Third-Order Nonlinear Optical Responses of Bis(15-crown-5)-stilbenes Binding to One-or Two-Alkali Metal Cation (Li+,Na+and K+)
Hai-Ling Yu,Tong Zhang,Tian-Liang Ma,Bo Hong,Zhi-Qiang Cheng
College of Resources and Environmental Science,Jilin Agricultural University,Changchun 130118,China
Bis(15-crown-5)-stilbenes containing crown ether parts have been widely used in a variety of chemical applications,such as cation detectors,because of their ability to selectively bind to alkali metal cations,Bis(15-crown-5)-stilbenes and its derivatives with complexation of oneor two-alkali metal cation (Li+,Na+ and K+) have been theoretically investigated by quantum chemistry methods.The coordination of alkali cations results in partial shrinkage of crown ethers,which directly affected natural distribution analysis charges and molecular orbital energy levels.The number of alkali metal ions has significant effects on absorption spectra and mean second hyperpolarizability.When one alkali metal ion was added to the anticonformer of bis(15-crown-5)-stilbene,the absorption spectra were obviously redshifted and the mean second hyperpolarizability values were slightly increased;while two alkali metal ions were added to bis(15-crown-5)-stilbene,the absorption spectra were obviously blue shifted and the mean second hyperpolarizability values decreased.On the other hand,as the radius of the alkali ions increased,the mean second hyperpolarizability values of the compounds increased gradually.It is indicated that the mean second hyperpolarizability value is sensitive to the number and radius of the alkali metal cations,thus the third order nonlinear optical response can be used as a signal to detect the number and type of alkali metal ions.
Key words: Bis(crown)-stilbene,Cation detector,Metal cation,Quantum chemistry,Second hyperpolarizability
I.INTRODUCTION
Crown ether is the earliest selective reagent used for colorimetric determination of alkali metal cations,and has been widely used in the field of alkali metal cationic optical molecular detectors [1-5].Crown ethers with chromogenic substituents (such as stilbene [6],anthracene [7,8],azobenzene [9,10] or trialkyl [11]) are common efficient multi-dentate ligands.Crown ethers have been shown to adapt to a wide variety of coordination requirements and media.Their flexibility confers some very interesting properties such as solubility in aqueous and lipophilic solvents,and fast,reversible ion binding properties.One aspect of crown ether chemistry is that these ligands can select metal cations to bind to,based on the size of the large ring [12] inside.The strongest binding affinity between crown ether and alkali metal cations has been fully reported at the molecular level [13-17].The cationic complexation process is controlled by crown ether cavity,cationic size and solvability of solvent.
Chromogenic substituents of crown ethers containing 1,2-bis(aryl/hexenyl)-ethylene fragments have been the subject of many studies due to their remarkable spectral fluorescence and photochemical properties.Because of these properties,they are widely used to design multi-photon absorption and second-order nonlinear optics (NLO) materials for optical information recording and storage [18-21].Bis(15-crown-5)stilbene is a typical representative of stilbene in crown ethers,which has three conformations (Scheme 1).Of these conformations,two anticonformations (1 and 2) are dominant,due to their relatively low energy barriers as the benzene ring rotates around the single bond in the form of ethylene fragment [17].Our theoretical calculations also confirm this prediction,and the energy barrier difference between them ranges from 0.41 kcal/mol to 0.74 kcal/mol (Table S1 in Supplementary materials(SM)).

Scheme 1.Conformers of bis(15-crown-5)-stilbenes.
When crown ethers with stilbenes are combined with ligands metal cations,more stable sandwich compounds can be formed.Gromovet al.have fully reported that the symmetrical bis(crown)stilbenes (L) and metal cations (Mm+) (such as Cs+,Rb+,K+,Ba2+,Sr2+,Ag+and Pb2+) can form 1(L):1(Mm+) or 1(L):2(Mm+) compounds in MeCN solutions [22-25].The coordination of the metal cation to a bis(15-crown-5)-stilbene moiety leads to “off-on” detecting signals(changes of properties) in the absorption spectrum and fluorescence response.These different spectral and excited state transition signals further lead to the change of NLO properties,so it has application prospects in the field of NLO [26-31].
However,to the best of our knowledge,the advantages of bis(15-crown-5)-stilbene ligand combined with alkali metal cations for the development of NLO-based detectors remain largely unutilized.With our continuing interests on the NLO compounds and NLO-based detectors [32-36],we have investigated third order NLO responses of crown ether derivatives formed by bis(15-crown-5)-stilbene with alkali metal cation (Li+,Na+and K+) by using density functional theory (DFT)(Scheme 2).In this work,stilbene is used to represent bis(15-crown-5)-stilbene for simplicity and clarity,while 1·Li+,1·Na+and 1·K+as well as 1·(Li+)2,1·(Na+)2and 1·(K+)2are used to represent one-and two-metal cation derivatives,respectively.
