Preparation and Supercapacitive Performance of CuFe2O4 Hollow-Spherical Nanoparticles
Yu Zhng,Qinggung Zhu,Yqi Zho,Xin Yng,Ling Jing
a.College of Chemistry and Chemical Engineering,Xinyang Normal University,Xinyang 464000,China
b.Xinyang Key Laboratory of Low-Carbon Energy Materials,Xinyang Normal University,Xinyang 464000,China
c.State Key Laboratory of Molecular Reaction Dynamics,Dalian Institute of Chemical Physics,Chinese Academy of Sciences,Dalian 116023,China
Spinel-type CuFe2O4 nanoparticles were synthesized by a solvothermal method using ethylene glycol as solvent and polyvinylpyrrolidone (PVP) as dispersant.The characterization results showed that the average diameter of the hollow-spherical CuFe2O4 was approximately 100 nm with homogeneous morphology and negligible agglomeration.CuFe2O4 was used as the active electrode material to explore its supercapacitive properties in different concentrations of KOH electrolytes.It was found that the CuFe2O4 hollow-spherical nanoparticles exhibit potential electronic performance in supercapacitor,with a specific capacitance of 368.2 F/g and capacitance stability retention of 91.0%after 2000 cycles at the current density of 5 A/g in 3 mol/L KOH electrolyte.The present findings demonstrate that the CuFe2O4 electrode materials can have important implications with practical prospects in energy storage systems.
Key words: Supercapacitor,Electrode material,Composite metallic oxide
I.INTRODUCTION
Energy is an important material basis for the sustainable development of society.With the consumption of limited fossil energy,it is significant to develop emerging energy.“How and when to replace oil” has been listed as one of the 125 most challenging scientific questions of the century by Science.Energy storage technology plays an important role in diverse applications,such as renewable energy grid connection,peak regulation and efficiency improvement of the power grid,regional energy supply,and electric auto mobiles[1,2].It is also a major strategic need to ensure energy security,implement energy conservation and emission reduction,and promote low carbon.Among various energy storage technologies,lithium-ion batteries have become the pillar technology for new energy development with their high energy density [3,4];sodium-ion batteries are placed great expectations in the large-scale energy storage field due to their low cost and high safety[5-7].With high power density,fast charge-discharge,and ultra-long cycle life,the supercapacitor is one of the key systems for achieving a stable output of renewable energy generation [8-15].The supercapacitor is popular as a high-power energy storage component and has been extensively researched by global experts and scholars.Its advantages make up for the shortcomings of other energy storage devices: short service life,high maintenance cost,high environmental pollution,narrow operating temperature range,etc.[16].Moreover,electrode materials are the core components of supercapacitors and directly affect their performance.
As is well known,the pseudocapacitance of transition metal oxides arises from the chemisorption/resolution of ions at the electrode/electrolyte interface and the Faradaic redox reaction.Due to the multiple valences available for charge transfer and reversible adsorption,higher specific capacitance of transition metal oxide can be obtained compared with carbon materials of the bilayer capacitance type.Several metal oxides(e.g.,RuO2,IrO2,Mn3O4,Co3O4,and Fe2O3) and hydroxides (e.g.,Co(OH)2and Ni(OH)2) showed the potentials as electrode materials for supercapacitors and energy storage [17-21].
The O2-ions in the structure of spinel-type transition metal composite oxide (AB2O4) are arranged in close-packed cubes.The A ions are located in the tetrahedral gap,and B ions are filled in the octahedral gap,i.e.,A2+ions are four-coordinated,while B3+ions are eight-coordinated [22].Due to the spatial structure of AB2O4,it exhibits more excellent physical and chemical properties than single component oxides,such as high-temperature resistance,excellent chemical stability,and high hardness.Therefore,it has great application prospects in electrode materials [23],catalytic materials [24],magnetic materials [25],etc.[26,27].
CuFe2O4ferrite is a typical spinel-type transition metal composite oxide.The O2-ions are in a dense cubic arrangement with Cu2+and Fe3+ions in two different crystal positions.CuFe2O4has attracted extensive attention for applications in sensors,batteries,and catalysts due to the unique electronic structure of Cu(3d104s1),low price,environmental friendliness,and large copper abundance [28].A variety of morphologies of CuFe2O4nanoparticles have been synthesized via a hydrothermal method.Zhuet al.synthesized CuFe2O4nanospheres with hierarchically porous structure through a facile solvothermal procedure,with high specific surface area,pore volume,and uniform pore size distribution [29].Very recently,Zhanget al.used carbonaceous materials as the matrix to inhibit the agglomeration of CuFe2O4nanoparticles [30].
