Resistance analysis of the rice variety Huaidao 5 against root-knot nematode Meloidogyne graminicola
FENG Hui ,ZHOU Can-rong ,ZHU Feng ,LE Xiu-hu ,JING De-dao ,Paul DALY ,ZHOU Dong-mei,WEI Li-hui#
1 Institute of Plant Protection,Jiangsu Academy of Agricultural Sciences,Nanjing 210014,P.R.China
2 Plant Protection and Quarantine Station of Jiangsu Province,Nanjing 210009,P.R.China
3 School of Landscape and Ecological Engineering,Hebei University of Engineering,Handan 056038,P.R.China
4 Zhenjiang Institute of Agricultural Science in Hilly Area of Jiangsu Province,Jurong 212400,P.R.China
Abstract Meloidogyne graminicola has emerged as one of the most destructive plant-parasitic nematodes affecting rice (Oryza sativa) production worldwide. Resistance to M.graminicola in rice could be the most effective option for its management.However,sources of germplasm with resistance to M.graminicola in rice remain limited. Here,we describe the root attraction,gall formation and genetic analysis of the resistance to M.graminicola in the rice variety Huidao 5. A nematode attraction assay showed that second-stage juveniles (J2s) of M.graminicola were attracted at the root tip of Huaidao 5 within 8 h without a significant reduction in attraction compared to the susceptible rice variety Nanjing 9108.Microscopic observation of the infection revealed that the J2s invaded root tissues 12 h after inoculation,but their subsequent movement to the root tip was hindered in Huaidao 5,resulting in decreased nematode number compared to Nanjing 9108. Additionally,we used the soil and hydroponic culture systems to simulate upland and flooding conditions in the paddy fields respectively,and found that gall number was significantly reduced,and nematode development was clearly suppressed in Huaidao 5. To investigate the genetic basis of this resistance,cross breeding was performed between the Huaidao 5 and Nanjing 9108 varieties. There was no reduction in the resistance of the F1 offspring to M.graminicola in the greenhouse or field trials,suggesting that a dominant gene could control resistance in Huaidao 5.In summary,this study provides a detailed characterization of a novel source of resistance to M.graminicola in rice,which is of great potential for use in crop breeding.
Keywords: root-knot nematode,Meloidogyne graminicola,rice,resistance
1.Introduction
Rice (OryzasativaL.) is the staple cereal for over half the global population and is therefore critical for food security(Muthayyaet al.2014). Three hundred nematode species belonging to 35 genera can infest rice,but one of the most important nematode infecting rice is the root-knot nematodeMeloidogynegraminicolaGolden &Birchfield.Currently,this pathogen has spread worldwide and has emerged as one of the most important biotic constraints to rice production in Asia and Africa (Upadhyayet al.2014;Mantelinet al.2017).
In the life cycle ofM.graminicola,the second-stage juveniles (J2s) penetrate the root system,induce root galling,and hijack plant metabolism to their own benefit by the establishment of giant cells. The symptoms and effects of the disease include stunting,chlorosis,reduction of tillering,and delayed maturation,which ultimately results in poor growth of the crop and substantial yield loss (Kyndtet al.2014). In India,M.graminicolawas reported to cause up to 21% yield loss in rainfed and welldrained soils (Pankaj and Prasad 2010). However,as much as 80% yield loss can occur in upland and irrigated rice fields in simulated conditions (Padghamet al.2004;De Waele and Elsen 2007). Additionally,rice losses may be aggravated along with other biotic stresses,such as the fungal pathogenMagnaportheoryzae(Kyndtet al.2017).
The management ofM.graminicola,including crop rotation,flooding,and the use of nematicides,is confronted with challenges in rice fields. Crop rotation with non-host plants is only partially effective because of the wide host range ofM.graminicola(Venturaet al.1981;Rahman 1990). Flooding at the early stage of nematode infection can kill nematodes but cannot be applied in water-limited areas. Chemical nematicides,such as carbofuran and methyl bromide have been used for nematode control (Soriano and Reversat 2003;Khanet al.2012). Nevertheless,because of their expense and harm to the environment,the use of these nematicides is limited and in some cases prohibited. In this context,the use of rice varieties with resistance and/or tolerance toM.graminicolaoffers a promising alternative to control this pathogen in rice production.