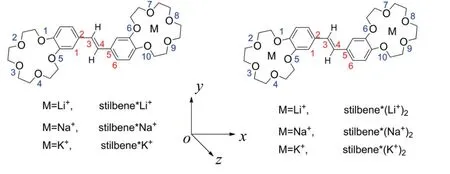
Scheme 2.Structures of the metal cation derivatives of bis(15-crown-5)-stilbenes.
II.COMPUTATION DETAILS
The geometric optimizations of these systems are fully optimized at the B3LYP/6-31G(d,p) level.The B3LYP [37,38] uses the non-local correlation provided by the LYP expression,and VWN functional III for local correlation.All the optimized structures reach the real local minimum stability point,which proves that there is no imaginary frequency.The interaction energy,enthalpy,entropy,Gibbs free energy and natural distribution analysis (NPA) charge are calculated at the B3LYP/6-31G(d,p) level.
Local orbital locators (LOL) are bond descriptors based on kinetic energy density used to describe the properties of chemical bonds.
whereφiis orbital wavefunction,ηiis occupation number of orbital,andD0(r) is energy density.
At the micro level,the energy of the system can be written as Taylor’s expansion for the uniform external electric fieldF:
where,μ0is the dipole moment,α is the polarizability(second-order tensor),and β is the first-order hyperpolarizability (third-order tensor,known as second-order nonlinear optical response (NLO) coefficient).γ is the second-order hyperpolarizability (which is a fourth-order tensor,known as the third-order NLO coefficient).The (hyper) polarizability tensor is directly related to the frequency of the outer fieldF.IfFis zero frequency(electrostatic field),the (super) polarizability is said to be static or frequency independent.Among them,is:
The γ values are calculated at the default field amplitude (0.0003 a.u.) by using analytic field and finite field hybrid methods.This method automatically obtains the higher-order derivatives by one or more finite difference methods based on the lower-order analytic derivatives.Here,the method is based on the third order analytic derivative,and then automatically performs a higher order finite difference to obtain the desired fourth derivative.It is more efficient than finite field method and saves a lot of computer cost.
The electron density can be written as the Taylor expansion [39]:
where,ρ(n)represents then-order response of electron density toF.According to the relationship between the dipole moment and the electron density,the relationship between the second hyperpolarizability and the electron density can be known [40]:
Due to too manyγcomponents,we only focus on the largestγxxxxalong thex-axis:
The CAM-B3LYP and BHandHLYP functions are better choices for the calculation of secondary hyperpolarizability.CAM-B3LYP functionality,which is a hybrid feature,improves the long-range properties [41].The BHandHLYP functional include the 50% exact exchange in the BLYP functional [42].Therefore,CAMB3LYP and BHandHLYP functions are used to compare the secondary hyperpolarizability.The basis set used is 6-311+G(d,p).Furthermore,the density of the second hyperpolarizabilityis calculated using the same function set and basis set as the second hyperpolarizability.
The excited states are evaluated using time-dependent density functional theory (TDDFT) [43,44].Since CAM-B3LYP functional is in good agreement with experimental values [45,46],the electron excited states of these systems are calculated at CAM-B3LYP/6-311 +G(d,p) level.
Both DFT and TDDFT are calculated using Gaussian 09W program package [47],and the results are analyzed by Multiwfn 3.3.8 software [48,49].