Here,the spinel CuFe2O4nanoparticles are synthesized by the hydrothermal method and characterized to have hollow-spherical structures by various analysis methods.It is found that the CuFe2O4hollow-spherical nanoparticles exhibit potential electronic performance in supercapacitor,with a specific capacitance of 368.2 F/g and capacitance stability retention of 91.0%after 2000 cycles at the current density of 5 A/g in 3 mol/L KOH electrolyte.This work provides a potential strategy for material design and synthesis and has important implications with practical prospects in energy storage systems.
II.EXPERIMENTS
A.Preparation of CuFe2O4 nanoparticles
The CuFe2O4nanoparticles were prepared by the solvothermal method.First,5 mmol of blue-green copper chloride (CuCl2∙2H2O) crystals were weighed and dissolved in 35 mL of ethylene glycol which was completely dissolved and dispersed by ultrasound.Then,10 mmol of brown-yellow ferric chloride (FeCl3∙6H2O)was dissolved in 35 mL of ethylene glycol and sonicated for 10 min until it was completely transparent;2 g of polyvinylpyrrolidone (PVP) was added and ultrasonically dispersed until it was completely dissolved.After that,the two solutions were mixed well with magnetic stirring,and 60 mmol of sodium acetate (NaAc∙3H2O)was added to the mixture and stirred well to form a precursor solution.The precursor solution was transferred to a 100 mL hydrothermal reactor and reacted at 180 ℃ for 12 h.After the reaction was completed,it was cooled to room temperature.The black precipitate obtained from the reaction was collected,centrifuged,and washed with deionized water and ethanol alternately.After being dried in an oven at 60 ℃,it was ground and standby.
B.Preparation of working electrodes
The working electrode was prepared by the coating method.The synthesized CuFe2O4sample was selected as the active electrode material,Ketjen black as the conductive agent,and polyvinylidene fluoride (PVDF)as the binder.They were weighed in the mass ratio of 7:2:1 before grinding and mixing well.After that,an appropriate amount ofN-methyl-pyrrolidone (NMP) was added,and the mixture was stirred and mixed into a slurry.The slurry was evenly coated on the nickel foam substrate (the effective area of the coating was about 1 cm×1 cm).After being dried at 60 ℃,it was pressed and weighed.
C.Structural characterization
The crystal structure information of the samples was measured using X-ray diffractometer (XRD,RIGAKU D/Max 2200 X) with a Cu-Kα radiation generator(λ=1.54 Å).The morphology and surface state were studied by scanning electron microscopy (SEM,JEOL,JSM6700F).The internal structure and properties of the samples were obtained by transmission electron microscopy (TEM,FEI Tecnai G2 F20 S-TWIN) and high resolution transmission electron microscopy (HRTEM,FEI Tecnai G2 F20 S-TWIN) where the accelerating voltage was 200 kV,since the elemental composition was confirmed by energy dispersive X-ray analysis(EDAX).
D.Supercapacitive performance test
The electrode prepared by the coating method served as the working electrode,the Pt electrode was the counter electrode,and the saturated calomel electrode (SCE) acted as the reference electrode to form a three-electrode system.Cyclic voltammetry (CV),galvanostatic charge-discharge (GCD) and electrochemical impedance spectroscopy (EIS) were performed in 1 mol/L,3 mol/L,and 6 mol/L KOH solutions with a RST5200F electrochemical workstation (Zhengzhou Shiruisi Technology Co.,Ltd.,Zhengzhou,China),respectively.
III.RESULTS AND DISCUSSION
A.Structure information
FIG.1shows the XRD pattern of CuFe2O4synthesized by the hydrothermal method.The diffraction peaks near 2θ=30.0°,35.2°,37.5°,43.5°,53.3°,57.3°,and 62.7° in the curves are attributed to the surfaces of cubic spinel CuFe2O4at (220),(311),(222),(400),(422),(511),and (440),which are perfectly matched with the standard card of spinel CuFe2O4(JCPDS No.77-0010) [31].The distinct and sharp diffraction peaks indicate the high crystallization degree of the synthesized crystals.In addition,the absence of diffraction peaks of other impurities indicates that the synthesized CuFe2O4is of high purity and free from impurities.