Natural resistance toM.graminicolahas been reported in non-cultivated African riceO.glaberrima,O.longistaminata(Sorianoet al.1999),American riceO.glumaepatula(Mattoset al.2019) as well as severalO.sativavarieties in South Asia (Kumariet al.2016;Singhet al.2021). However,only a limited number of Chinese rice cultivars,such asjaponicaZhonghua 11,hybrid Shenliangyou 1,and Cliangyou 4418,are of high resistance toM.graminicolaunder greenhouse conditions(Zhanet al.2018). The introgression of resistance genes fromO.glaberrimaintoO.sativahas not been successful(Sorianoet al.1999),while the F1progenies of Zhonghua 11 andO.sativaIR64 express the same degree of resistance observed in Zhonghua 11 (Phanet al.2018). Recently,the F2population of the resistantO.sativaLD24 and the susceptible Vialone Nano varieties were assessed forM.graminicolainfection,and a nematode resistance locus in chromosome 11 was identified by using the QTL-seq method (Lahariet al.2019).
Studies of the resistantO.glaberrimaandO.sativagenotypes revealed two types of defense response mechanisms toM.graminicolainfection. One is prepenetration inhibition,with reduced root invasion by J2s,and the other is post-penetration resistance,resulting in obstructed nematode development and collapse of the giant cells (Cabasanet al.2014). Petitotet al.(2017) observed that J2s penetration and development ofM.graminicolawere hindered in the resistantO.glaberrimaTOG5681 while Phanet al.(2018) found that whileO.sativaZhonghua 11 attracted large numbers of J2s to enter the roots at early stages,it subsequently expressed a hypersensitivity-like response to the feeding sites establishment and female development ofM.graminicola.
In this study,we describe a resistantO.sativasubsp.japonicavariety Huaidao 5 and characterized its resistance toM.graminicola. We further demonstrate using a genetic approach that the resistance genes can be used in breeding programs that develop new resistant rice varieties suitable for lowland and upland riceproducing agroecosystems.
2.Materials and methods
2.1.Rice varieties
Five rice varieties were firstly subjected to resistance assessment toM.graminicolainfection. Among these,O.sativajaponicaNipponbare (NPB) was used as a susceptible control,while thejaponicaZhonghua 11(ZH11) and the hybridindicaCliangyou 4418 (C4418) were selected as the resistant rice cultivars (Zhanet al.2018).ThejaponicaNanjing 9108 (NJ9108) and Huaidao 5(HD5),widely planted in Jiangsu and neighboring provinces,as well as their offspring (F1population)were tested and characterized in the experiments.Rice crosses were performed between NJ9108 as a female parent and HD5 as a male parent to produce F1progenies. Information on the rice varieties can be found at the China Rice Data Center (http://www.ricedata.cn).
2.2.Nematode inoculum preparation
The nematodeM.graminicolawas originally isolated from a paddy field in Taixing,Jiangsu Province,China,and identified through morphological description and molecular methods (Fenget al.2017). The nematode population was maintained and multiplied from a single egg mass in Nipponbare at 28°C in a greenhouse. Nematode eggs were extracted from roots with galls using 10% bleach and collected according to the method described by Teixeiraet al.(2016). Surface-sterilized eggs were then hatched in a modified Baermann funnel (De Ilarduyaet al.2001),and 2 d later,J2s were collected and counted.
2.3.Nematode reproduction and rice response
Rice seeds were sterilized with 10% bleach for 30 min,followed by rinsing several times with sterilized water.Seeds were then soaked in the sterilized water and germinated on a disposable Petri dish at 26°C. Two independent assays were carried out either under the greenhouse or in the field condition.
