一例具有单分子磁体行为的单核钴-氮氧自由基异自旋配合物
李宝毅 郝晓婷 李弘道*,
(1太原工业学院化学与化工系,太原 030008)
(2临县第一中学,吕梁 033200)
(3天津师范大学化学学院,天津 300387)
In recent years,remarkable achievements have been made in the design of single-molecule magnets(SMMs) toward high spin-reversal energy barriers and/or blocking temperatures[1-3].Typical cases can be observed in DyⅢ-SMMs with a magnetic blocking temperature of 80 K and an energy barrier up to 1 541 cm-1[2].Different from lanthanide compounds,transition metal possesses 3dvalence orbitals,as well as the ligand field can quench the orbital angular momentum with ease,which is in favor of the construction of compounds with simpler structures than adjusting structurefunction relationship[4-5].Especially,the transition metal-CoⅡion contains three unpaired electrons,ad7configuration,and unquenched orbital angular momentum during high -temperature regions.Recent investigations have verified that the CoⅡion is an excellent candidate for design of the transition metal-based SMMs[6-10].In 2003,the first d-block mononuclear SMM [Co(SCN)2(4-dzbpy)4] (dzbpy represents diazobenzylpyridine) was reported,which contains a single octahedral CoⅡion,coordinating to two NCS and four 4-dzbpy ligands[11].An air-stable star-shaped CoⅡCoⅢ3complex was constructed via a chiral Schiffbase ligand,exhibiting zerofield SMM behavior[12].Furthermore,interest in the CoⅡ-radical (CoⅡ-rad) SMM system also remains flourishing on account of strong 2p-3dmagnetic exchange couplings and the unquenched first-order angular momentum giving rise to large uniaxial magnetic anisotropy.In 2016,employing a bis-bidentate nitronyl nitroxide radical NIT-2-Pm,a nitronyl nitroxide-bridged CoⅡbinuclear compound was synthesized,furthermore,field-induced single-molecule magnet behavior was detected firstly in nitronyl nitroxide-bridged 3dcompounds[13].Long et al.recently introduced the CoⅡion into an exchange-coupled binuclear system by a semiquinone radical as the bridging ligand[14].Variation of radical ligand donor characteristics offers an avenue to adjust the magnetic anisotropy.
Along this line,to explore the transition metalradical chemistry,herein,we synthesized a new nitronyl nitroxide radical NIT-PyzMe-PhCHO,where NITPyzMe-PhCHO=2-(3-(1H-imidazol-1-ylmethyl)benzaldehyde)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide,and obtained a 2p-3dhetero-spin complex with the formula [Co(hfac)2(NIT-PyzMe-PhCHO)] (1),where hfac=hexafluoroacetylacetonate.The crystal structure and the static and dynamic magnetic susceptibilities of the CoⅡcomplex were investigated.Furthermore,the CoⅡ-based complex displays field-induced SMM behavior.
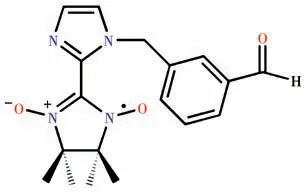
Scheme 1 Structure of NIT-PyzMe-PhCHO radical ligand
1 Experimental
1.1 Materials and instruments
Starting reagents including heptane and dichloromethane are commercially obtained.The nitronyl nitroxide NIT-PyzMe-PhCHO was synthesized based on the reported literature[15].The elemental analysis was performed on a Perkin-Elmer 240 elemental analyzer.Magnetic susceptibility measurements of the powder sample were undertaken on a Quantum Design MPMS magnetometer.Direct-current(dc)magnetic susceptibility was corrected for diamagnetism contribution in the light of Pascal constants[16].
