Exploring the nano-fungicidal efficacy of green synthesized magnesium oxide nanoparticles (MgO NPs) on the development,physiology,and infection of carrot (Daucus carota L.) with Alternaria leaf blight (ALB): Molecular docking
Lukman AHAMAD ,Azmat ALl KHAN ,Masudulla KHAN ,Orudzhev FARlD ,Mahboob ALAM
1 Plant Pathology/Nematology Laboratory,Department of Botany,Aligarh Muslim University,Aligarh 202002,India
2 Regional Plant Quarantine Station,Salt Lake,Kolkata 700097,India
3 Pharmaceutical Biotechnology Laboratory,Department of Pharmaceutical Chemistry,College of Pharmacy,King Saud University,Riyadh 11451,Saudi Arabia
4 Botany Section,Women’s College,Aligarh Muslim University,Aligarh 202002,India
5 Amirkhanov Institute of Physics,Dagestan Federal Scientific Center,Russian Academy of Sciences,Makhachkala 367003,Russia
6 Department of Safety Engineering,Dongguk University,Gyeongju-si 780714,Republic of Korea
Abstract In this research,green synthesized magnesium oxide nanoparticles (MgO NPs) from lemon fruit extracts and their fungicidal potential was evaluated against Alternaria dauci infection on carrot (Daucus carota L.) under greenhouse conditions. The scanning and transmission electron microscopy (SEM and TEM) and ultra-violet (UV) visible spectroscopy were used to validate and characterize MgO NPs. The crystalline nature of MgONPs was determined using selected area electron diffraction (SAED). MgO NPs triggered substantial antifungal activity against A.dauci when exposed to 50 and 100 mg L–1 concentrations but the higher antifungal potential was noticed in 100 mg L–1 under invitro conditions. In fungal inoculated plants,a marked decrease in growth,photosynthetic pigments,and an increase in phenol,proline contents,and defense-related enzymes of carrot were seen over control (distilled water). However,foliar application of MgO NPs at 50 and 100 mg L–1 resulted in significant improvement of plant growth,photosynthetic pigments,phenol and proline contents,and defense enzymes activity of carrots with and without A.dauci infection.Spraying of MgO NPs at 100 mg L–1 had more plant length (17.11%),shoot dry weight (34.38%),plant fresh weight(20.46%),and root dry weight (49.09%) in carrots when challenged with A.dauci over inoculated control. The leaf blight indices and percent disease severity were also reduced in A.dauci inoculated plants when sprayed with MgO NPs. The non-bonding interactions of Alternaria genus protein with nanoparticles were studied using molecular docking.
Keywords: green synthesis,MgO nanoparticles,alternaria leaf blight,photosynthetic pigments,defense enzymes,carrot
1.Introduction
Carrots (DaucuscarotaL.),which belong to the Apiaceae family of umbelliferous plants,are root vegetables. It is rich in antioxidants,vitamins,minerals,and fiber. Carrots are mainly rich in beta-carotene,which converts into vitamin A in the human body. The vitamin-A promotes eye health and is also important for growth,development,and immune function (Tanumihardjo 2011). It is a coolseason crop that requires a temperature of 7 to 24°C for germination and 18–24°C for growth and development.Typically,the carrot roots reach marketable size within 70 to 120 days. Carrots can grow in a variety of soil types,but loam is ideal for their uniform growth and development(Rubatzkyet al.1999).
Fungal diseases cause 70–80% of crop diseases (Chenet al.2022). In 1855,the fungusAlternariadauci(Kuhn)Groves and Skolko was first identified as the cause of carrot leaf blight in Germany. Carrot leaf blight brought on byA.dauciis one of the most important foliar diseases all over the world (Tahvonen 1978;Strandberg 1992). The infection caused byA.dauciresulted in considerable yield loss due to the loss of infected petioles during mechanical harvesting and a reduction in the photosynthetic area of the leaves. The fungus over seasons on or in infected seeds,carrot debris,and wild carrots. Additionally,during cultivation,contaminated carrot seeds may be dispersed into the fields. The pathogen can survive in the debris or contaminated seeds in the soil for up to two years after being introduced into the carrot fields (Rogers 2011). In addition,A.daucireproduce using asexual spores (conidia)produced conidiophores that were upright. During culture,A.daucihyphae are septate and subhyaline to olive-brown in color. Olive-brown conidiophores are straight to curved,with a single conidiogenous site at the end or 1–2 on the sides. Conidia are usually borne singly,but occasionally strong terminal secondary conidiophores with a single secondary spore are formed. Spores are medium to dark olive brown,ellipsoidal to obclavate,60–100 µm×15–25 µm (spore body),and less than half of the horizontal segments contain 7–11 transepta and 1–3 longisepta.The terminal filamentous beak of mature conidia is 80–250 µm×5 µm long and taper distally to about 2 mm,with up to 100 mm of lateral branching on occasion (Rajna and Paschapur 2019).