Rice seedlings were transferred into a pot (8-cm diameter and 17-cm height) in a greenhouse at (26±2)°C.Two plants were planted in a pot filled with an autoclaved soil matrix (2:1:1,river sand:paddy soil:commercial organic fertilizer). The 4-leaf-stage plant was inoculated with 150 J2s by pipetting the nematode suspension around the roots. Plants were watered every 3 d to maintain a non-saturated soil. At 35 d post-inoculation(dpi),plants were uprooted,and the roots were carefully washed to remove the soil. For each root system,the gall number was counted and the galling index (GI) was calculated according to Zhanet al.(2018). Then,the total number of eggs,J2s and females were recorded as the final population density (Pf). The reproductive factor (RF) per root system was calculated according to the following ratio: RF=Pf/Pi (Pi=initial population). To assess the rice responses to the nematodes,the root length and stem height were also measured. At least 10 replicate plants were examined for each rice variety and the experiment was repeated twice. A parallel test was carried out to obtain the RF at 28 dpi,as described above.
In the field assay,the 4-leaf-stage plants of NJ9108,HD5,and their F1progenies were transferred to a rice nursery with 15 cm distance between plants. At 3 d after transplanting,each plant was inoculated with 200 J2s.At least 20 plants in each variety or F1population were randomly selected for inoculation. At 45 dpi,nematodes were counted,and RF was then calculated.
2.4.Nematode attraction and penetration assays
The germinated seedlings of NJ9108 and HD5 were separately placed onto a 1% agar plate (13 cm×13 cm),and maintained in a plant growth incubator with 12 h light photoperiod at 25°C.
For the attraction assay,250 J2s mixed with 1 mL of 23% Pluronic gel (Pluronic F-127,Sigma-Aldrich,Shanghai,China) were pipetted onto a glass slide (2.6 cm×7.6 cm). A 2-cm long seminal root was then placed onto the gel,and covered immediately with another glass slide.After the gel solidified at room temperature,the number of nematodes within 2-mm diameter of the root tip was recorded at 1,2,4,6,and 8 h. At least 5 replications were set up for each rice variety.
For penetration assays,seedlings with a 2-cm long seminal root were selected for inoculation. Twelve seedlings growing on the 1% agar plate were inoculated with 150 J2s per seedling and maintained in a plant growth incubator as described earlier. Nematode penetration was evaluated by acid fuchsin staining at 12,24,and 48 h post inoculation (hpi) according to Teixeiraet al.(2016) with a minor modification. Briefly,seedlings were treated with 10% bleach for 3 min,washed with water and boiled for 10 s in acid fuchsin solution (3.5%acid fuchsin in 25% acetic acid). After destaining in the acidified glycerol (1:1:1 (v/v),acetic acid:glycerol:H2O),the nematodes inside the roots were counted using a stereo microscope.
2.5.Nematode development assessment
To further assess rice resistance toM.graminicola,the soil and hydroponic conditions were separately used for the nematode development survey. For the soil condition,rice seedlings were cultured and inoculated as described above. For the hydroponic condition,the rice seedlings at 3 dpi growing on plates as described above were transplanted into a 15-mL tube containing 10 mL Hoagland solution,and were then cultured under 12 h light photoperiod at 25°C. The Hoagland solution was changed every 2 d. At 3,7,14,and 21 dpi,the galls and the nematode development in each root system were surveyed as described above.
3.Results
3.1.Reproduction of M.graminicola on the rice varieties
To obtain resistant rice resources,we assessed dozens of Chinese rice varieties inoculated with J2s ofM.graminicolaunder greenhouse conditions and found that most of the selected varieties were susceptible toM.graminicola(unpublished data). Evaluation at 35 dpi revealed that both the root gall formation and nematode reproduction were significantly inhibited in HD5,in contrast to the susceptible controls NPB and NJ9108. Compared with the resistant control variety C4418,HD5 exhibited much lower values of gall index and RF,suggesting a closer resistance level to Zhonghua 11 (Table 1). Additionally,the growth of HD5 was not significantly impaired in terms of root and stem length and weight (Table 2).
Based on the results,we further surveyed the nematode reproduction on NJ9108,HD5,and their F1offspring under both greenhouse and field conditions.As expected,M.graminicolawas massively reproduced on NJ9108,with RF values of approximately 200 under both growing conditions. In contrast,significantly lower RF values were observed for HD5 and the F1plants.On HD5,the RF was less than 3,demonstrating that nematodes struggle to reproduce on this rice variety. The same level of resistance toM.graminicolainfection was also observed on the F1plants under both greenhouse and field conditions (RF<5) (Fig.1).

