil),which are significantly lower than that in the control oil(114.69 and 33.31 mg/g).For the content of TAG oligomers,no oligomer formation was observed in the yellow tea-added oil.Authors explained these phenomena as natural phenolic compounds are less volatile,while heating improves their solubility,and the increased temperature can also induce the formation of different or complementary secondary antioxidants.Therefore,natural phenolic compounds have anti-polymerization properties and are superior to synthetic antioxidants.
3.2.3.Trans fatty acids(TFAs)
Generally,the TFAs with harm to the human body mainly come from thermally refined oil,frying oil,and fried foods.Their excessive accumulation may lead to serious diseases,such as cardiovascular diseases triggered by increasing low-density lipoprotein cholesterol concentration and decreasing high-density lipoprotein cholesterol concentration[74].
So far,many studies have shown that phenolic extracts can effectively inhibit the formation of TFAs during deep frying.As reported by Gao et al.[42],after frying at 180◦C for 24 h,the increase in the content of TFAs in rapeseed oil with tea polyphenols(0.04%,W/W)was reduced in comparison with the control oil (tea polyphenols group: 3.36%,control group:3.85%).Moreover,Filip et al.[55]studied the effects of rosemary extract(1 g/kg oil)and lutein(0.1 g/kg oil)on the formation of TFAs in sunflower oil during deep frying at 180◦C for 120 h.Results exhibited that compared with the content of TFAs in the control group(0.45%),the formation of TFAs in oils added with rosemary extract and lutein decreased greatly to 0.32% and 0.24%,respectively.Recently,incorporation of apple pomace extract in soybean oil with a concentration of 200 mg/L significantly reduced the increase in the content of TFAs after 5-day frying(each day for 5 h) compared with the additivefree oil,such as the reduction by 21% at the last frying [75].In addition,this positive effect was better than that exhibited by adding the same concentration of TBHQ.Therefore,natural phenolic compounds can effectively retard the oxidation of unsaturated TAGs during deep frying.Considering that vegetable oils with high unsaturation can react strongly with oxygen during frying,more and more manufacturers want to reduce the unsaturation of oil by hydrogenation.However,it has been found that the hydrogenation of unsaturated oil will easily cause the formation of TFAs during frying,which would bring adverse effects on the health of consumers.Therefore,Hwang et al.[76] suggested the intake of oils containing unsaturated fats,especially polyunsaturated fats,can reduce potential health problems caused by trans fats and saturated fats.In this way,it is very important to add phenolic extracts to unsaturated edible oil to delay lipid oxidation and rancidity.
3.2.4.3-Monochloropropane-1,2-diol and glycidyl esters(3-MCPD and GE)
3-MCPD and GE found in a wide range of refined vegetable oils and fried and baked products [77] are potentially toxic to humans as foodborne process contaminants.Most studies have demonstrated that the formation of 3-MCPD and GE during frying is related to the chemical changes in TAGs,diacylglycerols,and monoacylglycerols [78].Wong et al.[56]reported that the content of 3-MCPD and GE is related to the composition of oil,heating temperature,and duration.Generally,the higher content of diacylglycerol is,the higher the content of 3-MCPD and GE formed,such as palm oil.In addition,the content of 3-MCPD is positively related to the frying temperature;however,when frying the temperature reaches 220◦C,the formation rate of 3-MCPD slows down and the formation rate of GE increases.The formation and decomposition of 3-MCPD and GE are simultaneous;however,when the temperature reaches a certain threshold,the decomposition speed of 3-MCPD and GE will be higher than the formation speed [79].