1.2 Syntheses of complex 1
[Co(hfac)2] hydrate (0.01 mmol) was dissolved in boilingn-heptane (98 ℃) and the mixture was refluxed for 5 h with continuous stirring.After cooling to 85 ℃,a CH2Cl2solution (6 mL) of NIT-PyzMe-PhCHO (0.01 mmol,0.003 0 g) was slowly added with refluxing for 0.5 h followed,then filtered.The blue filtrate was quiescence at room temperature,generating navy-blue rhombic crystals after three days.FTIR (KBr,cm-1):1 785(s),1 347(m),1 179(s),1 158(s),1 065(s),952(s),861(m),551(s).Elemental Anal.Calcd.for C28H23CoF12N4O7(%): C,41.29; H,2.84; N,6.87.Found(%): C,41.32;H,2.83;N,6.88.
1.3 Crystal structure determination
Crystal data of the mononuclear CoⅡcomplex was recorded on a Rigaku Saturn CCD diffractometer at 150(2) K using MoKαradiation (λ=0.071 073 nm).SADABS[17]was devoted to empirical absorption correction.The structure of the CoⅡcomplex was solved by direct methods and refined by least squares minimization with a suite of SHELX programs[18].Anisotropic refinement involving non-H atoms was implemented.In the meantime,H atoms were arranged at ideal positions.Additional single crystal data,selected bond lengths,and bond angles for the CoⅡcomplex are displayed in Table 1 and S1(Supporting information).
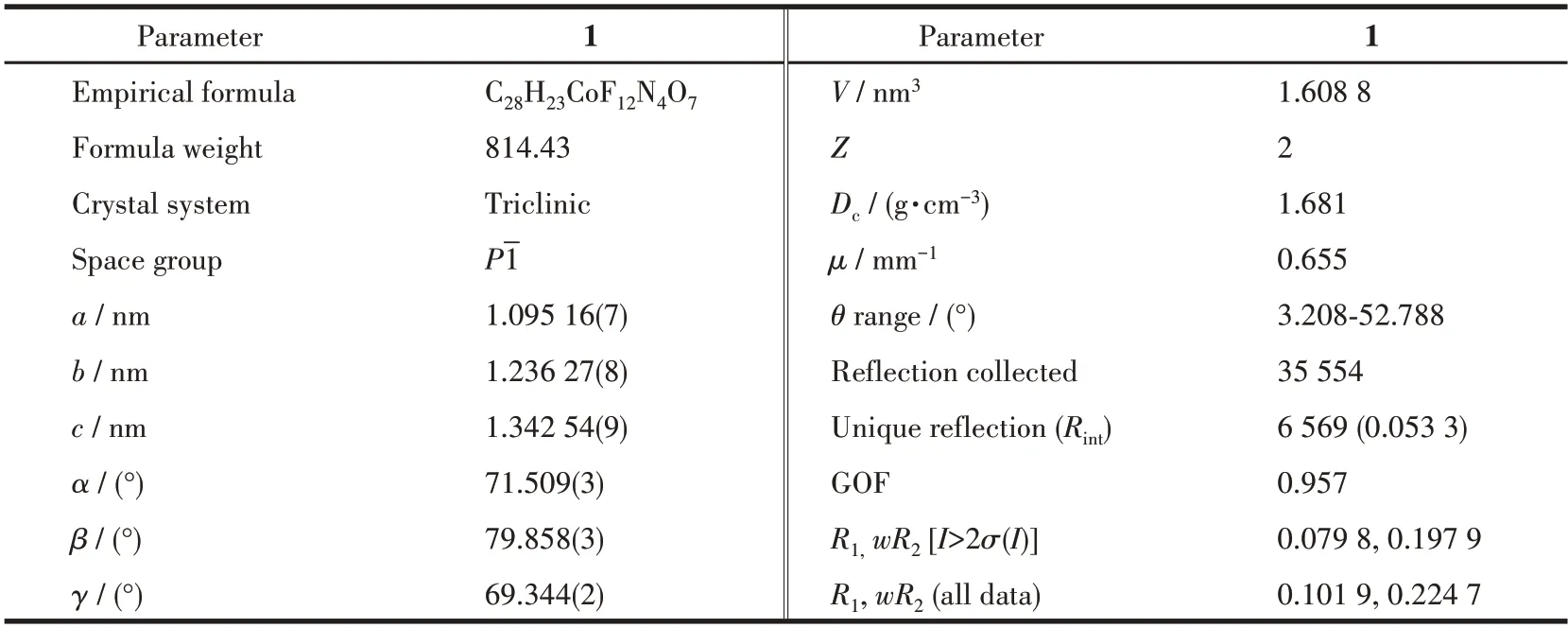
Table 1 Crystallographic data and structure refinement for complex 1
CCDC:2247534.