Fig.1 Reproduction of Meloidogyne graminicola in susceptible(Nanjing 9108 (NJ9108)),resistant (Huaidao 5 (HD5)) and hybrid Oryza sativa varieties (F1) in the greenhouse at 28 d post-inoculation (dpi) (A) and in field test at 45 dpi (B). Each data point represents the mean±SE of 2 replicates,in each comprising 8 individual plants. Different letters on the column indicate significant differences between mean at P<0.001 by the post-hoc Tukey’s test.
3.2.Attraction and penetration of M.graminicola on NJ9108 and HD5
During the early stages of infection,J2s ofM.graminicolafurther migrated to the root tip. However,the numbers of J2s attracted to root tips of NJ9108 and HD5 were not significantly different at 1,2,4,6,and 8 h in the PF-127 gel,suggesting thatM.graminicolacan locate both the hosts to the same extent (Fig.2).
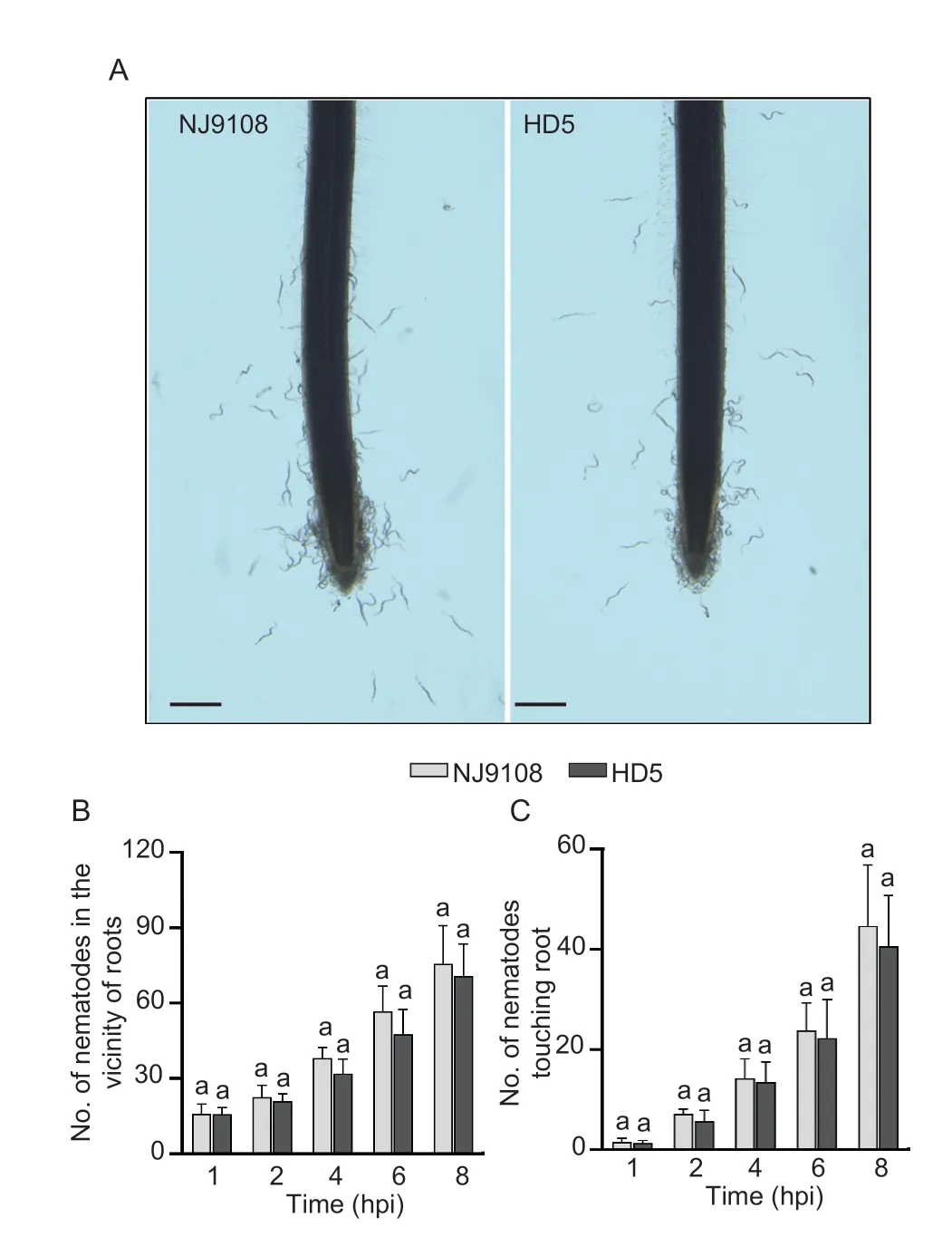
Fig.2 Comparative attraction of Meloidogyne graminicola to the root tips of Nanjing 9108 (NJ9108) and Huaidao 5 (HD5).A,photomicrographs showing attraction of the second-stage juveniles (J2s) in the PF-127 gel to the root tips of NJ9108 and HD5 at 4 h post-inoculation (hpi). B and C,numbers of M.graminicola J2s in a 2-mm diameter area around the root tips(B) and rice root tips (C) at 1,2,4,6,and 8 hpi. Data represent mean±SD (n=6) and bars with the same letter (within identical time point) indicate significant difference at P>0.05 by the posthoc Tukey’s test. Scale=20 µm.
