Targeting endoplasmic reticulum stress signaling in ovarian cancer therapy
Tianqing Yan, Xiaolu Ma, Lin Guo, Renquan Lu
1Department of Clinical Laboratory, Fudan University Shanghai Cancer Center, Shanghai 200032, China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China
ABSTRACT The endoplasmic reticulum (ER), an organelle present in various eukaryotic cells, is responsible for intracellular protein synthesis,post-translational modification, and folding and transport, as well as the regulation of lipid and steroid metabolism and Ca2+homeostasis.Hypoxia, nutrient deficiency, and a low pH tumor microenvironment lead to the accumulation of misfolded or unfolded proteins in the ER, thus activating ER stress (ERS) and the unfolded protein response, and resulting in either restoration of cellular homeostasis or cell death.ERS plays a crucial role in cancer oncogenesis, progression, and response to therapies.This article reviews current studies relating ERS to ovarian cancer, the most lethal gynecologic malignancy among women globally, and discusses pharmacological agents and possible targets for therapeutic intervention.
KEYWORDS Endoplasmic reticulum stress; unfolded protein response; ovarian cancer; targeted therapy
Introduction
Ovarian cancer (OC)
OC is the most mortality of gynecologic malignancy worldwide.Epithelial OC (EOC) accounts for approximately 90%of ovarian neoplasm cases1.According to GLOBOCAN 2018 database2estimates, 295,400 new cases of OC were diagnosed,and 184,800 deaths due to OC occurred.In China, population aging aggravates the cancer burden in urban and rural areas3.Statistics from 2016 indicated an ovarian carcinoma incidence and mortality in China as high as 57,200 cases and 27,200 deaths, respectively4.The 5-year overall survival rate is <45%and decreases to 25% for advanced OC5.Because of a lack of early screening methods and an absence of clear symptoms during early OC stages, more than 75% of patients are diagnosed in an advanced stage6.Debulking surgery with platinum-based chemotherapy is the first-line therapeutic strategy; however,most patients manifest recurrent disease within 18 months and develop drug resistance leading to therapeutic failure7.Notably,the histopathology of ovarian tumors is heterogeneous, and each OC subtype bears genetic mutations, which determine the efficacy of molecularly targeted treatments.Currently, targeted therapies such as antiangiogenic drugs (such as bevacizumab,a recombinant humanized monoclonal IgG1 antibody targeting vascular endothelial growth factor-A) or poly(ADP-ribose)polymerase (PARP) inhibitors are clinically applied to improve the outcomes of this malignancy.Nonetheless, this treatment is effective only in patients with homologous recombination deficiencies8.Therefore, the identification of molecules responsible for OC development and progression is essential for both early detection and the development of novel therapeutic approaches for OC.
Endoplasmic reticulum stress (ERS) and the unfolded protein response (UPR)
ERS occurs in tumor cells exposed to intrinsic factors (oncogenic activation, chromosome number alterations9, and exacerbated secretory capability10) and external triggers (hypoxia,nutrient deprivation, and acidosis) that alter protein homeostasis, thus resulting in the accumulation of unfolded or misfolded proteins in the ER lumen.Subsequently, 3 primary UPR signaling pathways, orchestrated by inositol- requiring enzyme 1 (IRE1), activating transcription factor 6 (ATF6), and protein kinase RNA-like endoplasmic reticulum kinase (PERK),are induced, thereby resulting in either adaptive restoration of homeostasis or cell death11.The critical roles and signaling networks of the UPR in ovarian carcinoma are illustrated in Figures 1 and 2.