Plant pathogenic fungi are major limiting factors for agricultural productivity and need to be controlled efficiently. So,the use of chemical-based fungicides has become one of the inevitable parts of the agro-food sector. These chemicals at higher doses in agriculture pose many environmental and health hazards (Rajna and Paschapur 2019). To overcome these problems,nanotechnology has opened up new opportunities in agriculture as a result of recent advancements.Nanotechnology has several advantages over traditional chemical-based pesticides in terms of plant disease control. As a result of these remarkable developments,the agricultural sector could also gain more precision and sustainability (Kahet al.2019). The effectively produced green synthesized magnesium oxide nanoparticles(MgO NPs),have high stability,are non-toxic,and offer a variety of opportunities for producing this material at the nanoscale (Abhinayaet al.2021). Because of their vast physiochemical and functionalization properties,they could be easily applied to the management of plant diseases (Kumari and Khan 2017). The MgO NPs have considerable antifungal activities towards the fungal pathogenFusariumoxysporumby morphological changes on hyphal morphology and integrity of fungal membrane (Abdel-Azizet al.2020). In addition,the utilization of MgO NPs also has the ability to provide resistance against the fungal pathogen,Phytophthora infestansthrough different toxic mechanisms such as continuous oxidative stress,cell surface distortion,inhibition of membrane transport,metabolic pathways,and regulation of hormonal signal transduction pathways in potato plants (Wanget al.2022). Nano-fungicides are anticipated to be a key component of future plant disease management strategies as more sustainable alternatives.
Several natural compounds including metabolic enzymes have been found to protect plants from pathogen infection and provide induced resistance to hosts against pathogens. It involves various signaling events that occur during pathogen infection. The induction of reactive oxygen species (ROS),and activation of the defense machinery of plants comprising of antioxidant enzymes,secondary metabolites,and pathogenesis-related proteins are the major induced changes in host plants (Kauret al.2022). However,the defense-related enzymes like peroxidase (POX),polyphenol oxidase (PPO),chitinase,β-1,3-glucanases,and phenolics were increased in response to fungal infection in banana plants (Thakkeret al.2013). In addition,metal-based NPs (CuO NPs)induced the defense enzymes inFusariumsolani-infected cucumber plants (Kamelet al.2022). A molecular docking method (Azamet al.2022;Çakmaket al.2022a,b;Yusofet al.2022) is a popular tool for studying hypothetical nonbonding interactions between nanoparticles and receptors in order to better understand their mechanistic aspects. The goal of the current study was to examine the function of environmentally friendly MgO NPs on thein-vitroantifungal activity and plant growth,photosynthetic pigments,phenol,proline contents and defense enzymes of carrots against leaf blight pathogen,Alternariadauciunder greenhouse conditions.
2.Materials and methods
2.1.Magnesium oxide nanoparticles synthesis
The lemon fruit was collected from the fruit Mandi of Aligarh,UP,India. A total of 1.6 g lemon fruits peels were dried and ground into a fine powder. Before being brought to room temperature,this fine seed powder was boiled for 30 min in 100 mL of deionized water. Resulting solution was filtered through Whatman filter paper. The filtered extract was stored in the fridge to be used later to make nanoparticles. Take 30 mL aqueous solution of magnesium nitrate into 10 mL of extract of strawberry extract in 250 mL in the flask and constant stirring on a magnetic stirrer at 50–60°C. After 3 h of vigorous stirring,the color of the reaction changes from transparent to white. After being collected,the nanoparticles were then dried at 40°C in a porcelain dish.
2.2.Characterization of MgO NPs
The scanning electron microscope (SEM) (JSM 6510 LV,JEO,Japan) equipped with an energy dispersive X-ray analyzer (EDX) was used to examine the element composition,morphology,and microstructure of MgO NPs samples. Transmission electron microscopy (TEM) (JEM-2100,JEOL,Japan) was used to examine MgO NPs to confirm their structure and particle size. The crystalline nature of biogenic NPs was confirmed by spectral studies such as selected area electron diffraction (SAED),which was followed by X-ray diffractometer (XRD). Fourier transforms infrared (FTIR) spectroscopy (Thermo Scientific NICOLET iS10,USA) with a 350–4 000 cm–1range of wave number was used to analyze the bond types in MgO NPs.
2.3.lsolation and identification of leaf blight fungus
Infected leaf samples of carrots having blighted symptoms were gathered from fields of carrots in Aligarh,India.Before processing,infected samples were put into bags of polyethylene and kept at 4°C in the freezer. The surface of the leaf samples was then treated with a 0.1% sodium hypochlorite (NaOCl) solution. Cut into small pieces and examined under a stereomicroscope,infected leaves were also placed in a Petri dish containing potato dextrose agar (PDA) in an aseptic manner for 15 days at 25°C.The fungus was identified asA.daucithrough cultural and microscopic examination. The identified fungus was subcultured on PDA media in pure form for performing further experiments. The composition of the growth media is as follows: 200.00,20.00,and 15.00 g L–1,and 5.6±0.2 for ingredients,potato infusion from boil extract potato,dextrose,agar,and final pH (at 25°C),respectively.
2.4.Nanofungicidal efficacy of MgO NPs on leaf blight fungus,A. dauci in lab conditions
The laboratory tests were performed on PDA media mixed with different concentrations (50 and 100 mg L–1)of MgO NPs. Before plating them in Petri plates (90 mm×15 mm),10 mL of each concentration of MgO NPs was poured into 100 mL of PDA medium. A BOD incubator was used to incubate media containing MgO NPs in each concentration at a temperature of (28±2)°C. Agar plugs with a diameter of 5 mm from a pure culture ofA.dauciwere inoculated simultaneously at the center of each Petri plate containing MgO NPs after 48 h of incubation and the plates were incubated at 28°C for 10 days. As controls,MgO NPs-free assays were used. After incubation,fungal colonies’ development was monitored. There were four repetitions of all treatments. Using the equation presented by Kaurgroup (Kauret al.2012),radial mycelial growth inhibition was calculated as a percentage:
where T and C mean fungal growth in treated plates and control,respectively.