The nematode penetration was traced in the roots of rice seedlings on the plates after acid fuchsin staining.The J2s initially infected the roots through the elongation region of both NJ9108 and HD5 at 12 hpi,and the number of nematodes counted inside the roots of both the rice genotypes was not significantly different. The J2s migrated to the root tip in both varieties at 24 hpi and unlike at the earlier time-point,the number of nematodes inside the roots of NJ9108 was significantly higher than in HD5. Later on,the J2s reached and assembled in the root tip of NJ9108 at 48 hpi,while the J2s suspended its movement and significantly decreased its penetration in the HD5 variety (Fig.3).
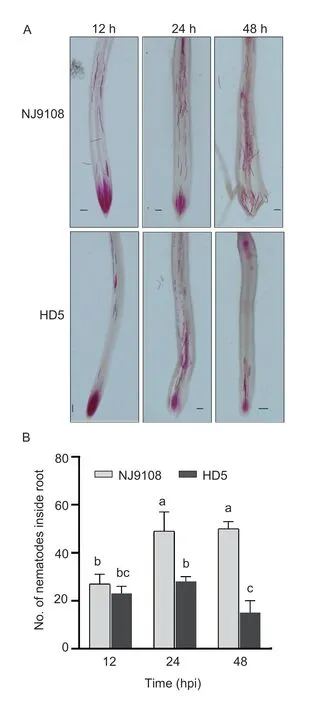
Fig.3 Early infection of Meloidogyne graminicola in the roots of Nanjing 9108 (NJ9108) and Huaidao 5 (HD5). A,photomicrographs showing penetration of the second-stage juveniles (J2s) in rice root tips at 12,24 and 48 h post-inoculation(hpi). B,number of nematodes inside roots. Data represents mean±SD (n=6) and bars with the different letters indicate a significant difference at P<0.05 by the post-hoc Tukey’s test.Scale=20 µm.
3.3.Development of M.graminicola on NJ9108 and HD5
In NJ9108,the gall formation was initiated at 48 hpi(Fig.3-A),while only slight root galling was observed in HD5 at 7 dpi (Fig.4-A and B). In contrast to the soil cultivation,the numbers of galls and nematodes inside the root systems were lower in the rice grown in the hydroponic system,however,development of the nematodes showed similar patterns in both NJ9108 andHD5 (Fig.4-C).