IRE 1 pathway
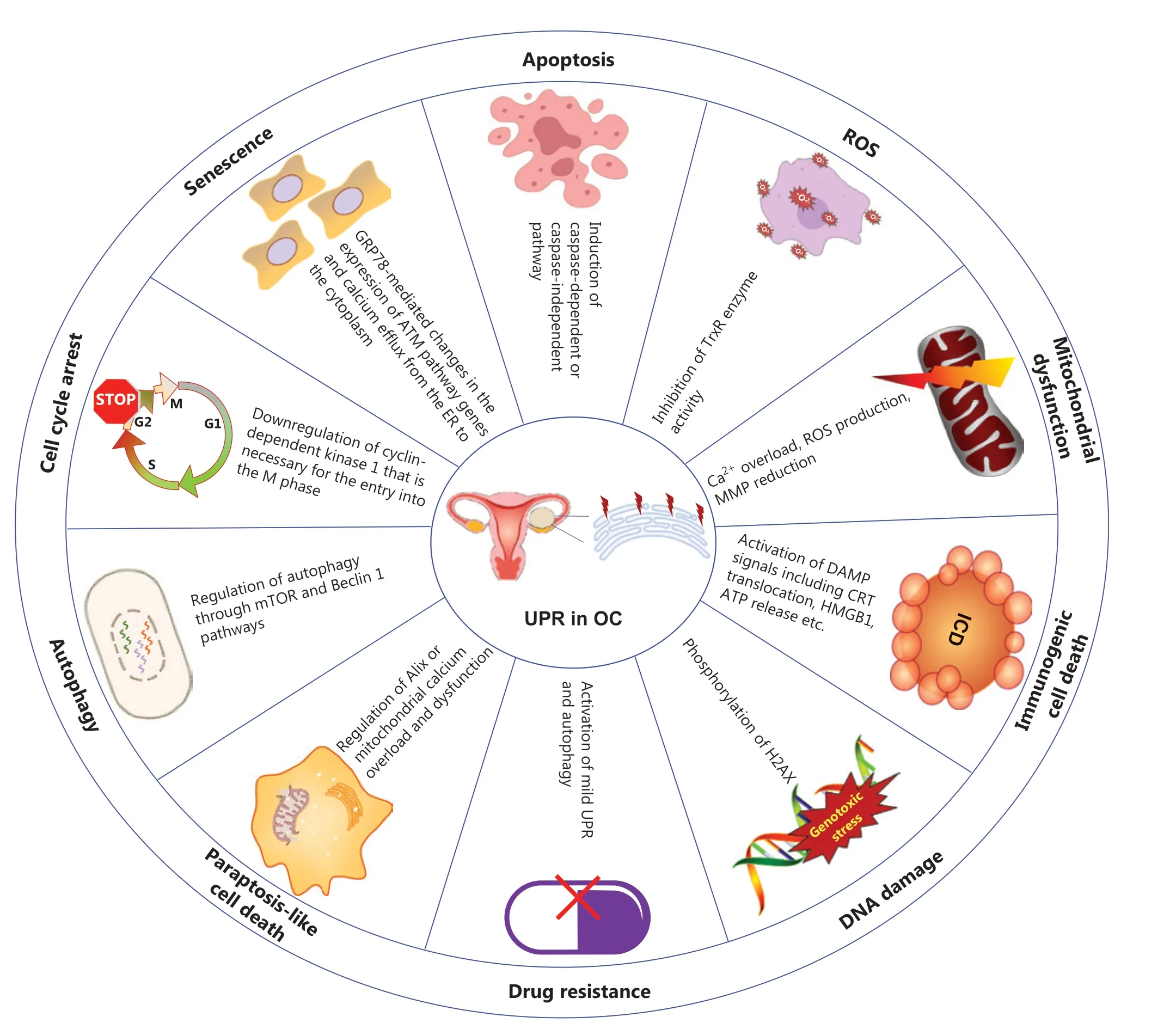
Figure 1 Critical roles of the endoplasmic reticulum unfolded protein response (UPR) in UPR in OC.The UPR is involved in various biological processes in OC that are closely associated with apoptosis12,13, ROS14, mitochondrial dysfunction15,16, non-apoptotic cell death17,18, DNA damage19,20, drug resistance21, autophagy22, the cell cycle23, and senescence24.OC, ovarian cancer; ROS, reactive oxygen species; ICD, immunogenic cell death; MMP, mitochondrial membrane potential; TrxR, thioredoxin reductase; DAMPs, damage associated molecular patterns;CRT, calreticulin; HMGB1, high mobility group protein B1; H2AX, H2A histone family, member X; Alix, apoptosis inducible factor 6 interacting protein; GRP78, glucose regulated protein 78; ATM, ataxia telangiectasia-mutated.