III.RESULTS AND DISCUSSION
A.Geometrical structure and thermodynamic analysis
The energies and energy defference (ΔE,kcal/mol) of three conformers of bis(15-crown-5)stilbene (1,2,and 3)are calculated at the B3LYP/6-31G(d) level (Table S1 in SM).It is found that 1 has the lowest energy and is the most stable conformer.The structures of compounds are optimized at the B3LYP/6-31G(d,p) level.Important geometrical parameters of compounds with 1 as their parent are listed inFIG.1and Table I.The geometrical parameters of compounds with 2 and 3 as their parents are listed in FIGs.S1 and S2 in SM.Since three parent compounds (1,2,and 3) are isomers,their geometrical parameters are similar.We select the most stable compounds 1 for detailed discussion.For 1,as compared with experimental data determined by X-ray analysis [21],the average O···O lengthes of optimized structures are slightly larger,whereas the C1-C2-C5-C6 dihedral angles are slightly smaller.This is because the environments are different in two cases (the measured data are in crystal,while the calculated results are in gas phase).However,the C2-C3,C3-C4 and C4-C5 bond lengthes agree sufficiently well with that of the experimental data,the deviations of them between DFT optimized structures and experimental data are 0.017,0.016,and 0.018 Å,respectively.The small bond deviations indicate that the B3LYP method for geometrical optimizations is reliable.For metal cation derivatives of stilbenes,the average O···O distances of crown ethers range from 2.597 Å to 2.798 Å,due to that metal cations have strong interactions with crown ethers.Moreover,the average distances between alkali metal ion and oxygen atom M+-O are 2.21 Å(Li+-O),2.31 Å (Na+-O) and 2.70 Å (K+-O).From the structures of the compounds with 2 and 3 as their parents (FIGs.S1 and S2 in SM),the average distances between alkali metal ion and oxygen atom M+-O are about 2.20-2.24 Å (Li+-O),2.31-2.33 Å (Na+-O),and 2.70-2.72 Å (K+-O).It is indicated that as the radius of alkali metal ion increases,the distance between alkali metal ion and oxygen atom increases.

FIG.1 The structures of the compounds with 1 as the parent and their localized orbital locator (LOL) maps obtained by B3LYP/6-31G(d,p) level.
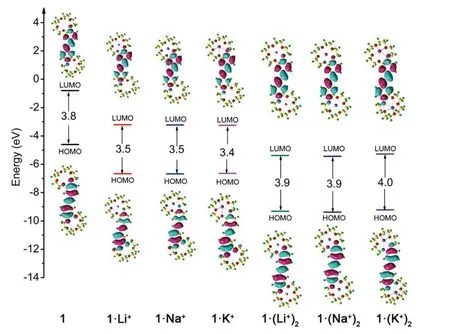
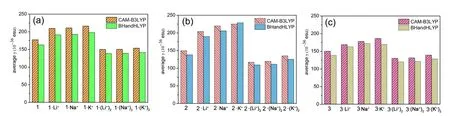
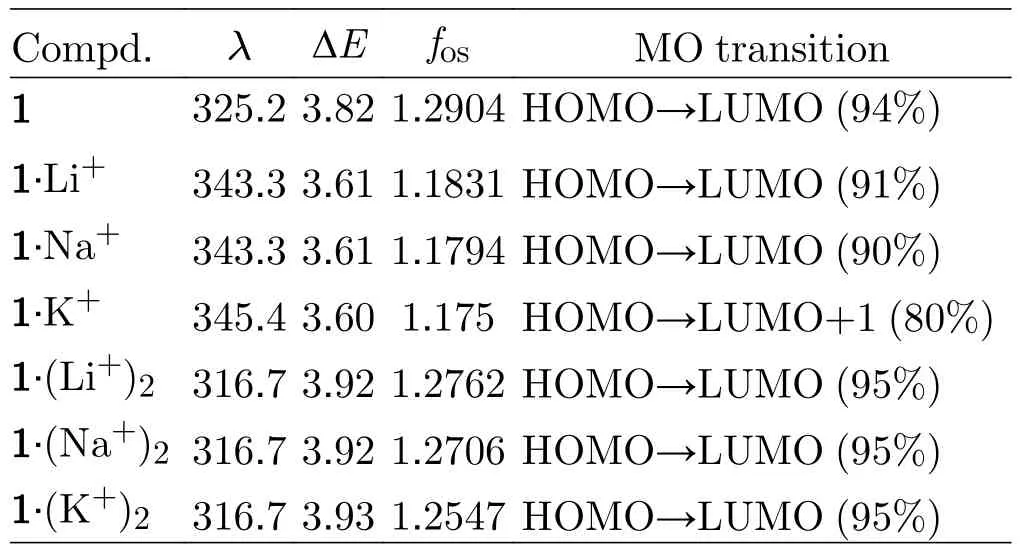
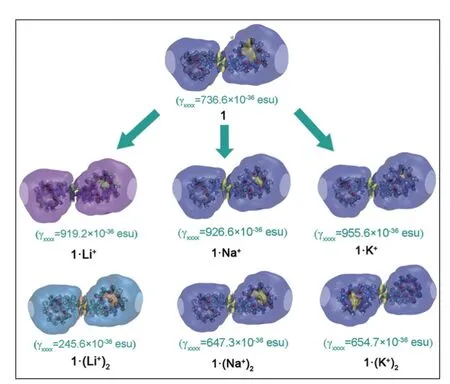
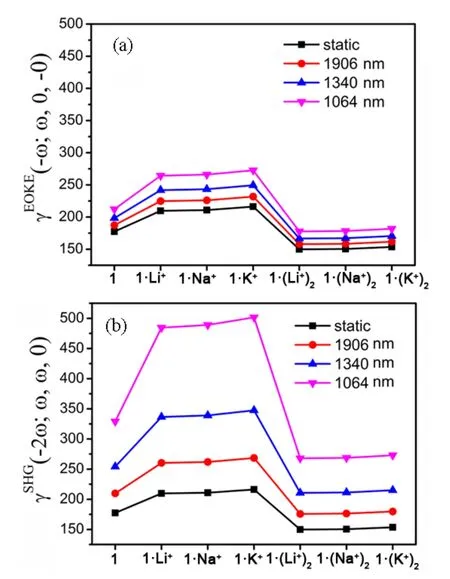
Localized orbital locator (LOL) maps are used to explain structures and bonds clearly and visually.As showm in FIG.1,the LOL >0.5 regions in yellow-red correspond to a typical covalent bond.The LOL ≈ 0.5 regions shown in pale green characterize delocalized electrons.The 0.0 In order to understand the interaction between ligand and metal cation,the interaction energy is calculated.Table I shows that the absolute value order of interaction energy between metal cation and crown ether is 120.0 kcal/mol (1·Li+) >93.7 kcal/mol (1·Na+) >66.9 kcal/mol (1·K+) and 221.1 kcal/mol (1·(Li+)2>180.1 kcal/mol (1·(Na+)2) >124.3 kcal/mol (1·(K+)2),indicating that the addition of alkali metal ions leads to stronger interaction energy between ligands and metal ions.The interaction energy between ligand and metal ions decreases with the increase of the radius of homophonic alkali metal ions.This is because the bond between the coronal ion and the metal cation is essentially electrostatic,while the Coulomb force is inversely proportional to the square of the radius;and the small-er the radius,the smaller the distance between ions is,therefore the stronger the electrostatic interaction is. TABLE I The selected geometrical parameters and interaction energies Eint calculated at the B3LYP/6-31G(d,p) level. Table II summarizes the main thermodynamic data of cation