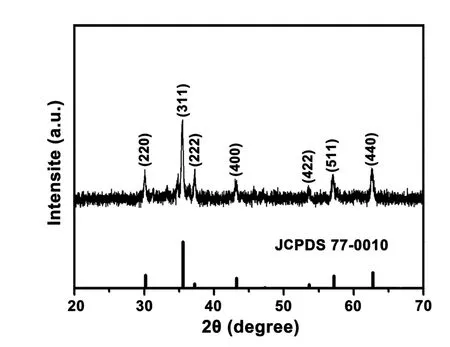
FIG.1 XRD pattern of CuFe2O4.
Microscopic morphology,particle size and surface state directly affect the electrochemical performance of materials.We use SEM,TEM and HRTEM to observe the microscopic morphology and internal structure of the synthesized CuFe2O4samples and analyze the possible effects of morphology on electrochemical performance.FIG.2shows the SEM images of CuFe2O4at different magnifications.According toFIG.2(a),CuFe2O4particles are regular spherical with a particle size of about 100 nm.The spherical structure is relatively homogeneous,without any obvious agglomeration.As shown in the high-magnification diagram(FIG.2(b)),the spherical particles are uniformly dispersed,and the spherical surface is rough.As can be seen from the broken spherical particles,the inside of the sphere is hollow.
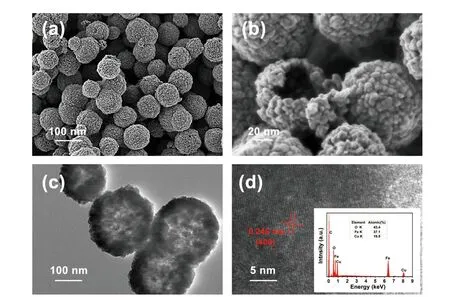
FIG.2 (a,b) SEM spectra of CuFe2O4 at different magnifications.(c) TEM image of CuFe2O4 and (d) HRTEM image of CuFe2O4 (the insert shows EDAX analyses of CuFe2O4).
To further determine the internal structure of CuFe2O4particles,TEM tests are performed.As shown inFIG.2(c),the outer edge of the spherical particle is black,while its inner color is light or even colorless.Thus,we infer that the particles are hollow nanoparticles.The lattice characteristics of CuFe2O4particles are determined by the HRTEM test.The regular lattice fringes can be clearly seen inFIG.2(d),indicating the high crystallinity of CuFe2O4particles.The lattice spacing of the CuFe2O4sample is measured to be 0.246 nm,which corresponds to the (400) crystal plane of the spinel CuFe2O4.Moreover,the EDAX spectrum insert inFIG.2(d) shows obvious Cu,Fe,O,and the atomic ratios are estimated to be 19.5%,37.1%,43.4%respectively,which are in stoichiometry and verify the high purity of CuFe2O4.
B.Electrochemical test
The CV tests are performed in 1 mol/L,3 mol/L and 6 mol/L KOH solutions with the voltage window from-0.2 V to 0.6 V and the scan rate of 5-100 mV/s.The results are displayed inFIG.3.As shown inFIG.3(a-c),all the CV curves possess a pair of typical redox (Cu+/Cu2+,Fe2+/Fe3+) peaks,which are assigned to the reversible Faradaic reaction that takes place on the surface of the CuFe2O4hollow spheres in the charge and discharge processes [32].Additionally,as the CV scan rate increases,the integral area encompassed by the CV curves also gradually increases.Even at high scan rates (100 mV/s),CV curves undergo insignificant deformation,revealing excellent rate capability.For comparison,the CV curves at the same scan rate (20 mV/s) for CuFe2O4in different concentrations of KOH electrolyte are displayed inFIG.3(d).The integral area of the CV curve in 3 mol/L KOH electrolyte is calculated to be the highest,demonstrating the largest specific capacitance.However,the polarization effect is more obvious at relatively higher concentration [33].

FIG.3 CV curves of CuFe2O4 samples in electrolytes with different concentrations of (a) 1 mol/L KOH,(b) 3 mol/L KOH,(c) 6 mol/L KOH.(d) CV comparison of different concentrations at scan rate of 20 mV/s.