IRE1α and IRE1β are 2 isoforms of IRE in mammals.IRE1α is ubiquitously expressed and has been extensively studied,whereas IRE1β is expressed primarily in the gastrointestinal and respiratory tracts25.IRE1α is both a kinase and an endo-ribonuclease (RNase), which dimerizes/oligomerizes and auto-trans-phosphorylates under ERS, thus leading to the activation of endo-RNase.Active IRE1α catalyzes the excision of a 26-nucleotide intron within the X-box binding protein-1(XBP1) mRNA, and RNA-splicing ligase RTCB-mediated ligation of the remaining 5′ and 3′ fragments26shifts the reading frame, thus resulting in translation of a stable and active transcription factor known as XBP1s (spliced form).XBP1s modulates the expression of several UPR target genes involved in ER folding, glycosylation, and ER-associated degradation (ERAD)27.In addition, IRE1/RNase activity targets other mRNAs and microRNAsviaregulated IRE1-dependent decay (RIDD), a novel UPR regulatory pathway that controls cell fate under ERS28.In addition to activating ribonuclease activity, IRE1α recruits the adapter target c-Jun N terminal kinase 1 cytoplasmic receptor-associated factor 2 (TRAF2),which in turn activates apoptosis signal-regulating kinase 1(ASK1) and its downstream target c-Jun N terminal kinase 1 (JNK/MAPK8/SAPK1)29.This signaling pathway subsequently activates the nuclear factor-κB (NF-κB) pathway under ERS30.
ATF6 pathway
ATF6 is a type II transmembrane protein exhibiting transcription factor activity in its cytosolic domain.Under ERS,ATF6 shuttles to the Golgi apparatus and is cleaved by specific site 1 and 2 proteases (S1P and S2P), thus leading to the release of the cytosolic fragment of the protein ATF6.In cooperation with XBP1s, ATF6f up-regulates many genes that increase ER size and protein-folding capability, as well as genes associated with ERAD of misfolded proteins11,31.Under irreversible ERS, ATF6 decreases levels of antiapoptotic proteins, such as myeloid cell leukemia-1 (Mcl-1)32.Nevertheless, the role of ATF6 in ERS-induced cell death remains to be better explored.
PERK pathway
After ERS activation, PERK inhibits global protein translationviatrans-autophosphorylation and phosphorylation of the eukaryotic translation initiation factor (eIF2α) at serine 51, thereby decreasing the burden of newly synthesized proteins.Furthermore, activating transcription factor 4 (ATF4)mRNA is selectively translated; this mRNA plays an important role in amino acid metabolism, antioxidant response,autophagy, and protein folding27.ATF4 expression is also essential for the activation of apoptosisviathe regulation of C/EBP-homologous protein (CHOP), which upregulates pro-apoptotic members of the B-cell lymphoma-2 (BCL-2)protein family33, thereby inhibiting cell growth and promoting DNA damage19.Activation of the ATF4-CHOP pathway induces growth arrest and expression of DNA damageinducible protein 34 (GADD34), an adaptor of eIF2α phosphatase PP1c, which in turn modulates eIF2α dephosphorylation, and recovery from stress or proteotoxicity34,35.Nuclear factor erythroid 2-associated factor 2 (Nrf2)36is also phosphorylated by PERK, and consequently transcriptionally up-regulates antioxidants and other components that protect against oxidative stress.The PERK-mediated translational cascade is also required for the activation of NF-κB in cancer cells37.Overall, the UPR is a central player in tumor progression38,39representing an attractive therapeutic target in many solid and blood neoplasms40.In the next section, we summarize studies associating the UPR with the evolution of ovarian carcinoma.
Overview of components participating in ERS signaling in OC
Chronic ERS and defective UPR signaling are emerging as critical players in an increasing numbers of human diseases,including OC.