2 Results and discussion
2.1 Description of crystal structure
Complex 1 crystallizes in the triclinicspace group with one independent Co(hfac)2moiety and one NIT-PyzMe-PhCHO radical ligand.As shown in Fig.1,the radical NIT-PyzMe-PhCHO behaves as a bidentate ligand to chelate one CoⅡion to form one mononuclear structure.The CoⅡion adopts distorted octahedral geometry,encircled by four O atoms from twoβ-diketonate co-ligands (Co—Ohfac: 0.205 5(4)-0.208 7(4) nm)and one N atom from imidazole ring (Co1—N4:0.209 0(4) nm) and one O atom from the nitroxide group (Co1—O3: 0.205 5(3) nm).The dihedral angle between the imidazole ring and O—N—C—N—O heterocyclic plane of the PyzMe-PhCHO radical is 30.2(1)° ,and the Co—O—N—C torsion angle is 38.5(6)°.The crystal packing of the CoⅡ-rad complex is depicted in Fig.2.The closest CoⅡ…CoⅡintermolec-
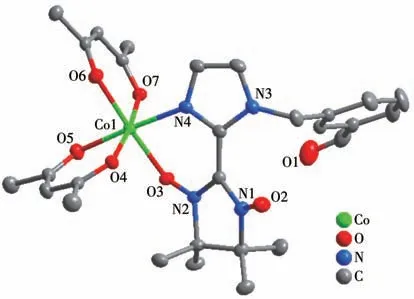
Fig.1 Crystal structure of complex 1
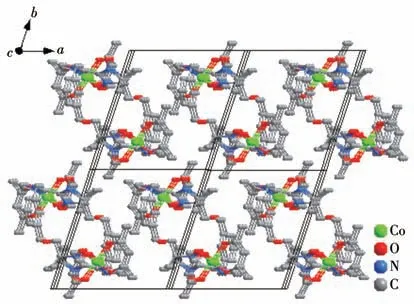
Fig.2 Crystal packing of complex 1
ular contact is 0.845 nm,and the shortest uncoordinated NO…ON interval is equal to 1.095 nm.
2.2 Magnetic properties
Static magnetism of the mononuclear CoⅡcomplex was recorded under an external field of 1 000 Oe during a temperature range of 2-300 K.As shown in Fig.3,theχMTproduct at 300 K was 2.33 cm3·mol-1·K,which was distinctly lower than the theoretical value for one uncoupled CoⅡion (χMTproduct of octahedral high-spin CoⅡ:3.0-3.4 cm3·mol-1·K)[19]and one radical(radical:S=1/2,g=2.0,C=0.375 cm3·mol-1·K).This phenomenon is indicative of extraordinarily strong CoⅡ-radical antiferromagnetic interaction.
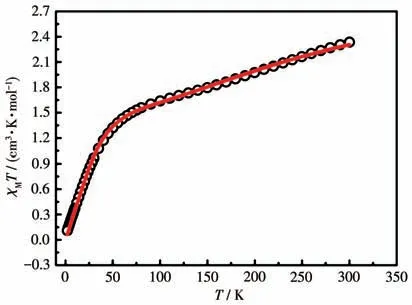
Fig.3 χMT versus T plot of complex 1
For 1,on lowering the temperature,the value ofχMTcontinuously decreased to 0.110 cm3·mol-1·K at 2 K.This phenomenon verifies the prevailing dominant antiferromagnetic coupling.Field dependence of the magnetization (ca.70 kOe) at 2 K was measured.TheMvalue displayed a gradual increase and reached 0.46Nβat 70 kOe(Fig.S1),which was significantly lower than the predicted saturation value deriving from the spin-orbit effect of CoⅡion and/or strong antiferromagnetic coupling.