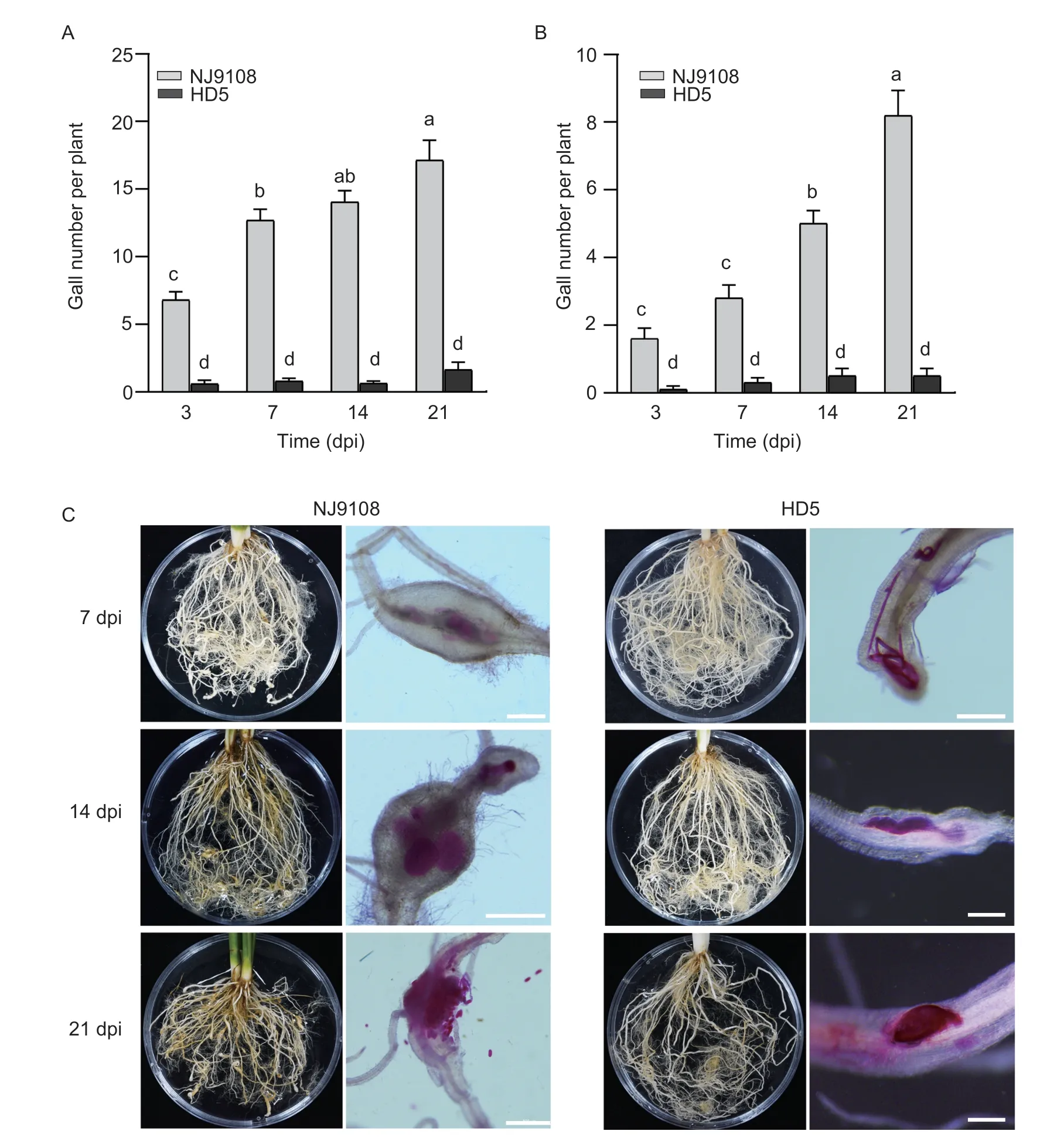
Fig.4 The comparative development of Meloidogyne graminicola on Nanjing 9108 (NJ9108) and Huaidao 5 (HD5). A and B,gall number of the plants cultured in the soil (A) and the hydroponic conditions (B). C,different developmental stages of M.graminicola in roots of the susceptible rice NJ9108 and resistant HD5 post-inoculation with M.graminicola at different days. Data represents mean±SE (n=8) and bars with the different letters indicate a significant difference at P<0.05 by the post-hoc Tukey’s test. Scale=500 µm (NJ9108) and 200 µm (HD5).