complexation reactions of stilbenes with alkali metals.The Gibbs free energy of the reaction(T=298.15 K,P=1 atm) is exothermic,and the most exothermic reactions are coordination reactions(1·(Li+)2,1·(Na+)2and 1·(K+)2).The evolutionary order of the absolutevalues was 114.4 kcal/mol(1·Li+) >87.1 kcal/mol (1·Na+) >57.9 kcal/mol (1·K+)and 221.6 kcal/mol (1·(Li+)2>168.1 kcal/mol(1·(Na+)2) >107.2 kcal/mol (1·(K+)2).The Gibbs free energy (absolute value) changes monotonically with the cation size,decreasing from Li+to K+.This is partly due to the similarity of reaction enthalpy and the small change of reaction entropy.In addition,the evolution trend of absolutevalues value is completely consistent with the binding energyEint between cation and ligand.It can be seen that the alkali metal ions with smaller radius are more likely to combine with crown ether to form complexes,and the binding strength with crown ether is greater.The complex formed by the alkali metal ions with smaller radius and crown ether is more stable. Atomic charge is one of the simplest and most intuitive descriptions of charge distribution,which is of great significance in theory and practical applications.In theis work,all the compounds are divided into five fragments (F1-F5).The total charges of different fragments and their metal cation derivatives are calculated at the B3LYP/6-31G(d,p) level using the NPA method(Table S2 in SM).The negative charges of all com-pounds are mainly located on crown ethers (F1,F5),which are attributed to the electronegativity of oxygen and the abundance of π-bound electrons,respectively.The negative charge and cavity of crown ether confer its coordination ability with alkali metal cations.After the coordination of the alkali cation,the positive charge of all fragments (F1 to F5) increases and the electron density decreases (FIG.2),that is,the complexation results in the transfer of electrons from all fragments to the alkali cation.Among all compounds,the most modified charge distributions are crown ether (F1,F5) and benzene (F2,F4) groups.It is worth noting that the NPA charge of the crown ether group decreases monotonically with the size of the cation from Li+to K+in the coordination of alkali metal cations.But vinyl (F3)is almost unaffected by complexation,which is just playing a bystander,because this part is away from the alkali cation. TABLE II The enthalpy and Gibbs free enthalpy(kcal·mol-1·K-1) and entropy (in kcal/mol of the complexation reaction calculated on B3LYP/6-31G(d,p) at 298.15 K and 1 atm. FIG.2 The fragments (F1 to F5,left panel) of the compounds and their NPA charges (right panel) obtained at the B3LYP/6-31G(d,p) level,∆ q=q(L·(M+/2+)2)-q(fL) (L was the ligand). The frontier molecular orbitals are often used to obtain qualitative information about the optical and electrical properties of molecules [50].Further,the energy gap between the highest occupied molecular orbital(HOMO) and the lowest unoccupied molecular orbital(LUMO) is used to understand the charge transfer interaction within the compound.The frontier molecular orbital energies are given in Table S3 in SM and the plots of energy levels are presented inFIG.3.For stilbene,the HOMO energy is-4.60 eV,while the LUMO energy is-0.80 eV,thus resulting in the energy gap of 3.80 eV.However,for 1·Li+,1·Na+,and 1·K+,a significant decrease of both HOMO and LUMO energy levels is noticed.It is due to that the addition of alkali metal ions makes it easier for crown ethers to gain electrons and less likely for them to lose electrons.The energy level of HOMO reflects the electron loss ability of cluster molecules.According to Koop Manns theorem,the negative value of HOMO energy represents the first ionization energy of the molecule.The lower the ionization energy is,the higher the HOMO energy