TheχMT-Tdata of 1 was fitted by the PHI software[20]with a spin Hamiltonian ofwhereJrepresents the magnetic coupling between the CoⅡcenter and the directly coordinated nitroxide group,zero-field splitting (ZFS)parametersDandEare used to record magnetic anisotropy.The reasonable fitting generates the following magnetic parameters:grad=2.00 (fixed),gCo=2.47,J=-82.90 cm-1,D=49.30 cm-1andE=3.31 cm-1.The largeJvalue confirms strong antiferromagnetic CoⅡ- rad interaction,which is in accordance with other reported CoⅡ-nitronyl nitroxide compounds[6,13,21].The positiveDvalue declares the easy-plane magnetic anisotropy.In the system,the Co—O—N angle is equal to 120.4°,and the Co—Oradbond length of 0.205 4 nm is short,which is conducive to the efficient overlap of magnetic orbitals.
The alternating current (ac) susceptibility of complex 1 was tested for probing spin dynamics.As shown in Fig.S2,the out-of-phase susceptibility merely displayed frequency dependent at low-temperature region under 0 Oe due to strong quantum tunneling of magnetization (QTM).To weaken QTM,under an external field of 2 000 Oe,the temperature- and frequencydependent ac behaviors were investigated in detail.For the CoⅡ-rad complex,evident peaks ofχ″ signals were discerned (Fig.4 and S3),a typical SMM characteristic.The relaxation time (τ) and distribution coefficient (α)were simultaneously deduced fromχ″=f(ν) curves with a generalized Debye model.The α values lay in a range of 0.18-0.23 (Fig.5),giving a moderate distribution (α)of relaxation time.Linear fitting of the lnτvsT-1data with the Arrhenius lawτ=τ0exp[Δ/(kBT)] acquired the energy barrierUeff=9.0(3) K and pre-exponential factorτ0=3.0(2) μs (Fig.4b),which was comparable to observed typical SMMs[21-26](10-10-6μs).

Fig.4 (a)χ″=f(ν)plot of complex 1 under 2 000 Oe;(b)ln τ vs T-1 plot under 2 000 Oe for 1
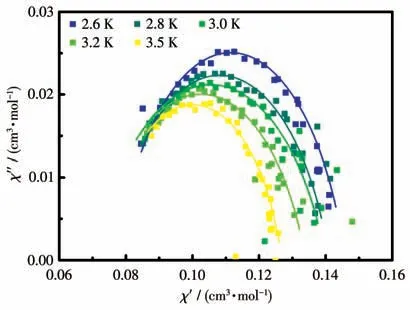
Fig.5 Cole-Cole plot for complex 1 under 2 000 Oe external field
Generally,strong magnetic exchange coupling between spin carriers is known to contribute to reducing QTM effects and is desirable for the design of better SMMs.Thus,in complex 1,strong 2p-3dmagnetic exchange represents a key step toward the realization of SMM behavior.However,complex 1 still requires an external dc field to display slow relaxation behavior.To improve the magnetic behavior of complex 1,one strategy is to design a more ideal ligand-field environment to generate more strict axial symmetry around the CoⅡion,hence weakening transverse anisotropy and restraining QTM[27].At this point,modulation of a lowercoordination number at CoⅡion may optimize the SMM behavior of complex 1.
3 Conclusions
We successfully isolated a 2p-CoⅡhetero-spin SMM using nitronyl nitroxide,in which the NIT-PyzMe-PhCHO radical acts as a bidentate ligand to chelate one CoⅡion,generating a mononuclear structure.Furthermore,strong antiferromagnetic CoⅡ-nitronyl nitroxide coupling was discerned.The 2p-3dhetero-spin complex showed field-induced SMM behavior withτ0of 3.0 μs andUeffof 9.0 K.To explore the effect of different 3dmetal ions on SMM behavior,the construction of analogous complexes with other 3dions is on the way.
Supporting information is available at http://www.wjhxxb.cn