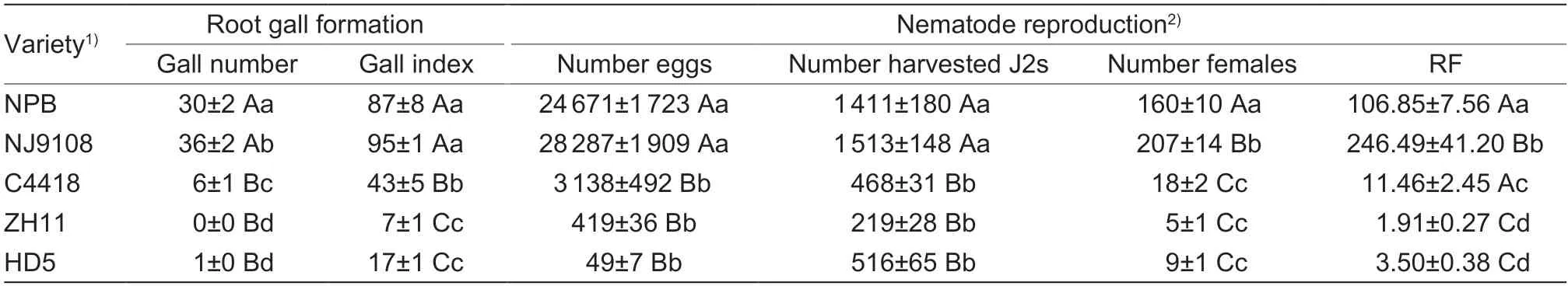
Table 1 Comparison of root gall formation and nematode reproduction of Meloidogyne graminicola on different rice varieties at 35 d post-inoculation
During the nematode development,gall numbers were almost unchanged in HD5 but progressively increased in NJ9108 (Fig.4-A and B). In the roots of NJ9108,J2 developed into J3/J4 at 7 dpi,and then developed into the female at 14 dpi,and finished oviposition at 21 dpi.In HD5,most of the nematodes inside the roots were J2 at 7 dpi,J3/J4 at 14 dpi and then the female at 21 dpi(Fig.4-C;Table 3).
4.Discussion
OryzasativajaponicaHD5 is the primary rice variety grown widely in Jiangsu Province of China. Studies have showed that this cultivar is tolerant to salt and low temperature stresses (Aliet al.2021;Liuet al.2022),but susceptible to pests and pathogens,such as rice white tip nematodeAphelenchoidesbesseyi(Fenget al.2014),the small brown planthopperLaodelphaxstriatellusand rice black streaked dwarf virus (Hajanoet al.2016). In this study,HD5 was firstly reported as a potential-resistant source toM.graminicola.

Table 3 Comparison of Meloidogyne graminicola development in Nanjing 9108 (NJ9108) and Huaidao 5 (HD5)
A drastic reduction inM.graminicolafitness in terms of the numbers of galls,eggs,harvested J2s,females,gall index and RF at 35 dpi,was observed in HD5 when directly compared with the susceptible varieties Nipponbare and NJ9108. Notably,the resistance level was similar to that of Zhonghua 11 but higher than that of Cliangyou 4418,based on the data of gall index and RF (Table 1). Meanwhile,the growth of roots and above parts of plants was not suppressed significantly in HD5 compared to Nipponbare and NJ9108. Furthermore,the F1hybrid offspring of the NJ9108 and HD5 also exhibited high resistance toM.graminicolaboth under greenhouse and in the field conditions,indicating that resistance is probably conferred by dominant genes. Lahariet al.(2019) assessed 175 F2cross progenies toM.graminicolaand identified a resistance locus on chromosome 11 from the resistant cultivars LD24 and KPM using QTLseq. However,recessive inheritance for resistance toM.graminicolashould not be excluded because it was also suggested in the hybrid progeny with resistance against other root knot nematode species (Wanget al.2004). Conclusions about the dominant expression of resistance toM.graminicolain HD5 need to be evaluated using the F2and F3progenies. Nevertheless,our results demonstrate the potential of HD5 as a useful donor in developingM.graminicolaresistant and/or tolerant rice varieties for breeding programs,and thus prompted us to further characterize its resistance to this nematode.