is,and the more easily the substance loses electrons.Because the addition of alkali metal ions makes the crown ether less likely to lose electrons,the HOMO level decreases.The negative value of LUMO energy level represents the electron affinity potential of the molecule.The higher the affinity potential,the lower the LUMO energy level is,and the more easily the substance can get electrons,while the alkali metal ions make it easier for crown ether to get electrons,so the LUMO energy level decreases. FIG.3 The frontier molecular orbital energy levels of 1 and compounds with 1 as their parent. As compared with stilbene,the energy levels of LUMOs of 1·Li+,1·Na+,and 1·K+decrease more in contrast to HOMOs,thus their smaller energy gaps are obtained.It is due to that the frontier molecular orbital distribution represents the electron cloud configuration of the complex.When the crown ether bonds to one alkali metal cation,the electron cloud distributions at both ends of 1 are no longer equal.Therefore,the dipole moment increases,leading to the decrease of the energy gaps.These small energy gaps demonstrate the facility of the electronic transition from lower occupied MOs to unoccupied MOs,suggestting that these derivatives of stilbenes containing one alkali metal cation may exhibit large second hyperpolarizability. While for 1·(Li+)2,1·(Na+)2,and 1·(K+)2,it is evident that two alkali metal cations still significantly reduce the HOMO and LUMO energy levels.For a comparison with stilbene,the HOMO energy levels of 1·(Li+)2,1·(Na+)2,and 1·(K+)2are more sensitive to reduction,as compared to that of LUMOs,leading to the larger energy gaps.This is due to that when the crown ether bonds to two alkali metal cation,the electron cloud distributions at both ends of 1 decrease almost equally,leading to the larger energy gaps with respect to that of 1.Such a large energy gap will cause the difficulty of the electronic transition,implying small second hyperpolarizability. With regard to compounds with 2 as its parents(FIG.S3 in SM),the order ofEgap values is following as 3.4 eV (2·Li+,2·Na+,and 2·K+) <3.6 eV (2) <4.0 eV(2·(Li+)2,2·(Na+)2,and 2·(K+)2).A similar result is found for compound 3 as its parents (FIG.S4 in SM),the order ofEgap values is following as 3.3-3.4 eV(3·Li+,3·Na+,and 3·K+) <3.8 eV (3) <4.0 eV (3·(Li+)2,3·(Na+)2,and 3·(K+)2).It is found that these derivatives of stilbenes containing one alkali metal cation may exhibit large second hyperpolarizability,while that containing two alkali metal imply small second hyperpolarizability. The UV-Vis absorption spectra and the electron transitions of the compounds are studied using the timedependent density functional theory (TD-DFT)method.In order to study electron transitions,the electron density difference map (EDDM) corresponding to the key electron transitions is given inFIG.4.The calculation results of electron transition parameters of the main excited states are shown in Table III.The UV-Vis absorption spectra of the studied compounds have a similar shape,with only one peak in the range of 316.7-345.4 nm.For 1,the maximum absorption peak is located at 325.2 nm.Since the accumulation and depletion regions of electron density are delocalized on the styrene,but less on the crown ether,it can be determined as intramolecular π-π* charge transfer of the styrene unit.The maximum absorption peaks of 1·Li+,1·Na+,and 1·K+are located at 343.3,343.3,and 345.4 nm,respectively,which can also be considered as intramolecular π-π* charge transfer of stilbene unit.After alkali metal ions are added,the absorption spectra are redshifted about 18.1-20.2 nm.The maximum absorption peaks of 1·(Li+)2,1·(Na+)2,and 1·(K+)2are all located at 316.7 nm,which is also considered as intramolecular charge transfer of styrene.This