2.5.Preparation of soil and carrot seedling management
The loamy soil originated from the agricultural areas of Aligarh District,Uttar Pradesh,India. The soil was filtered through a 10-mesh sieve. After that,soil plus cow dung manure was blended in a certain proportion (3:1 by volume),and then 1 kg of the soil mixture was placed in 15 cm-diameter clay pots. Before steam sterilization at 137.9 kPa for 20 min,some water was poured into each pot to moisten the soil surface. Before sowing in the greenhouse,sterile pots were brought to room temperature.
The seeds of carrot (DaucuscarotaL.)cv.Red Rose were washed three times with distilled water after being disinfected for 2 min with 0.1% sodium hypochlorite.In a soil-filled clay pot,5 disinfected seeds were sown. Thinning was done 1 week after germination to the healthiest seedlings per pot. The healthy carrot sprout was kept under greenhouse conditions with temperature (20±2)°C,humidity 96% and photoperiod 18 h/6 h (day/night).
2.6.Preparation and inoculation of A. dauci inoculum
For obtaining liquid inoculum ofA.dauci,a sterile inoculation needle was used to introduce fungi into Richard’s liquid medium (Riker and Riker 1936),which contained 10 g potassium nitrate,5 g potassium dihydrogen phosphate,2.5 g magnesium sulphate,0.02 g ferric chloride,50 g sucrose,and 1 000 mL distilled water. A total of 80 mL of the Richards liquid medium was prepared and filtered through a muslin cloth before being sterilized in 250-mL Erlenmeyer flasks for 15 min at 103.4 kPa. As previously mentioned,the fungi were each cultured separately in a flask and incubated at (25±1)°C for roughly 15 days. Following that,the fungus growing in the medium was filtered through No.1 Whatman filter paper. Excess water and nutrients were blotted with blotting paper after washing the fungal mycelium mat on filter paper with distilled water. The inoculum was made by blending 10 g of fungal mycelium for 30 s at 10 000 r min–1in a Waring blender with 100 mL of distilled water. A total of 10 mL of the suspension containing 1 g ofA.dauciinoculum was applied for the inoculation of the carrot seedlings. Six treatments were used in the greenhouse experiment,which was conducted using a fully randomized block design: T1,control;T2,A.dauci;T3,MgO NPs (50 mg L–1);T4,MgO NPs (50 mg L–1)+A.dauci;T5,MgO NPs (100 mg L–1);and T6,MgO NPs (100 mg L–1)+A.dauci. Five times of each treatment were repeated,and the experiment contains 30 pots.
2.7.Evaluation of carrot growth characters
90 days after inoculation (DAI),plants were harvested,and plants treated with MgO NPs and infected with pathogens were harvested and cleaned to remove soil adhesions. Plant length (cm),plant fresh weight(g),and shoot and root dry weight (g) were the growth characteristics. The plant length was recorded by measuring the top of the shoot and the bottom of the root.However,moisture was taken out using blotting sheets before weighing to monitor the plant fresh weight. The uprooted plants were divided into roots and shoots using a knife in the initial zone of the roots for the analysis of dry weight,and then they were kept in an oven at 60°C for 7 to 10 days.
2.8.Determination of photosynthetic pigments
Mackinney’s method (Mackinney 1941) was used to determine the amount of chlorophyll and carotenoid in the fresh plant samples. After pouring 20 cm3of 80%acetone over 1 g of freshly cut leaves,the leaves were ground to a fine pulp using a mortar and pestle. For 5 min,the mixture was centrifuged at 5 000 r min–1. In volumetric flasks measuring 100 cm3,the supernatant was collected. 80% acetone was used to wash the residue three times. The same volumetric flask was used to collect each wash,and 80% acetone was used to adjust the volume to the desired level. On a spectrophotometer (Shimadzu UV-1700,Tokyo,Japan),the absorbance was measured at 645 and 663 nm for chlorophyll and 480 and 510 nm for carotenoid against a blank (80% acetone).
2.9.Estimation of proline content
Bates method (Bateset al.1973) was used to calculate the proline content of fresh leaves. A total of 300 mg sample of fresh leaves was homogenized with 3 mL of 3% sulphosalicylic acid,filtered,and the filtrate was then diluted with 1 mL of ninhydrin and 1 mL of glacial acetic acid for 1 h in a test tube set in a warm water bath at 100°C. After adding toluene to the sample,it was placed in an ice bath and read at 520 nm with L-proline used as a standard.
2.10.Estimation of phenol content
The total phenolics were calculated using the Folin-Ciocalteau reagent (Singleton and Rossi 1965). A total of 2.0 g of fresh leaf material was homogenized with 80%liquid ethanol at room temperature. The supernatant was taken out after 15 min of centrifugation at 10 000×g.The residue was further extracted twice with 80%ethanol,and the supernatants were collected,placed in evaporating dishes,and dried at room temperature. A total of 5 mL of distilled water was used to dissolve the residue. This extract was diluted to a volume of 3 mL with water and 0.5 mL of the Folin-Ciocalteau reagent. After 3 min,2 mL of 20% sodium carbonate was added and thoroughly mixed. After 60 min,the color was developed,and the absorbance at 650 nm was measured with a spectrophotometer (Shimadzu UV-1700,Tokyo,Japan)with catechol as a standard. The results were expressed in mg catechol g–1of FW.