ER-resident components involved in OC
Multiple molecular chaperones are enriched in the ER, where they ensure normal folding of newly synthesized proteins.The major ER chaperone glucose regulated protein 78 (GRP78)is extensively expressed in human neoplasms.Accordingly,elevated levels of GRP78 in OC tissues are correlated with poor patient prognosis41.Functionally, GRP78 is weakly expressed in cisplatin-sensitive OC cells, and it mediates cisplatin- induced senescence24.Another ER chaperone protein, disulfide isomerase (PDI), is also highly abundant in OC tissues and predicts poor prognosis in patients diagnosed with OC41.Furthermore, tumor suppressor candidate 3 (TUSC3),an ER localized protein responsible for N-glycosylation of proteins, is often lost in epithelial cancers, thus triggering ERS and inducing hallmarks of the epithelial-to-mesenchymal transition (EMT) in OC cells42.In our previous study, the UPR signaling component XBP1 was found to be upregulated in OC cell lines.Knockdown of XBP1 significantly inhibits cell propagation and enhances the sensitivity of OC cells to H2O2by elevating intracellular ROS levels43.Inhibition of the IRE1α/XBP1s branch alone or in combination with immune checkpoint blockade provides a therapeutic strategy for several cancer types with frequent coactivator-associated arginine methyltransferase 1 (CARM1) overexpression, including OC44.Furthermore, pharmacological inhibition of the IRE1a/XBP1 pathway alone or coupled with histone deacetylase 6(HDAC6) inhibition is urgently needed therapeutic strategy against AT rich interactive domain 1A (ARID1A)-mutant OCs45.Moreover, key functions of UPR signaling have been established in the regulation of tumor stromal cells.For example, activation of IRE1α-XBP1s reprograms tumor-associated dendritic cells and T cells, thereby impairing anti-tumor activity in OC46,47.
Molecules participating in ERS signaling in OC
Beyond the ER-resident components involved in OC, several molecules have been confirmed to participate in the chemoresistance of OCviathe ERS signaling.For instance, overexpression of ankyrin repeat domain 1 (ANKRD1) or pleckstrin homology like domain family A member 1 (PHLDA1) in ovarian carcinoma correlates with poor survival, and upregulation of these proteins in OC cell lines modulates cell apoptosisviathe ERS pathway48,49.The ubiquitin-binding protein p62/SQSTM1 (sequestosome 1) is abundant in cisplatin- resistant SKOV3 cell lines and prevents ERS-mediated cell apoptosis,thereby leading to cisplatin resistance.Knockdown of p62 re-sensitizes resistant cells to cisplatin50.Twist expression is strongly associated with the expression of DNA damage response proteins, whose upregulation contributes to cisplatin resistance in OC cells.Notably, the combination of niraparib and cisplatin has been found to be considerably effective against 3D cultures of Twist silenced, cisplatin- resistant OC cells with upregulated ERS, thus leading to the initiation of mitochondrially mediated cell death51.WW domaincontaining oxidoreductase (WWOX), which is frequently lost in several cancers, sensitizes EOC to paclitaxelviaERS-induced apoptosis, and is predictive of clinical outcomes in patients52.Therefore, ERS response mechanisms can be targeted to resolve chemoresistance in cancer.Additionally, the dysregulation of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1),receptor tyrosine kinase-like orphan receptors (ROR2), and angiotensin II receptor (AGTR1) in OC has been found to predict poor outcomes in patients, thus suggesting that strategies targeting ERS relevant components may provide potential therapeutic benefits53-55.All the above factors mediating OCviaERS signaling are summarized in Table 1.Therefore, targeting UPR components or factors relevant to ERS signaling as a therapeutic strategy to combat ERS-associated pathologies is a promising future research direction.
Studies on pharmacological agents targeting ER homeostasis in OC
UPR signaling is believed to be a self-protection mechanism in cells.Nevertheless, if the intensity or duration of cellular stress is elevated, these pathways instead activate cell death.Therefore, regulation of UPR signaling components has the potential to either stimulate or attenuate protein folding, and to have therapeutic effects in diseases such as diabetes and neurodegenerative diseases, or in the induction of apoptosis,thus enabling anticancer strategies38.To date, the mechanisms defining the threshold that switches UPR signals from adaptive cellular protection to proapoptotic cell death or vice versa remain to be elucidated.ERS activation is intricately involved in signaling pathways including cellular autophagy56,57, oxidative stress58,59, Ca2+homeostasis60,61, apoptosis57, metabolic disorders12,62, and inflammatory responses30,37.Thus, clarification of the ERS pathway, and the rationale for drug design and implementation, are key challenges.We next review the pharmacological agents targeting the ERS signaling in ovarian carcinoma.