indicates that the absorption spectra of 1·(Li+)2,1·(Na+)2,and 1·(K+)2are blue shifted by 8.5 nm when the two alkali metal ions are added.Therefore,the absorption spectrum can be used as a probe to detect the number of bound alkali ions,but it cannot effectively distinguish the types of alkali ions. FIG.4 The absorption spectra and electron density difference diagram of the studied compunds obtained at TD-CAMB3LYP/6-311+G(d,p) (purple and blue indicate electron density accumulation and depletion,respectively). The second static hyperpolarizability is calculated to further study the signal that can distinguish the types of alkali metal ions.Due to the centrosymmetric structure of these compounds,the first hyperpolarizability is close to zero,so the discussion is skipped.The second hyperpolarizability (γ) is an obvious characteristic parameter that contributes to the third-order NLO response at the molecular level.It is speculated that crown ethers are good metal ion detectors because of their feasibility in controlling γ values.To further improve our understanding of these predictions,we theoretically analyze the static γ values using CAM-B3LYP and BHandHLYP functionals of the 6-311+G(d,p) basis set listed in Tables S4 and S5 in SM.It is clear that the results of the two functions are basically the same,that is,the results are very close and show a similar trend(FIG.5).For convenience,we only analyze the results of the CAM-B3LYP functional,as we have found this functional to be a more suitable method for calculating hyperpolarizability. FIG.5 Static mean second hyperpolarizabilities () of compounds with (a) 1,(b) 2,and (c) 3 as the parent computed by the CAM-B3LYP and BHandHLYP functionals. TABLE III The wavelength of the main absorption peak(λ,nm),the transition energies from S0 to S1 state (∆E,eV) and the corresponding oscillator strengths (fos),as well as the major contributions obtained at the CAMB3LYP/6-311+G(d,p) level. For all of the compounds,the origin of the Cartesian coordinate system located at the center of the C=C bonds of the stilbene which is placed in thexy-plane with the longitudinalxaxis pointing to the stilbene moieties (Scheme 1).The static mean second hyperpolarizabilities () of these compounds along with the corresponding components are positive (Table S4 and S5 in SM).We confine our attention to the longitudinal componentγxxxx,since it dominates the response of the.The magnitude ofγxxxxagrees with the order of thevalues.The order of thevalues is 177.4×10-36esu(1) <209.7×10-36esu (1·Li+) <210.9×10-36esu(1·Na+) <216.2×10-36esu (1·K+).A most interesting aspect to be stressed is that thevalues of 1·Li+,1·Li+,and 1·K+slightly increase with the complexation of one alkali metal cation to the bis(15-crown-5)-stilbene crown ether ligand.On the other hand,it is found that with the increase of the radius of alkali metal ions,thevalues of the compounds increase gradually.Therefore,the static second hyperpolarizability can be used as a signal to detect the type of alkali metal ions.However,when the stilbene is binding with two metal ions,thevalues of 1·(Li+)2,1·(Na+)2,and 1·(K+)2are 149.9×10-36esu (1·(Li+)2),150.4×10-36esu (1·(Na+)2),and 153.6×10-36esu (1·(K+)2),respectively.It is indicated that the addition of two metal ions reduces thevalue of the stilbene ligand (177.4×10-36esu).This apparent discrepancy actually reveals that the second hyperpolarizability was sensitive to the nature and number of the alkali metal cation. For compounds with 2 as the parent,the order of thevalues is 149.5×10-36esu (1) <204.2×10-36esu(1·Li+) <219.7×10-36esu (1·Na+) <225.1×10-36esu(1·K+),indicating that with the increase of the radius of alkali metal ions,thevalues of the compounds increase gradually.Therefore,the static second hyperpolarizability can be