2.11.Estimation of defense enzymes activities
To determine the enzyme activities,5 mL of 50 mmol L–1phosphate buffer containing 1% polyvinylpyrrolidone was used to homogenize 500 mg of fresh leaf tissue. An enzyme extract was obtained using the supernatant of the homogenate centrifuged for 10 min at 5°C at 15 000×g for 10 min.
Fresh leaf samples were tested by Chance and Maehly(1955) for enzyme activity peroxidase (POX,EC 1.11.1.7).A total of 0.1 mL of enzyme extract,0.5 mL of 1% H2O2,and 3 mL of pyrogallol phosphate buffer were mixed in a cuvette. Absorbance at 420 nm was measured at 20-s intervals for 3 min.
A method of Mayeret al.(1966) was used to measure polyphenol oxidase activity in fresh leaves. The reaction mixture contained 1.5 mL of 0.1 mol L–1sodium phosphate buffer (pH 6.5) and 200 µL of the enzyme extract. A spectrophotometer was used to record the change in absorbance at 495 nm at 30-s intervals at the start of the reaction using 200 µL of 0.01 mol L–1catechol. All of the aforementioned ingredients,except the enzyme extract,were used to make the control set. Both enzyme activities,such as POX and PPO,were expressed as U mg–1FW.
2.12.Disease assessment
Indicators of leaf blight severity were determined by visually observing disease symptoms. A scale of 0 to 5 was used to assess the severity of leaf symptoms. Where 0 represents no disease (no blight symptoms observed);1 represents up to 12.5% blight symptoms on leaves and roots;2 represents 12.6 to 25% blight symptoms on leaves;3 represents 25.1 to 37.5% blight symptoms on leaves;4 represents 37.6 to 50% blight symptoms on leaves;and 5 represents greater than 50% blight symptoms on leaves(Nesha and Siddiqui 2013).
2.13.Molecular docking
The effect of magnesium oxide nanoparticles on the very similar sequence of amino acids in the protein of genusAlternaria,obtained from the RCSB PDB website (https://www.rcsb.org/) named Crystal Structure of genusAlternaria(PDB: 4AUD),was studied using molecular docking. Discovery Studio was used to identify the active receptor sites that are important for docking studies as well as the active amino acids that are most likely involved in nonbinding interactions. The retrieved protein was prepared by several procedures,including adding hydrogen,adding missing amino acids,and energy minimization. After adding it to the PyRx Software,additional preparation steps were automatically completed. The Cartesian coordinates of the cluster(MgO)36as ligand obtained from previous work (Chen 2014) were used as the MgO NP Model for docking to the receptor. The best pose of docking was obtained for visualizing and analysis of protein using Discovery Studio(Dassault Systèmes BIOVIA 2017).
2.14.Data analysis
The data collected were analyzed through R Software(2.14.0). The significance of the treatments was evaluated using analysis of variance (ANOVA). Duncan’s multiple range test (DMRT) and least significant differences (LSD) were utilized to demonstrate significant differences between treatments. SigmaPlot_14 was used to display the graphs. The presence of error bars on the graphs represents standard error (±SE). All the observed parameters were also subjected to analyze through principal component analysis (PCA,OriginPro_2022).
3.Results
3.1.Characterization of MgO NPs
The size and shape of the as-prepared MgO NPs were analyzed through SEM and TEM imaging. The singlecrystalline nature of the NPs was confirmed by the TEM grid-recorded micrograph. The majority of MgO NPs were spherical with an average diameter of 100 nm,while some particles had a larger scale range,as evidenced by TEM micrographs. The single-crystalline structure of the biogenic MgO NPs was confirmed by SAED research.The results demonstrate UV-Vis absorption spectrum of synthesized MgO NPs. MgO particles’ nano-range dimensions were confirmed by a broad absorption peak between 270 and 320 nm (Fig.1-A–D).
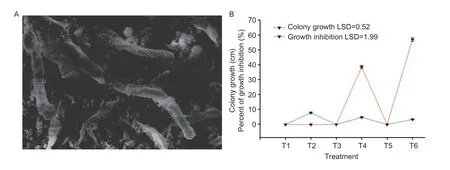
Fig.3 Antifungal activity of magnesium oxide nanoparticles (MgO NPs). A,SEM image showing the deleterious effects on conidiophores of Alternaria dauci. B,the fungal colony growth and percent of growth inhibition in in-vitro condition. T1=control;T2=A.dauci;T3=MgO NPs (50 mg L–1);T4=MgO NPs (50 mg L–1)+A.dauci;T5=MgO NPs (100 mg L–1);T6=MgO NPs (100 mg L–1)+A.dauci. Data are mean±SE (n=5).
3.2.Efficacy of MgO NPs on the growth of Alternaria dauci in laboratory conditions
The antifungal activity of MgO NPs (50 and 100 mg L–1)was assessed against leaf blight fungus,A.dauciunderin-vitroconditions. MgO NPs at the tested concentrations significantly (P≥0.05) inhibited the growth ofA.dauci(Figs.2 and 3). The decrease in fungus growth was also influenced by the medium’s NP concentration. After 10 days of incubation,the growth ofA.dauciwas observed to be inhibited by 38.60 and 56.96% at 50 and 100 mg L–1,respectively. The adverse effects of MgO NPs onA.dauciare significantly dependent on the quantity of MgO NPs,while the highest inhibition level was noticed 100 mg L–1of MgO NPs.