Phenolic extracts can inhibit the formation of 3-MCPD and GE by inhibiting the formation of free radical intermediates.Wong et al.[56]studied the effects of BHT,BHA,TBHQ,oleoresin rosemary,and sage extract (200 mg/kg) on the formation of 3-MCPD and GE in RBD palm oil after frying at 180◦C for 10 min.Results showed that all five antioxidants could alleviate the formation of both 3-MCPD and GE.Among the five frying systems,the largest loss of GE was the oil added with TBHQ,followed by the oleoresin rosemary,sage extract,BHA,and BHT,with losses of 32.88%,30.45%,26.53%,26.21%,and 23.49%,respectively.Yildirim et al.[80]reported that the contents of 3-MCPD and GE in the potato crisps-fried oil were not significantly affected by different sodium chloride and rosemary extract concentrations during short-term frying.However,the content of 3-MCPD in potato crisps was low when rosemary extract was added during repeated frying cycles.Therefore,plant phenolic extracts,such as rosemary extract,possess the potential to inhibit the formation of 3-MCPD and thus guarantee the safety of fried foods.
3.2.5.Acrylamide
So far,acrylamide is mostly formed by a heat-induced reaction between the amino group of free asparagine and the carbon group of reducing sugar,which is degraded by the Maillard reaction [81].Besides,acrylamide can also be derived from the oxidation of acrolein produced during deep frying[82].Generally,the content of acrylamide increases with the increase of frying time,and when the heating temperature is higher than 175◦C,the formation of acrylamide is accelerated.Therefore,the amount of acrylamide is related to the type of oil sample,heating temperature,and concentrations of amino and carbonyl reactants.Wherein,the temperature>120◦C is the main reason for the formation of acrylamide because of the occurrence of the Maillard reaction [83].
The addition of antioxidants to potatoes is commonly suggested to reduce the formation of acrylamide;for instance,mixed spices added to potato chips before frying could decrease the formation of acrylamide by 50% after frying [84].However,when frying,the addition of potatoes will promote the formation of hydroperoxides in oil.Due to the presence of water in potatoes,TAGs are more prone to hydrolysis,producing diglycerides,monoglycerides,and FFAs.The more hydrolysis products form,the faster oxidation occurs[31].
Adam et al.[57] reported that the addition of 0.3 g/mL rosemary extract to oil significantly reduced the amount of acrylamide after frying(P<0.05).A similar result was observed when rosemary extract was added to reduce the formation of acrylamide during deep frying of potato in sunflower oil[85].In addition,Gertz et al.[83]also reported that the flavonoids mixed flavor reduced the formation of acrylamide by 50%in frying oil.As described by Zhang et al.[58],potato chips were soaked in 0.01% (W/W) bamboo leaf extracts for 60 s and then fried in oil at 170◦C,and the result showed that the formation of acrylamide decreased by nearly 74.1%.The main antioxidant components of bamboo leaves are flavonoids,lactones,and phenolic acids.Recently,lypophilized pomegranate peel nanoparticles extract was also proven to possess a significant reduction (54%) in acrylamide content in potato chips after 20 frying cycles in sunflower oil with an additional amount of 1000 mg/kg [59].According to these studies,plant phenolic extracts can effectively reduce the amount of acrylamide formed during deep frying.
4.Antioxidant mechanisms of phenolic extracts during deep frying
According to the positive effects of natural phenolic compounds on the physicochemical properties of frying oil or fried foods,the antioxidant mechanisms of phenolic extracts mainly include i) inhibiting the oxidative degradation of lipids as free radical scavengers,ii)interfering with the oxidation process as metal chelating agents,and iii)undergoing acid-catalyzed deposition reaction as polymerization inhibitors.Combined with the possible products formed during deep frying,three possible mechanisms for the exertion of antioxidation of plant phenolic compounds include scavenging free radicals,trapping toxicological aldehydes,and retarding the formation of polymerized TAGs via nonradical reactions [2].Antioxidant activities of phenols mainly vary with the number and position of hydroxyl groups in molecular structure[20].