used as a signal to detect the type of alkali metal ions.However,when the stilbene is binding with two metal ions,thevalues of 1·(Li+)2,1·(Na+)2,and 1·(K+)2are 117.1×10-36esu (1·(Li+)2,119.5×10-36esu (1·(Na+)2),and 135.0×10-36esu(1·(K+)2),respectively,suggesting that the addition of two metal ions reduces thevalue of the stilbene ligand.It reveals that the second hyperpolarizability is sensitive to the nature and number of the alkali metal cation.This fact inspires us to further investigate the NLO-based detection of cations associated with an obviousγcontrast as compared with stilbene. In order to understand the actual transitions responsible for the origin of theγin the molecules,two-level model that links theγvalue and the low-lying CT character has been used earlier by other workers [51].According to this model,theγvalue was inversely proportional to the fifth power of the transition energy and proportional to the second power of the oscillator strength.The spectroscopic parameters corresponding to the low-lying transition energy and oscillator strength as well as MO transitions are listed in Table III.Thefos2/∆E5varies in the following order of 12.62×10-36esu (1·(Li+)2)<13.11×10-36esu (1·(Na+)2) <13.22×10-36esu (1·(K+)2) <15.38×10-36esu(1) <17.05×10-36esu (1·Li+) <17.16×10-36esu(1·Na+) <17.17×10-36(1·K+).This is quantitatively consistent with their order of value.The results show that the addition of alkali metal ions reduces the electron transition energy of the main excited states and increases the third-order nonlinear optical response of the compounds.The addition of two alkali metal ions increases the electron transition energy of the main excited state and reduces the third-order nonlinear optical response. The second hyperpolarization density is often used to elucidate the contribution of space electrons to γ.The positive and negative γ densities correspond to the increase and decrease of the field-induced third-order electron density.Thus,the γ density represents the relative phase and magnitude change of the third-order electron density [50].Eq.(7) gives the relationship between the γ density andγxxxx.This means that-x×is much easier to characterize the spatial contribution to the dominatingγxxxxcomponent as compared with.The plots of-x×for 1,1·Li+,and 1·(Li+)2are listed inFIG.6.The negative contribution toγxxxxis essentially on the molecular valence shell region,whereas the enlarged positive contribution is on the outer regions.Obviously,the positive enlarged area is larger than that of the negative,this is why the current compounds ofγxxxxare large positive values.For 1·Li+,as compared to stilbene,the negative contribution toγxxxxis shrunken,leading to the largerγxxxxvalue.However,1·(Li+)2shows much smaller positive contribution area as compared to stilbene,though small negative contribution is also observed,which coincides with smallerγxxxxvalue.As for theγxxxxvalues of Na+and K+cation derivatives,the effect of the complexion of one-and two-alkali metal cation to stilbene are similar in FIG.S2 in SM. FIG.6 Schematic diagram of the-x× for the compounds computed at the CAM-B3LYP/6-311G(d,p)level.Blue and purple colors indicate the positive contribution,while yellow and orange colors indicate the negative contribution. To make our calculations more useful to experimentalists,we investigate the frequency dependent second hyperpolarizability [52].The frequency dependent second hyperpolarizability associated with the photoelectric Kerr effect,γEOKE(-w;w,0,0),and the second harmonic generation,γSHG(-2w;w,w,0),were performed at the CAM-B3LYP/6-311+G(d,p) level(FIG.7).To avoid over-measurement ofγvalues,we calculate the frequency-dependent second hyperpolarizability at 1906 nm,1340 nm,and 1064 nm.There is a tiny increase in