3.3.Effects of MgO NPs on plant growth characters
Plant growth parameters like plant length (27.30%),plant fresh weight (39.07%),shoot dry weight (42.23%),and root dry weight (55.52%) decreased significantly afterA.dauciwas inoculated (P=0.05) (Fig.4). MgO NPs sprayed at 50 and 100 mg L–1,plant growth was significantly higher(P=0.05) than in sprayed inoculated control. However,plants sprayed with MgO NPs at 100 mg L–1againstA.daucihad higher growth followed by MgO NPs at 50 mg L–1. The application of MgO NPs at 100 mg L–1increased the plant length (17.11%),shoot dry weight (34.38%),plant fresh weight (20.46%),and root dry weight (49.09%) when challenged withA.dauciover inoculated control (Fig.4).

Fig.4 Foliar spray of magnesium oxide nanoparticles (MgO NPs) on the growth,chlorophyll,and carotenoid contents of carrot infected with Alternaria dauci. T1=control;T2=A.dauci;T3=MgO NPs (50 mg L–1);T4=MgO NPs (50 mg L–1)+A.dauci;T5=MgO NPs (100 mg L–1);T6=MgO NPs (100 mg L–1)+A.dauci. Data are mean±SE (n=5). Different letters represent the significance at P≤0.05 by using Duncan’s multiple range test (DMRT).
3.4.Effects of MgO nanoparticles on photosynthetic pigments
Inoculation ofA.daucion carrot plants caused a significant reduction (P=0.05) in chlorophyll content and carotenoid contents by 21.18 and 10.17% respectively over control (water). Comparing the treatment with water as the control,the foliar application of MgO NPs (50 and 100 mg L–1) substantially raised the chlorophyll content by 25.96 and 28.88%,respectively,and the carotenoid content by 15.53 and 19.14%. Foliar application of MgO NPs at 100 mg L–1withA.dauciimproved the chlorophyll and carotenoid contents followed by spraying of MgO NPs at 50 mg L–1over control (Fig.4).
3.5.Effects of MgO nanoparticles on proline and phenol content
In plants,the proline and phenol contents were enhanced by 24.87 and 21.05% respectively after pathogen inoculation over the control (distilled water). Spraying of MgO NPs with 50 and 100 mg L–1improved the proline by 30.75 and 37.90% and phenol contents by 30.46 and 35.38% respectively over the control (distilled water).Spraying of plants with MgO NPs at 100 mg L–1withA.daucihad greater proline and phenol contents followed by 50 mg L–1of MgO NPs in comparison to the control(Fig.5).
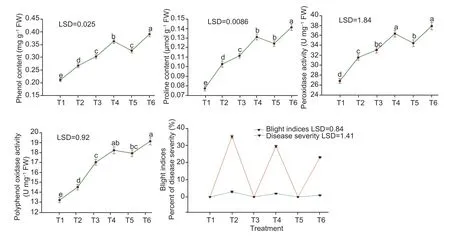
Fig.5 Foliar spray of magnesium oxide nanoparticles (MgO NPs) on phenol,proline,peroxidase,polyphenol oxidase contents and blight indices of carrot infected with Alternaria dauci. T1=Control;T2=Alternaria dauci;T3=MgO NPs (50 mg L–1);T4=MgO NPs(50 mg L–1)+A.dauci;T5=MgO NPs (100 mg L–1);T6=MgO NPs (100 mg L–1)+A.dauci. Data are mean±SE (n=5). The letters that appear in the graphs represent the significance at P≤0.05 by using Duncan’s multiple range test (DMRT).
3.6.Effects of MgO nanoparticles on defense enzymes of carrot
InA.dauciinfected plants had increased POX and PPO activity by 14.87 and 8.96% respectively over the control(distilled water). Foliar application of MgO NPs at 50 and 100 mg L–1improved the POX (18.78 and 22.02%),and PPO (22.35 and 26.25%) in comparison to control.Spraying of MgO NPs at 100 mg L–1had higher POX and PPO activity withA.daucifollowed by spraying of MgO NPs at 50 mg L–1over control (Fig.5).
3.7.Effects of MgO nanoparticles on disease parameters
Plants inoculated withA.daucishowed indices of 3 for carrot leaf blight. Disease indices caused byA.dauciwere reduced by 2 when MgO NPs at both concentrations but a bigger drop in disease indices was 1 where MgO NPs were applied as a spray at concentration of 100 mg L–1. However,the percent (%) disease severity was observed 35.2% inA.dauciinoculated plants alone. Foliar spray with MgO NPs at 50 and 100 mg L–1concentrations reduced the disease severity to 23.1 and 29.5% respectively (Fig.5).
3.8.Principal component analysis
All treatments on carrot plants with MgO NPs andA.dauciand their effects on various studied parameters were subjected to PCA. The obtained PCA clarified 98.58%of the data variability (PC1=59.33%;PC2=39.25%)(Fig.6). Plant growth,photosynthetic pigments,phenol and proline contents,and carrot defense enzymes all showed significant positive correlations. These attributes demonstrated a negative association with disease indices and disease incidence in percentages (%). According to the segregation of various treatments in the biplot,the PCA also determined that the maximum diseasesuppressing effect of MgO NPs at 100 mg L–1,followed by MgO NPs spray at 50 mg L–1(Fig.6).