ERS-mediated autophagy induced by pharmacological agents resulting in either protective or anti-tumor effects in OC
The UPR is indispensable for the adaptation of cancer cells to rapid growth, hypoxia, nutrition deprivation, and chemotherapies.The UPR restores cellular homeostasis, thereby leading to degradation of unfolded and/or misfolded proteinsviaautophagy or ERAD.Nonetheless, the UPR also results in cell death under certain circumstances63.For instance,OC cell apoptosis induced by metformin (a first-line treatment for type 2 diabetes) has been found to be abrogated by autophagy and PERK activation64; however, in another study, metformin has been found to promote the apoptosis of OC cellsviaERS induction65.Similarly, quercetin(3,3′,4′,5,7-pentahydroxyflavone) has been reported to induce ERS, thus concomitantly promoting protective autophagy by activating the signal transducer and activator of transcription 3 (p-STAT3)/BCL-2 axis66.Intriguingly, one study has demonstrated that quercetin suppresses DNA double-strand break repair and enhances the radiosensitivity of human OC cellsviaa p53-dependent ERS pathway67.Another study has indicated that quercetin enhances the apoptosis of OC cells exposed to tumor necrosis factor- associated apoptosis-inducing ligand (TRAIL) by upregulating death receptor 5 (DR5) expression after ERS68.Furthermore, the HIV protease inhibitor saquinavir induces ERS-regulated cellular autophagy through the mTOR and Beclin 1 pathway, and decreases the sensitivity of SKOV3 to cisplatin69, whereas saquinavir has also been reported to promote cell death in OC cells characterized by ERS activation and autophagy22.These contradictory results suggest that a balance may exist between cell death and survival, as mediated by ERS involved in autophagy, according to the degree and duration of drug stimulation.Some pharmacological compounds exert anti-tumor effectsviainduction of ERS and autophagy.For example, the flavonoid kaempferol inhibits cell propagation and induces apoptosis in A2780 cells by triggering ERS-mediated cytotoxic autophagy56.B19 (a novel monocarbonyl analogue of curcumin) induces apoptosis in human OC cellsviaactivation of ERS70and the autophagy signalingpathway71.Trans10, cis12-conjugated linoleic acid (occurring naturally in dairy products and red meat) has also been identified to inhibit the proliferation and migration of OC cells through activating ERS and autophagy72.Mifepristone sensitizes OC cells to proteasome or lysosome inhibitors by inducing ERS and autophagic flux73.The aforementioned studies have indicated that ERS signaling and autophagy may be used by OC cells to survive in the hostile tumor microenvironment; however, extensive stress and autophagy might result in cell death in OC, thereby suggesting a need for therapeutic strategies targeting ERS signaling or autophagy in cancer therapy.

Table 1 Components in ERS signaling implicated in OC
Pharmacological agents inducing ERSmediated anti-tumor effects involve apoptosis and non-apoptotic cell death in OC
As described above, ERS has antipodal functions in the progression of OC.Beyond the protective effects of ERS on OC cell fate, most compounds like ABT-737, GYY4137 and Garcinone E etc.tend to exert anti-tumor effects directlyviaERS induction74-82.ERS mediated OC cell death also includes caspase-dependent12,15,16,60,83-85or caspase- independent cellular apoptosis13,86-88, and non-apoptotic cell death such as immunogenic cell death (ICD)17,89-91and paraptosis- like cell death18,92.
Apoptosis of OC cells mediated by ERS
The 3 main sensors (PERK, IRE1, and ATF6) and their downstream cascades are involved at different levels in cell death induced by unresolved ERS, among which the PERK/ATF4/CHOP pathway plays a critical role in cell destruction13,20,93-95.The pharmacological agent cucurbitacin I induces OC cell deathviaCHOP- and caspase-12-dependent ERS-associated apoptosis86.α, β-thujone leads to cell deathviaactivation of ERS, DNA damage, and caspase-dependent apoptotic pathways12.ERS- and caspase-dependent apoptosis is also induced in OC cells treated with pimaric acid83or valosin- containing protein inhibitors84.Furthermore, caspase-independent pathways such as the JNK branch of the IRE1 signaling also promote cell death96.For example, low levels of glucose and metformin have been reported to induce apoptosis of human OC cellsviaactivation of the ERS-associated ASK1-JNK pathway65.Sodium 4-carboxymethoxyimino-(4-HPR)(a novel water-soluble derivative of 4-oxo-4-HPR) exhibits anticancer activity against solid tumorsin vivoandin vitrothrough ERS-activated p-JNK signaling, and fenretinide(a synthetic retinoid) induces apoptosisviaa ROS-dependent mechanism involving ERS and JNK activation97-99.