bothγEOKE(-w;w,0,0) a ndγSHG(-2w;w,w,0).Although the frequency dependence onγSHG(-2w;w,w,0)is slightly larger as compared toγEOKE(-w;w,0,0),both of them show the same trend when comparing with the static second hyperpolarizability.Thus,for all of the studied compounds,the frequency-dependent effect is weak. FIG.7 The frequency-dependent second hyperpolarizabilities associated with the photoelectric Kerr effect γEOKE(-ω; ω,0,0)(a),and frequency-dependent second hyperpolarizabilities associated with the second harmonic generation γSHG(-2ω; ω, ω,0) (b). In order to find sensitive probes and signals for the detection of alkali metal ions,the density functional theory methods have been performed for the bis(15-crown-5)-stilbenes ether ligand and its derivatives with complexation of one-or two-alkali metal cation (Li+,Na+,K+).Geometric analysis reveals that the alkali metal cations and the dihedral angle of the stilbene ring together make the crown ethers shrink obviously.The addition of one alkali metal cation to 1 leads to the smaller energy gaps of 1·Li+,1·Na+,and 1·K+with respect to that of 1,while the addition of two alkali metal cation to 1 leads to the larger energy gaps of 1·(Li+)2,1·(Na+)2,and 1·(K+)2than that of 1.Different energy gaps produce an obvious mean second hyperpolarizabilitycontrast between one-and two-alkali metal cation derivatives,which can be explained by two-level model and second hyperpolarizability density.It is found that the addition of an alkali metal ion slightly increases theγ¯values of 1,and with the increase of alkali metal ions radius,thevalues of the compounds increase gradually.However,the addition of two alkali metal ions reduces thevalues of 1.Frequency-dependent second hyperpolarizability calculation reveals that the photoelectric Kerr effect and the second harmonic generation frequency-dependent effect are weak.These results have inspired us to further investigate the NLO-based molecular detector due to their high optical selectivity and will broaden the scope and the efficiency of the optical molecular detectors. Supplementary materials:The components of total polarizabilities and second hyperpolarizabilities for bis(15-crown-5)-stilbene and its derivatives with complexation of one-or two-alkali metal cation (Li+,Na+,K+) are available. This work is surported by the Jilin Province Science and Technology Development Project (No.2022 0203017SF),Industrialization Project of the ‘‘13th Five-Year” Education Department of Jilin Province(No.JJKH20200334KJ),and the National Natural Science Foundation of China (No.11704143).Additionally,we gratefully acknowledge the software support from Prof.Jian Lv at College of Physics of Jilin University.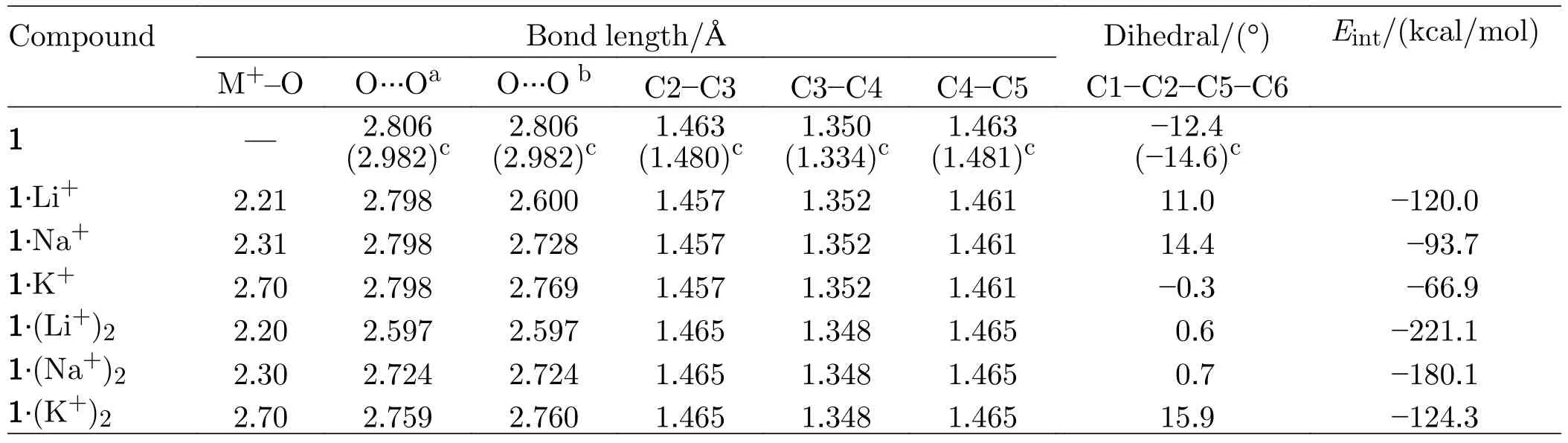
B.Charge distribution analysis

C.Frontier molecular orbital analysis
D.The absorption spectra

E.Static second hyperpolarizability
F.Second hyperpolarizability density
G.Frequency-dependent second hyperpolarizability
IV.CONCLUSION
V.ACKNOWLEDGMENTS
 CHINESE JOURNAL OF CHEMICAL PHYSICS2023年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2023年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Minimum-Modified Debye-Hückel Theory for Size-Asymmetric Electrolyte Solutions with Moderate Concentrations
- Quantum Dynamics Calculations on Isotope Effects of Hydrogen Transfer Isomerization in Formic Acid Dimer
- Design Strategy of Infrared 4-Hydroxybenzylidene-imidazolinone-Type Chromophores based on Intramolecular Charge Transfer: a Theoretical Perspective
- Photothermal Catalytic Selective Oxidation of Isobutane to Methacrylic Acid over Keggin-Type Heteropolyacid
- Controllable Modulation of Morphology and Property of CsPbCl3 Perovskite Microcrystals by Vapor Deposition Method
- On-the-Fly Nonadiabatic Dynamics of Caffeic Acid Sunscreen Compound