FIG.4displays the GCD profiles of CuFe2O4electrode in different concentrations of KOH electrolyte tested under current density of 0.5-5 A/g.At the current density of 0.5 A/g,the GCD curve exhibits redox plateaus and nonlinear shapes,which is consistent with the CV test results,demonstrating battery-type electrode nature for CuFe2O4[34].Moreover,the GCD curves exhibit the longest charge-discharge time at the current density of 0.5 A/g.With the increase of the current density,both charge-discharge plateaus and chargedischarge time gradually disappear.The reason may be that the current density increases and the electrolyte ions cannot make sufficient contact with the active material,resulting in an inefficient electrochemical reaction [35,36].Notably,the charge and discharge curves are basically symmetrical,indicating the good reversibility of the CuFe2O4electrode.

FIG.4 GCD curves of CuFe2O4 in electrolytes with different concentrations of (a) 1 mol/L KOH,(b) 3 mol/L KOH,(c)6 mol/L KOH.(d) Specific capacitances,(e) cycling stabilities at 3 A/g,and (f) EIS spectra of CuFe2O4 in electrolytes with different concentrations,the insert in (f) displays equivalent circuit diagram.
The specific capacitance of the electrode at different current densities can be calculated according to the following equation [37]:
whereCsdenotes the mass specific capacitance (in unit of F/g),Iis the response current,mis the mass of active material on the working electrode (in g),Δtis the difference in discharge time in the discharge curve (in s),ΔVis the discharge voltage window in the discharge curve (in V).
According to Eq.(1),the calculation results of specific capacitance values at various current densities are shown inFIG.4(d).The specific capacitance is negatively correlated with the current density.All of the specific capacitances of the CuFe2O4electrode decrease with the acceleration of current density.Presumably,at the large current,the internal resistance of the electrode increases,resulting that the active material can not fully contact the electrolyte solution,and the reaction is insufficiently proceeded during the discharge.As expected,at 0.5 A/g,the CuFe2O4electrode presents higher specific capacitance of 368.2 F/g in 3 mol/L KOH electrolytes compared to that in 1 mol/L KOH
(276.5 F/g) and in 6 mol/L (300.3 F/g) KOH solution electrolytes,which is in agreement with the CV analysis result.Even at high current densities (5 A/g),the mass specific capacitance remains 252.7 F/g in a 3 mol/L KOH electrolyte,and the capacitance retention is 74.4%.In addition,the comparison of electrochemical parameters for CuFe2O4supercapacitor with electrode active materials reported recently is listed in Table I.Amazingly,the elaborately designed hollowspherical CuFe2O4shows an excellent electrochemistry performance for the supercapacitor electrode material.
FIG.4(e) shows the cycling stability of the CuFe2O4electrodes at 3 A/g for consecutive 2000 cycles.In particular,the CuFe2O4electrode in 3 mol/L KOH electrolytes presents a remarkable cycling performance with 91.0% capacity retention after 2000 GCD cycles,which is higher than that of 1 mol/L (85.7%) and 6 mol/L(81.1%) KOH electrolytes at the same test conditions.Long-term repeated Faraday reaction would slightly change the crystal structure of CuFe2O4,resulting in a decrease in chemical stability [40].In turn,the reduced surface-active reaction sites for the Faradic reactions lead to the decay of the charge and discharge capacities.The highest cyclic stability in 3 mol/L KOH electrolyte benefits from the hollow-spherical structure of CuFe2O4,which can effectively relieve the tension generated by the redox reaction during the charge and discharge process.However,the intense polarization in 6 mol/L KOH electrolyte might prevent it from retaining capacitance well.
The interfacial properties of CuFe2O4electrode in different concentrations of KOH electrolyte are studied by EIS under frequencies of 0.01-106Hz and an AC amplitude of 5 mV (FIG.4(f)),the inset shows the corresponding equivalent circuit.As shown inFIG.4(f),in the high-frequency region,the impedance curves possess essentially the same value of the intersection point with the real part.The intercepts of the real impedance corresponding to the solution resistance (Rs) are calculated to be 0.3 Ω,0.4 Ω,and 0.6 Ω in 1 mol/L,3 mol/L,and 6 mol/L KOH electrolyte,respectively.The diameter of quasi semicircle in the high-frequency region represents the charge transfer resistance (Rct),which was evaluated to be 0.05 Ω,0.08 Ω,and 0.13 Ω in 1 mol/L,3 mol/L,and 6 mol/L KOH electrolyte,respectively.Such small resistance values reveal the rapid electron transfer at the electrode-electrolyte interface.By contrast,the slopes of the three curves differ considerably in the low-frequency region.The slope of the straight line in the low-frequency region is associated with the Warburg impedance (Zw),which represents the cation diffusion resistance in the electrode material.As the electrolyte concentration increases,the straight line in the low-frequency region becomes increasingly steeper,implying that the ionic diffusion resistance of the electrode gradually decreases.The curve in 3 mol/L KOH electrolyte shows a most vertical line,indicating the lowest ionic diffusion resistance.Therefore,the hollowspherical CuFe2O4electrode in 3 mol/L KOH electrolyte exhibits the superior electrochemical properties including the high specific capacitance,low resistance,and superior cycling stability,affording attractive cathodes for supercapacitor applications.