Nonapoptotic cell death mediated by ERS in OC
Beyond ERS-mediated caspase-dependent/independent apoptosis, some agents induce ICD or paraptosis-like cell death.ICD denotes a specific variant of regulated cell death driven by stress and the induction of adaptive immunity against the antigens of dead cells.For instance, ERS induced by thapsigargin or doxorubicin partially regulates the release and binding of calreticulin (CRT, an ER chaperone) to the surfaces of OC cells,where it releases an “eat me” signal and activates anti-tumor adaptive immune responses89.CRT exposure on the surfaces of primary and metastatic high grade serous OC cells is driven by a chemotherapy-independent ERS response and culminates in the establishment of a local immune microenvironment characterized by Th1 polarization and cytotoxic activity,thus enabling superior clinical benefits89.Benzenesulfonamide(a mitochondrial uncoupler) activates ERS sensors, as well as growth inhibition and apoptosis promotion, thus resulting in ICD and anti-tumor immune effects17.Lau et al.have reported that paclitaxel induces ICD-associated damage-associated molecular patterns (DAMPs, such as CRT exposure, ATP secretion, and high mobility group box 1 release) in OCin vitroand elicits significant anti-tumor responses in tumor vaccination assaysin vivo90.In addition, paraptosis, first reported in 2000100, is a caspase-independent form of programed cell death, characterized by the absence of classical apoptotic features such as apoptotic body and chromatin agglutination100,101.The morphological features of paraptosis are also distinct, including swollen ER or mitochondria and cytoplasmic vacuolization102.De novosynthesis of proteins and ERS are also essential for paraptosis.Morusin (a prenylated flavonoid extracted from the root bark ofMorus australis) induces paraptosis-like cell deathviaactivation of ERS and mitochondrial Ca2+overload and dysfunction in EOC92.Another study has found that the novel rhein derivative 4a induces paraptosis-like cell death by ERS in OC cells18.Cucurbitacin I has also been proposed to mediate ERS-dependent autophagy, and caspase- independent nonapoptotic cell death86.Several pharmacological agents that target ERS signaling for the potential therapy of OC, described above or in prior studies14,103-109, are summarized inTable 2.
Concluding remarks and future perspectives
On the basis ofin vitroandin vivoexperiments, the activation of UPR has been shown to modulate processes including the cell cycle, oxidative stress, autophagy, cell death, and chemoresistance in OC (Figure 1).This review summarizes studies on UPR components and pharmacological compounds that target ERS-associated pathways in OC.Small molecules that specifically target components of the UPR signaling network are promising potential therapeutic interventions.Therefore,the UPR is emerging as an appealing therapeutic target; however, the benefits and risks of modulating the UPR in anytumor type require further evidence.Numerous compounds are being developed to target the 3 UPR sensors; however, the factors determining the behavior of a particular sensor as a pro- or anti-apoptotic signal remain unclear.On the one hand,cancer cells use adaptive responses to survive excessive stress,which are accompanied by tumor initiation, progression,metastasis, immune escape, and chemoradiotherapy resistance.On the other hand, excessive or sustained stress results in tumor killing123.Strategies for blocking tumor stress relief or elevating stress-induced cell mutation may achieve optimal therapeutic outcomes.The new compound ERX-41 has been documented to exacerbate ERS, thus leading to several types of cancer deaths with elevated ERS115.The concept of increasing ERS by ERX-41 in cancer cells for therapeutic purpose has been licensed to Dallas-based EtiraRx, and is expected to enter clinical trials soon.Moreover, monitoring the adaptive response on multiple scales is necessary to help design optimal treatment schedules and balance on-target toxicity with tumor eradication.Finally, potential combinatorial therapies with clinical chemotherapeutic drugs are also appealing and promising.Future studies addressing these issues are expected to pave the way to novel avenues for treating ERS-associated diseases.