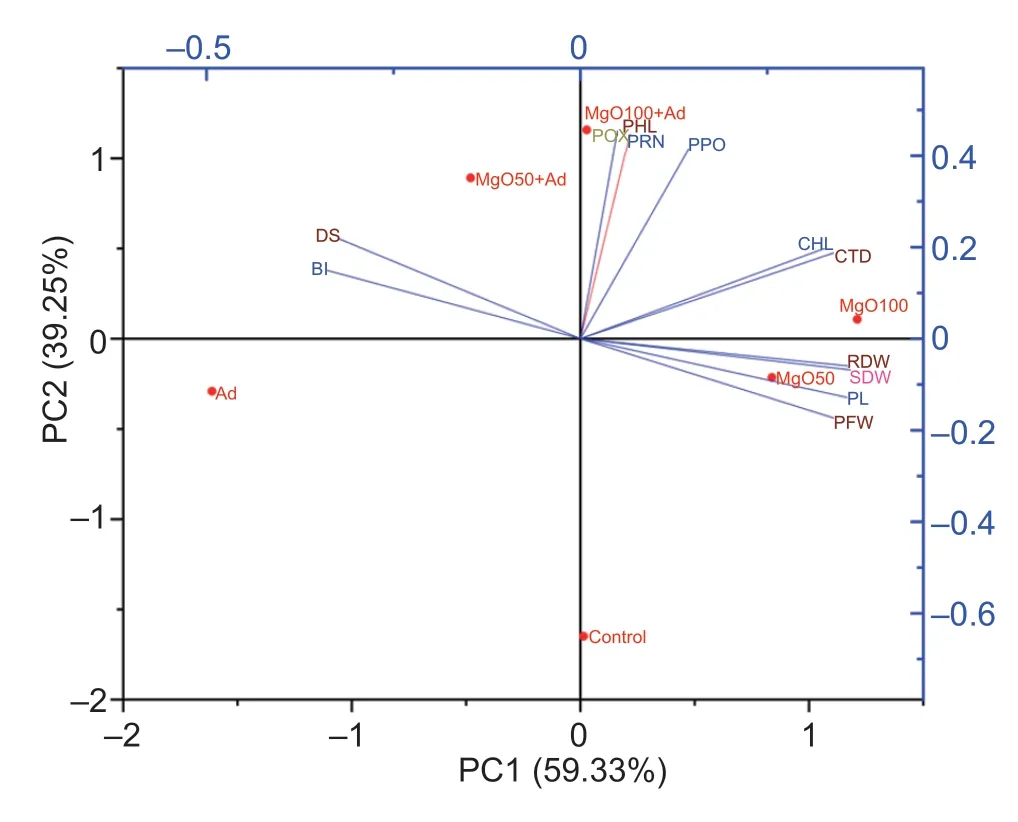
Fig.6 Principal component analysis (PCA) biplots comparing the effects of applying MgO nanoparticles (MgO NPs) to carrot leaves with and without Alternaria dauci on various measured parameters. CHL=chlorophyll content;PL=plant length;SDW=shoot dry weight;PFW=plant fresh weight;RDW=root dry weight;CTD=carotenoid content;PHL=phenol content;POX=peroxidase;PPO=polyphenol oxidase;BI=blight indices;DS=% disease severity;Ad=Alternaria dauci;MgO50=MgO NPs at 50 mg L–1;MgO100=MgO NPs at 100 mg L–1.
3.9.Molecular docking analysis
The docking pose with high–9.5 kcal mol–1binding energy was selected among the other poses.Magnesium atoms of MgO NPs interacting with ASP10 and TYR11 to form electrostatic forces as an attractive charge as well as a Pi-Cation involving MG29,MG46,MG61,MG31,and MG46 atoms of nanoparticles are shown in Fig.7;while oxygen atoms of MgO NPs involved in the formation of hydrogen bonding with active amino acids;TYR11,THR42,LEU43,TRP13,and ASN39 leading close interaction as well as stabilization of protein–NPs complex. The hydrophobic,neutral,and hydrophilic amino acids of the receptor approached the nanoparticles’ surface,forming a variety of non-interacting bonds that stabilized the complex.