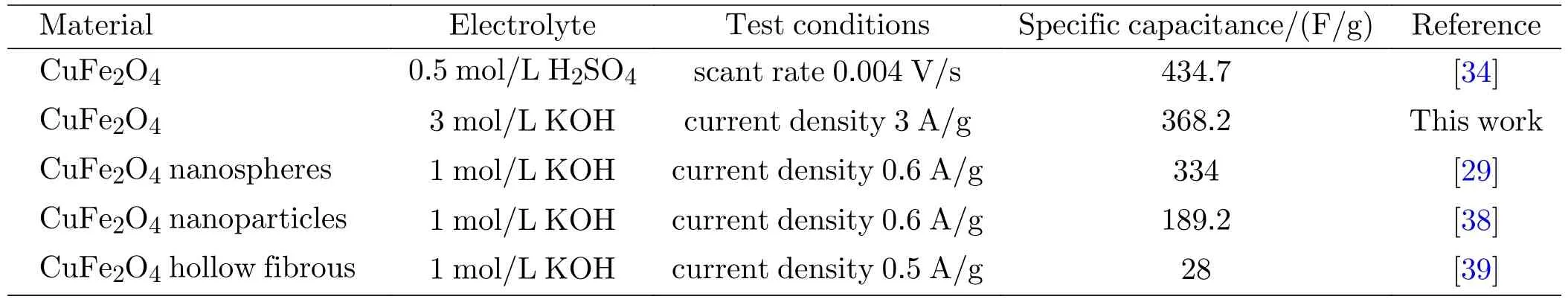
TABLE I Comparison of the electrochemical parameters for supercapacitor with CuFe2O4 as electrode active material.
IV.CONCLUSION
The spinel CuFe2O4samples with a hollow-spherical structure are synthesized by one-step solvothermal method using copper chloride and ferric chloride salt as raw materials and PVP as the dispersant.In a threeelectrode system,their electrochemical performance is tested at different concentrations of electrolytes.The results reveal that the CuFe2O4electrode exhibits superior supercapacitive properties and good rate performance in 3 mol/L KOH electrolyte.Its intrinsic resistance and mass transfer resistance are low,which is favorable for charge movement and ion transport.The prepared particles are spherical CuFe2O4with a particle size of about 100 nm and a rough surface.The hollow-spherical structure of CuFe2O4benefits large specific surface areas and numerous active sites for electrochemical reactions.Thus,the high efficiency of the redox reaction is readily achieved in the Faradaic charge storage process.Overall,the CuFe2O4samples have a promising application prospect as supercapacitors due to the low cost of raw materials,simple sample preparation methods,high specific capacitance,good rate performance,and low environmental pollution.
V.ACKNOWLEDGMENTS
This work was supported by Henan Province Key Research and Development and Promotion of Science and Technology Project (No.222102320336),Application Research Plan of Key Scientific Research Projects of Higher Education Institutions of Henan Province(No.22B150018),and the National Natural Science Foundation of China (No.92061203).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2023年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2023年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Minimum-Modified Debye-Hückel Theory for Size-Asymmetric Electrolyte Solutions with Moderate Concentrations
- Quantum Dynamics Calculations on Isotope Effects of Hydrogen Transfer Isomerization in Formic Acid Dimer
- Design Strategy of Infrared 4-Hydroxybenzylidene-imidazolinone-Type Chromophores based on Intramolecular Charge Transfer: a Theoretical Perspective
- Photothermal Catalytic Selective Oxidation of Isobutane to Methacrylic Acid over Keggin-Type Heteropolyacid
- Controllable Modulation of Morphology and Property of CsPbCl3 Perovskite Microcrystals by Vapor Deposition Method
- On-the-Fly Nonadiabatic Dynamics of Caffeic Acid Sunscreen Compound