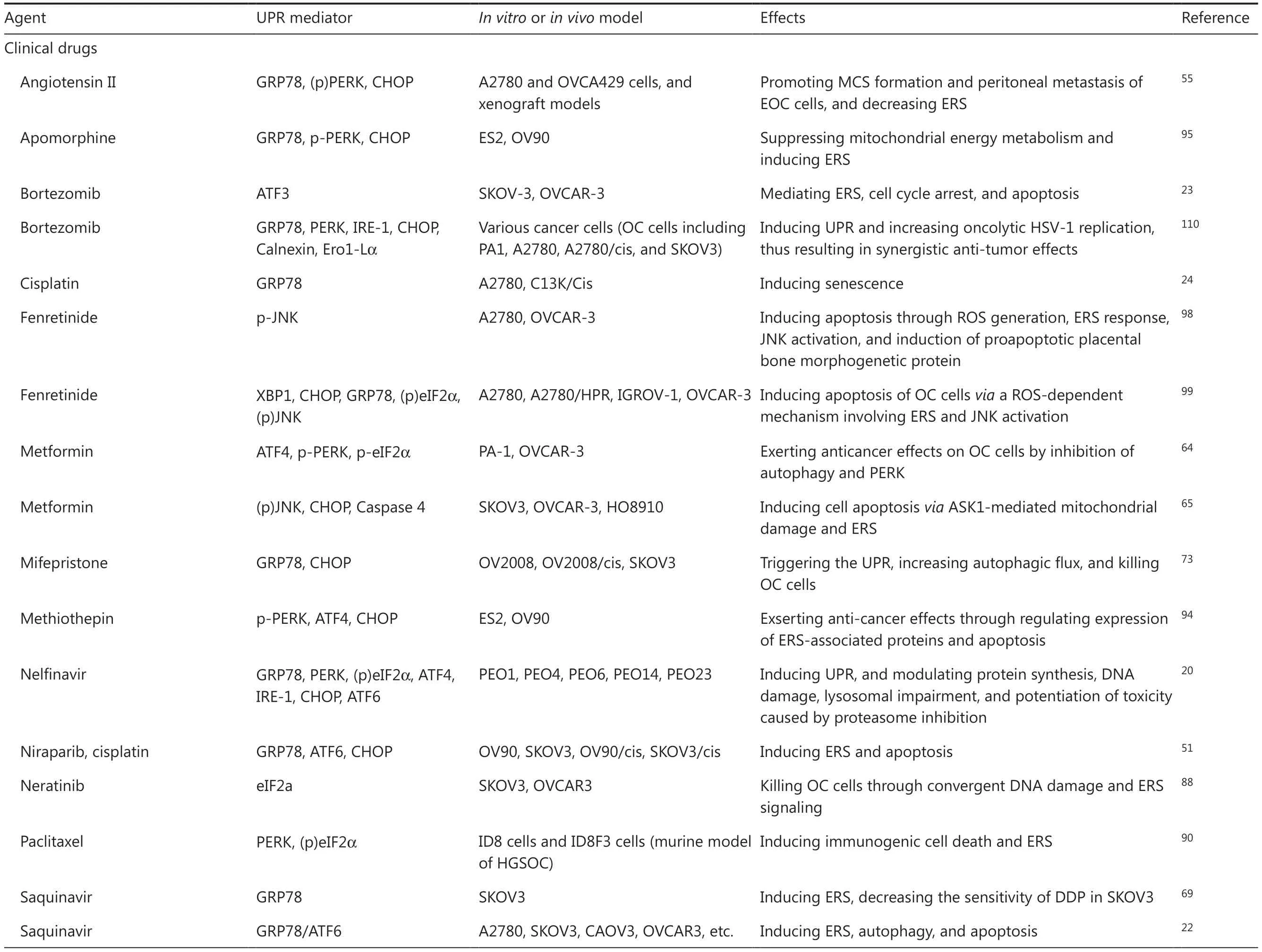
Table 2 Pharmacological agents targeting ER homeostasis in OC

Table 2 Continued

Table 2 Continued
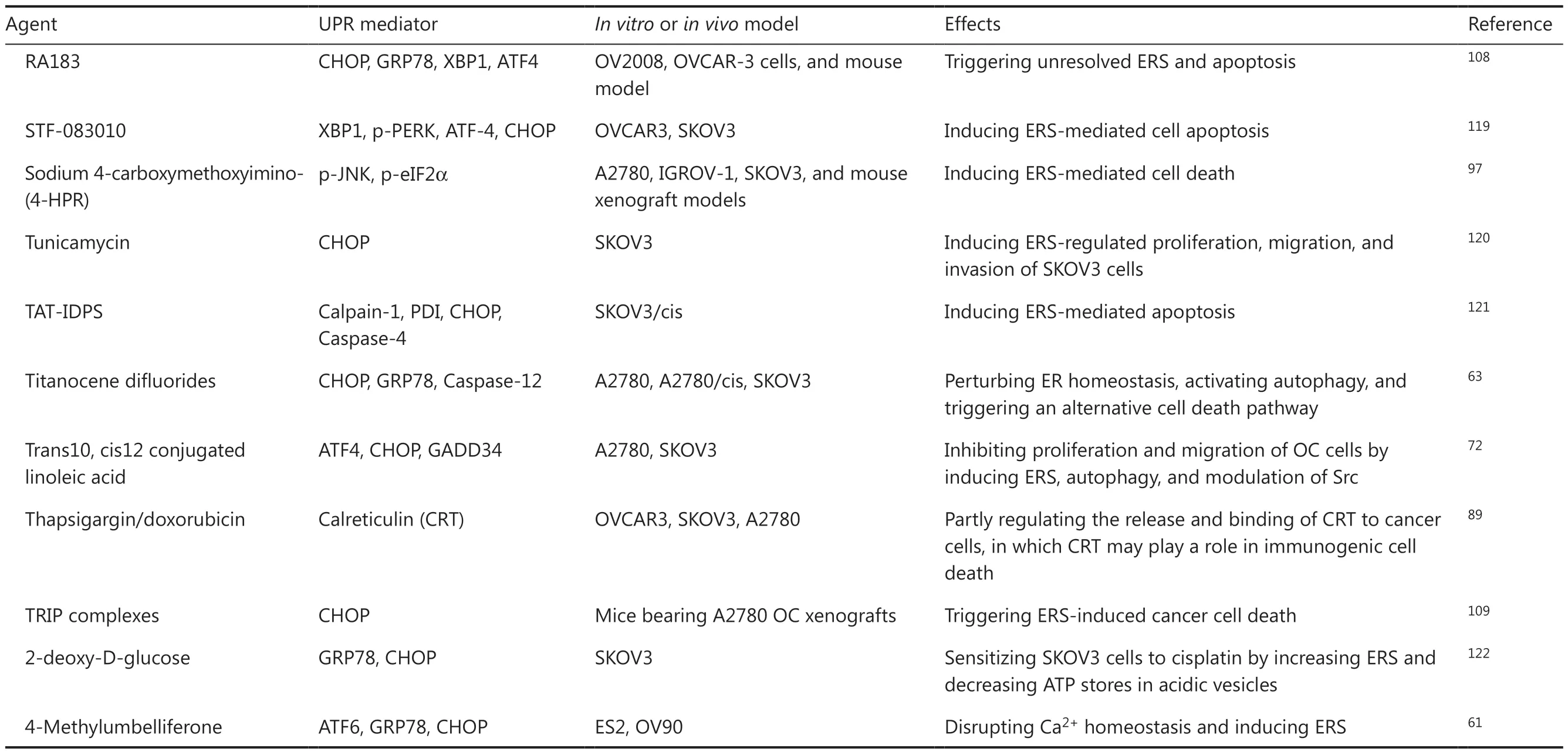
Table 2 Continued
Acknowledgements
Some images in figures in this work were created by ScienceSlides and the website www.biorender.com, accessed on May 1, 2023.
Grant support
This work was supported by the National Natural Science Foundation of China (Grant Nos.NSF-82072876 and NSF-82002618).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Literature searching and manuscript writing: Tianqing Yan.Reviewing and editing: Xiaolu Ma.
Supervision and project administration: Lin Guo, Renquan Lu.
 Cancer Biology & Medicine2023年10期
Cancer Biology & Medicine2023年10期
- Cancer Biology & Medicine的其它文章
- Immunotherapy for multiple myeloma: new chances and hope
- Opportunities and challenges of immunotherapy for dMMR/MSI-H colorectal cancer
- Cohort profile: design and methods of the Chinese colorectal,breast, lung, liver, and stomach cancer screening trial(C-BLAST)
- Recent research hotspots in sequencing and the pancreatic neuroendocrine tumor microenvironment
- Emerging roles of plasmacytoid dendritic cell crosstalk in tumor immunity
- Next-generation antibody–drug conjugates revolutionize the precise classification and treatment of HER2-expressing breast cancer