The classical ways of lipid oxidation,as shown in Fig.3,include initiation (production of lipid free radicals),reproduction,and termination (production of non-free radical products).Phenolic extracts can provide hydrogen atoms to lipid free radicals,thus producing lipid derivatives and antioxidant free radicals [63],and interrupting the reproductive stage of the oxidation chain reaction.Meanwhile,phenolic compounds can act as free radical receptors and terminate free radicals at the initial stage.For instance,BHT,BHA,TBHQ,and tocopherol can delay or inhibit the initial step by reacting with lipid free radicals[86].Compared with the radical-scavenging mechanism,the metal chelation mechanism(Fig.4)is considered to be a minor reaction,that is,during the frying process,some metal ions which are called lipid oxidation initiators(e.g.Fe2+,Fe3+,Cu2+,…),catalyze the oxidation process and lead to the formation of hydroxyl radicals.These metal ions reduce the activation energy of oxidation,especially in the initial step,to accelerate the oxidation of the oil [64].Some phenolic extracts can react with initiators as metal chelating agents [87].As ligands,phenolic compounds interact with metal ions to form a stable complex structure,thus preventing metal-catalyzed free radical reactions,especially the anion of polyphenols has a significant chelating ability for transition metal ions[88].Flavonoids and phenolic acids have the ability to act as prooxidants under certain conditions,that is,to promote the oxidation of other components.The pro-oxidant activity of natural phenolic compounds depends on their chelating behavior,metal-reducing properties,and O2-reducing ability [36].Sakihama et al.[89] showed that in an environment containing redox-active metals (i.e.,Cu and Zn),phenolic components can act as pro-oxidants,leading to the formation of phenoxy radicals and ROS.In the presence of O2,transition metals,such as Cu and Fe,catalyze the redox cycle of phenolics,leading to the formation of reactive oxygen species and other organic radicals,which destroys lipid molecules.Therefore,the addition of low concentrations of natural phenolic compounds may inhibit lipid oxidation during deep frying,but may also play a pro-oxidant role under certain conditions.The reactions during deep frying are dynamic and complex,involving not only free radical reactions but also non-free radical reactions.When frying at high temperatures with limited supply and food ingredients,the reaction of forming C–C linked dimer,polymer,or cyclotriacylglycerol catalyzed by non-free radicals is dominant [90].Some natural phenolic compounds,such as sesamin,can be converted into a variety of antioxidant compounds in the presence of acid and moisture.Despite their lower radical scavenging activity,they are effective inhibitors against polymerization which can undergo acid-catalyzed deposition reaction to increase the frying performance of oils under frying conditions[91].
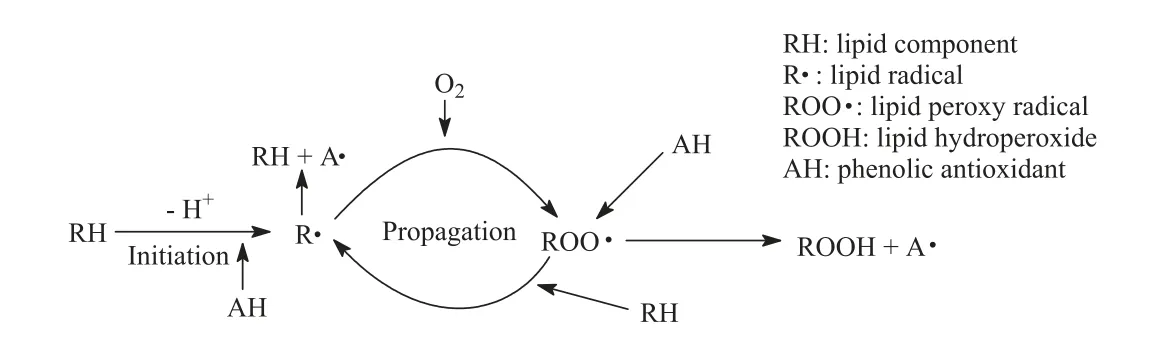
Fig.3. Alkyl radical scavenging mechanism of phenolic antioxidants during deep frying [63].