The plant root exudates may contain allelochemicals that can regulate nematode chemotaxis in terms of attraction,repellence,motility inhibition,or even death(Curtiset al.2009;Duttaet al.2012;Shivakumaraet al.2019). In this study,J2s were chemotaxed alike towards the root exudates of either NJ9108 or HD5 in the PF-127 based assay system,suggesting that the root excretions from HD5 did not have the repellent effect onM.graminicola. However,while a massive penetration of J2s occurred in HD5 at 12 hpi,subsequently there was a decreased nematode number at 24 and 48 dpi.These results demonstrated that HD5 did not display pre-penetration resistance,but could initiate a strong defense response to kill the nematodes and/or force their movement out of the roots at the early stage of J2 infection. The similar phenotype was also found the in resistant variety Zhonghua 11 (Phanet al.2018) and oat lines (Yaoet al.2020).
The soil conditions and water management influence rice plants towardM.graminicolain different agroclimatic zones. Previous research showed that maximum reproduction ofM.graminicolawas found in upland rice fields,while early flooding of rice crops minimizes the yield losses caused by such nematodes (Tandinganet al.1996;Sorianoet al.2000;Fernandezet al.2013). To further characterize the resistance of HD5,we adopted the soil and hydroponic system to simulate the upland and flooded conditions,respectively. Our results showed that although the gall number per root tissue in hydroponic conditions was less than in soil,the gall and the nematode development ofM.graminicolain HD5 were remarkably inhibited,compared with NJ9108 at 3,7,14 and 21 dpi. These data confirmed that the resistance toM.graminicolawas stable and reliable in both upland and lowland fields. Additionally,the delay of gall formation and the nematode development in HD5 suggested the presence of defense reactions,which restrain nematode development and thus limit giant cell formation. The inhibition of the development ofM.graminicolahas also been observed in previous studies of other resistant rice genotypes includingO.glaberrima(Cabasanet al.2018),O.longistaminata(Sorianoet al.1999),O.glumaepatula(Mattoset al.2019),and severalO.sativavarieties(Kumariet al.2016;Phanet al.2018;Zhanet al.2018;Singhet al.2021). Among most of these varieties,the resistance is explained as the post-infection mechanism suppressing the development and reproduction of nematodes. However,it is needed to further investigate whether and how the resistance from these rice genotypes constrainsM.graminicolaparasitism when the nematodes have invaded and initiated the feeding sites in the roots.
Resistance to root-infecting nematodes could be largely determined by nematode species. For example,the Asian rice genotype KPM and LD24,which are equally resistant toM.graminicola,exhibited the opposite resistance phenotypes to the root-lesion nematodePratylenchuszeae(Lahariet al.2020). Our attempt to assess rice resistance to other root-knot nematode species showed that HD5 did not confer resistance toM.incognitaandM.javanica(Appendix A). If the resistance in HD5 is indeed specific toM.graminicola,there could be a novel resistance mechanism whereby HD5 specifically recognizes this nematode and triggers robust immunity in roots. Future research should focus on understanding the underlying molecular mechanism of such a specific resistance toM.graminicola.
5.Conclusion
A new rice source was characterized in dealing with the root-knot nematodeM.graminicola,which provides a better understanding of resistant mechanisms of this HD5 genotype. The desired features of resistance elucidated in this study are of huge potential for future management of rice breeding.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (31871943),the Jiangsu Agricultural Science and Technology Innovation Project,China (CX(21)1011),and the General Program of Hebei Natural Science Foundation,China (C2019402344).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendixassociated with this paper is available on https://doi.org/10.1016/j.jia.2022.11.008
 Journal of Integrative Agriculture2023年10期
Journal of Integrative Agriculture2023年10期
- Journal of Integrative Agriculture的其它文章
- The association between the risk of diabetes and white rice consumption in China: Existing knowledge and new research directions from the crop perspective
- Linking atmospheric emission and deposition to accumulation of soil cadmium in the Middle-Lower Yangtze Plain,China
- Genome-wide association study for numbers of vertebrae in Dezhou donkey population reveals new candidate genes
- Are vulnerable farmers more easily influenced? Heterogeneous effects of lnternet use on the adoption of integrated pest management
- lnfluences of large-scale farming on carbon emissions from cropping:Evidence from China
- Spatio-temporal variations in trends of vegetation and drought changes in relation to climate variability from 1982 to 2019 based on remote sensing data from East Asia