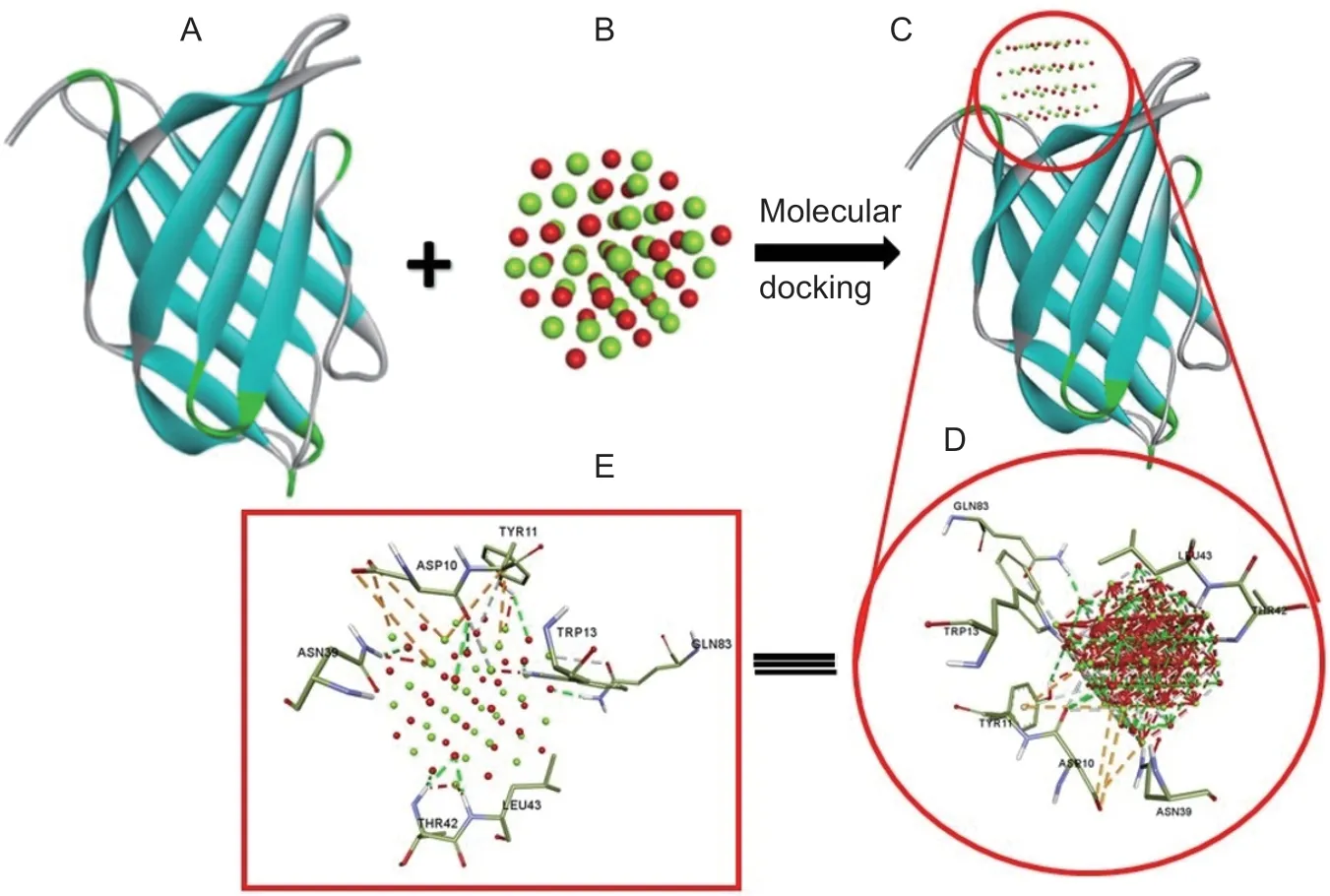
Fig.7 Molecular docking analysis. A,crystal structure of Alternaria (PDB: 4AUD) of the receptor. B,nanoparticles (MgO)36 as ligand. C,ligand in the active pocket of receptor. D,various amino acids on the nanoparticles (NPs) surface and their interactions.E,nonbonding interactions including hydrogen bonds,vdW interactions between receptor and NPs.
4.Discussion
Carrots are a popular vegetable that is susceptible to a variety of diseases,including leaf blight affected by the necrotrophic fungusAlternariadauci(Lecomte 2013).Alternaria leaf blight (ALB) is a widespread disease that is one of the most damaging to carrot leaves. Premature foliage loss or collapse may result in significant yield reductions in carrot fields due to photosynthesis impairment (Rogers 2011). In addition,it was also studied thatA.dauciproduces the phytotoxin named as zinniol,which degrades the structure of cell membranes and chloroplasts (Barashet al.1981). Cells of carrot leaf adjoining to mycelium lose chlorophyll and get necrotic during colonization. Other studies also showed thatA.dauciinfection in carrot plants decreases the growth parameters,chlorophyll,and carotenoid contents (Ahmad and Siddiqui 2019).
Management ofA.dauciinfection in carrots is necessary to improve plant health and productivity. So,green nanotechnology is a promising option to destabilize the pathogenic infection as well as to improve plant health. In green nanotechnology,magnesium oxide nanoparticles were finally synthesized using a green synthesis that is safe for the environment,non-toxic,more stable,and offers a variety of options for producing this substance at the nanoscale (Abhinayaet al.2021). The biophysical properties (Jeevanandamet al.2017) of the phytosynthesized MgO nanoparticles were determined by the UV-visible spectrum and dynamic light scattering techniques. However,morphological characteristics of NPs were described using TEM and SEM. In addition,SAED patterns was applied to confirm that the synthesized MgO nanoparticles have a single crystalline structure.
In laboratory tests,we found that MgO NPs found inhibitory toA.dauciin both applied doses (50 and 100 mg L–1) but greater inhibition was achieved at higher concentration of MgO NPs. MgO could prevent fungi from growing,spores from germinating,andPhytophthoranicotianaeandThielaviopsisbasicolafrom producing sporangium,as per toin-vitrostudies (Chenet al.2020). Additionally,MgO and ZnO NPs at various concentrations significantly inhibited spore germination ofA.alternata,F.oxysporum,R.stolonifer,andM.plumbeus in-vitro. However,greater inhibition in the germination of all the tested fungi was noticed from higher to lower concentrations of nanoparticles with MgO found as more effective than ZnO in reducing spore germination (Wani and Shah 2012).