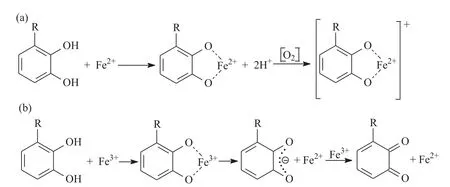
Fig.4. Metal chelation mechanism phenolic antioxidants.(a) Coordination of Fe2+ by polyphenols and subsequent electron transfer reaction in the presence of oxygen-generating the Fe2+-polyphenol complex;(b)Coordination of Fe3+by polyphenols,subsequent iron reduction and semiquinone of formation,and reduction of Fe3+ to form a quinone species and Fe3+;R=H or OH [88].
Besides the possible antioxidant mechanisms mentioned above,the antioxidant activities of phenols may also be related to the following factors: i) strong stability at high temperatures,such as rosemary extract;ii) forming decomposition products to be acted as secondary antioxidants.For example,the thermal degradation of quercetin produces protocatechuic acid that can continue to act as an antioxidant to delay lipid oxidation [8].Therefore,the selection and control of appropriate frying conditions should be considered for the exertion of antioxidant activities of plant polyphenols.
Based on the intrinsic antioxidant activity of polyphenols,the possible mechanism by which the plant phenolic extracts exert the antioxidant effects to protect frying oil from thermal oxidation is the effective contact between the single polyphenol and the unsaturated TAGs assisted by the molecular thermal motion.As indicated by Gao et al.[42],tea polyphenols could reduce the breaking degree of=C–H,C–O–C,and C=C in TAGs and inhibit the oxidation of unsaturated oils by inhibiting the generation of free radicals or eliminating free radicals during deep frying.Therefore,the dispersibility or solubility of plant polyphenols in oil matrix determines the antioxidant capacity of the plant extracts.
5.Challenges for the use of plant phenolic extracts
At present,there are many methods to extract phenolic compounds from natural plants,such as liquid-liquid extraction,supercritical CO2extraction,and subcritical water extraction;however,solvent extraction is the most commonly used method.Commonly used extraction solvents are alcohols (methanol and ethanol),acetone,diethyl ether,and ethyl acetate [92].The yields of phenolic compounds extracted by different solvents are significantly different,which may affect their antioxidant activity.Therefore,an appropriate solvent should be selected to maximize the yield when plant phenolic extracts are prepared.For instance,strongly polar phenolic acids(benzoic,and cinnamic acids)could not be extracted completely with pure organic solvents;therefore,mixtures of alcohol-water or acetone-water are recommended[93].In addition,the solvent extraction rate of phenolic compounds is not positively correlated with polarity.Solvents with high polarities,such as water,do not have a high extraction rate for phenolic compounds[92].Methanol and ethanol have been found to be the most suitable solvents for extracting antioxidants from plant materials.However,the safety and cost of the solvents should be carefully considered for the application of plant polyphenols in food frying.
The effective protection of frying oil from thermal stress needs the effective contact between the polyphenols of plant extracts and the unsaturated TAGs,indicating that the plant extracts must thoroughly dissolve into the oil matrix.However,the incorporation of plant extracts in frying oil is not the simple mixing of these two matters.When actually applied,plant phenolic extracts should be initially dissolved in organic solvents (such as ethanol) to guarantee effective dissolution and dispersion in the frying oil[94].If the phenolic extract is directly added to the oil matrix,inhomogeneity of dissolution or even insolubilization might occur,leading to the emergence of visible flocculation during deep frying.This might be related to the certain hydrophilicity or impurity of the phenolic extract.Therefore,the solvent used to obtain plant extracts is usually selected to promote the dissolution of plant extract components in frying oil.Generally,the amount of ethanol used to dissolve phenolic extract is not fixed but depends on the total volume of the frying oil.Another advantage of using ethanol as a “bridge” is that ethanol can be volatile away from the frying system during heating.
It should also be noted that the appropriate concentration should be considered when natural phenolic extracts are selected.Unlike the high content of lipids in foods,antioxidants at low concentrations significantly delay or inhibit the oxidation of oxidizable substrates,but usually lose their activity at high concentrations,and act as pro-oxidants by participating in the initiation reaction[4].In addition,natural phenolic compounds not only have antioxidant effects during deep frying but also play the role of a flavoring agent[95].As described by Chammem et al.[20],a pleasant taste was detected in the frying oil containing 0.08%of rosemary extract.Vanillin,the most widely used flavoring agent,can improve the flavor of PUFA-enriching foods.More recently,the essential oil ofCoriandrum sativumwas added into SFO at 0.16 g/kg to fryCaijiaoand results indicated that the sensory attributes ofCaijiaowere obviously improved[48].However,it should be noted that not all phenolic extracts of natural plants contribute to flavor.For instance,apple extract has a great influence on the flavor of products because of its high level of bitterness [96].Therefore,it is necessary to consider the flavor impact on frying oil before using plant phenolic extract.
6.Conclusion and outlook
Plant phenolic extracts attract increasing attention because of their excellent antioxidant effects (such as the reduction of POV,IV,oil darkening,and rancidity),non-toxicity to the human body,and improving the sensory quality of food.Herbs,berries,and agricultural and sideline products are all effective sources of plant phenolic extracts.Among the numerous plant sources,rosemary is one of the most commonly used plant in food processing at present and its extract also considered to be the most effective plant extract for the protection of frying oil.Antioxidant effects of plant phenolic extracts are also related to extraction solvents and their concentrations because some antioxidants might even lead to oxidation promotion.Solvents with high polarity have low extraction rates for phenolic compounds.At present,methanol is recommended as an effective extraction solvent for phenolic extracts.The possible antioxidant mechanisms of phenolic extracts include free radical scavenging,metal chelating,and synergistic effects with other compounds.However,on the basis of the improvement of quality attributes of frying oil by phenolic extracts,some challenges still exist as follows:
i) The lipophilicity and thermal stability of phenolic extracts need to be improved via modifying natural phenols with specific functional groups to produce semi-synthesized antioxidants and the corresponding practical application in quality protection of frying oil during deep frying;
ii) It may be extracted/contaminated with potentially toxic or allergic compounds during the extraction process;
iii) The direct impact of plant phenolic extracts or their thermally chemical products on the color of frying oil or the fried foods needs to be elucidated to support the application reliability of plant extracts in food frying.
iv) Digitization of the changes in physicochemical properties of frying oil and fried foods during the antioxidant process or the evolution of natural phenolic extract;
v) Phenolic extracts can effectively prolong the induction period when added to any oil not deteriorated,but they are ineffective in delaying the decomposition of deteriorated lipids;
vi) Synergistic effects might exist when two or more plant phenolic extracts are simultaneously used.
vii) Natural antioxidants may produce toxic decomposition products under deep frying conditions;thus,the potential toxicity of antioxidants needs to be evaluated.
viii) Although phenolic compounds can improve the properties of frying oil,most compounds containing hydroxyl groups have poor solubility in oil.It is important to improve its solubility and diffusivity in the substrate.
Therefore,in the future,the solubility,stability,purity,effectiveness,and pertinence of plant phenolic extracts applied in oils during deep frying needs further research.
CRediT Authorship Contribution Statement
Fa Wang:Conceptualization,Methodology,Investigation,Writing–original draft.Yixi Sun:Investigation.Shanshan Li:Writing–review&editing.Jing Yan:Writing– review &editing.Wen Qin:Supervision.
Ahmed S.M.Saleh:Writing– review &editing.Qing Zhang:Supervision,Funding acquisition,Project administration,Writing– review&editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgement
This work was financially supported by the National Natural Science Foundation of China (Grant No.32101981).
 Grain & Oil Science and Technology2023年3期
Grain & Oil Science and Technology2023年3期
- Grain & Oil Science and Technology的其它文章
- Encapsulation of lipases on coordination polymers and their catalytic performance in glycerolysis and esterification
- Drying kinetics of soy protein isolate-corn starch film during preparation and its moisture adsorption characteristics during storage
- Effect of pressure cooking on phenolic compounds of quinoa
- Peanut proteins: Extraction,modifications,and applications: A comprehensive review