Plants require Mg as a mineral element,and it is nontoxic to other organisms. Mg2+,when used as a foliar and soil treatment,not only interacts with cellular proteins and denaturing proteins,but also serves as a critical mineral element for plant growth. Our study also suggests that foliar application of MgO NPs at 50 and 100 mg L–1concentrations improved the growth characteristics and photosynthetic pigments inA.dauci-infected or noninfected plants. The results also correlated with the study of MgO NPs improved the growth and yield of cotton plants sprayed with varied concentrations (Kanjana 2020). It was also studied that Mungbean plants treated with MgO NPs showed improvements in growth attributes when infected with soil-borne pathogens,F.solaniandF.oxysporum(Abdallahet al.2022). In addition,foliar spraying with MgO NPs increased the chlorophyll contents in pepper plants when infected with the powdery mildew pathogenOidiopsis sicula(Ismailet al.2021).
In the findings,it was observed that spraying with MgO NPs at both concentrations increases the phenol and proline contents as well as defense enzyme activities inA.dauci-inoculated or un-inoculated plants. A previous study showed that some nanoparticles including MgO NPs increase the total phenol contents and polyphenol oxidase activity in sugar beet when challenged with damping-off and root rot pathogens (El-Argawyet al.2017). Other studies also suggested that spraying of ZnO and GO nanoparticles on carrot plants increases the photosynthetic pigments and proline contents under stress conditions posed by pathogenic fungi (Siddiquiet al.2019). In a similar study,Suriyaprabhaet al.(2014) discovered that Si NPs were effective at inducing phenols,peroxidase,and PPO in maize,all of which were associated with increased resistance toAspergillusspecies. Additionally,it was discovered that bean pods treated with NPs had significantly increased chlorophyll content,and total phenols infected withSclerotinia sclerotiorumas compared to non-infected pods (Abdel-Halim and El-Ghanam 2019).
The blight indices and disease incidence were often correlated with the overall health of the plant.The disease indices and percent disease incidence increased when infected leaf blight pathogen,A.dauci.Foliar application of MgO NPs played a protective role in decreasing blight indices and percentage disease incidence in carrot plants. Furthermore,MgO NPs and ZnO NPs were found to reduce disease severity (DS)and the area under the disease progress curve (AUDPC)in pepper plants infected withOidiopsis sicula(Ismailet al.2021). In addition,the disease of the black root and shank of tobacco decreased when plants were treated(500 mg mL–1) with MgO NPs (Chenet al.2020). During analysis with PCA,a total 98.58% of the data’s variability was observed inA.dauci-infected carrot plants. These results are following Sneath and Sokal (1973) that data should represent at least 70% variability. Plant growth parameters,photosynthetic pigments,proline,and phenol content,and defense enzymes of carrots sprayed with MgO NPs under pathogenic stress all correlated positively. On the other hand,a negative correlation in blight indices and % disease incidence with other studied attributes was also observed which suggests that MgO NPs played a protective role in disease suppression.The MgO cluster was docked against the amino acid sequence ofAlternaria,and the results demonstrated the significance of key amino acids for the toxicity of carrot leaf blight,including ASP10,TYR11,THR42,LEU43,TRP13,and ASN39. These interactions may control the spread of diseases causing carrot fungal leaf blight.
5.Conclusion
To lessen the deleterious effects of synthetic fungicides,green synthesized nanoparticles are one of the emerging alternatives to improve plant health and productivity.Here,we examined the green MgO NPs for their fungicidal efficacy againstAlternaria dauci,which adversely affects plant health. Moreover,when the fungus was grown in the presence of NPs,micro-morphological characteristics of fungal hyphae were changed. In addition,nanofungicidal potential of MgO NPs was proved when applied against Alternaria leaf blight of carrots in pot-soils. The MgO NPs suppressed the blight indices and disease severity caused by the fungus and improved growth attributes,photosynthetic pigments,phenol and proline contents,and defense enzymes activity of carrot. As a result of this study,MgO NPs with sufficient anti-mycotic activity might be utilized as a safe and effective alternative to chemical fungicides. The nano fungicidal potential of MgO was also explained using molecular docking and various non-interactions with pathogenic fungi. However,more research in this area is required to assess the phytotoxicity level under field conditions,which must be carried out before application.
Acknowledgements
This work was funded by the Researchers Supporting Project Number (RSP2023R339) at King Saud University,Riyadh,Saudi Arabia.
Declaration of competing interest
The authors declare that they have no conflict of interest.
 Journal of Integrative Agriculture2023年10期
Journal of Integrative Agriculture2023年10期
- Journal of Integrative Agriculture的其它文章
- The association between the risk of diabetes and white rice consumption in China: Existing knowledge and new research directions from the crop perspective
- Linking atmospheric emission and deposition to accumulation of soil cadmium in the Middle-Lower Yangtze Plain,China
- Genome-wide association study for numbers of vertebrae in Dezhou donkey population reveals new candidate genes
- Are vulnerable farmers more easily influenced? Heterogeneous effects of lnternet use on the adoption of integrated pest management
- lnfluences of large-scale farming on carbon emissions from cropping:Evidence from China
- Spatio-temporal variations in trends of vegetation and drought changes in relation to climate variability from 1982 to 2019 based on remote sensing data from East Asia
