The nitrate-responsive transcription factor MdNLP7 regulates callus formation by modulating auxin response
LI Tong ,FENG Zi-quan ,ZHANG Ting-ting ,YOU Chun-xiang ,ZHOU Chao ,WANG Xiao-fei#
1 Apple Technology Innovation Center of Shandong Province/Shandong Collaborative Innovation Center of Fruit &Vegetable Quality and Efficient Production/National Key Laboratory of Wheat Improvement/College of Horticulture Science and Engineering,Shandong Agricultural University,Tai’an 271018,P.R.China
2 National Key Laboratory of Wheat Improvement/College of Life Sciences,Shandong Agricultural University,Tai’an 271018,P.R.China
Abstract Under appropriate culture conditions,plant cells can regenerate new organs or even whole plants. De novo organ regeneration is an excellent biological system,which usually requires additional growth regulators,including auxin and cytokinin. Nitrate is an essential nutrient element for plant vegetative and reproductive development. It has been reported that nitrate is involved in auxin biosynthesis and transport throughout the growth and development of plants.In this study,we demonstrated that the ectopic expression of the MdNLP7 transcription factor in Arabidopsis could regulate the regeneration of root explants. MdNLP7 mainly participated in the regulation of callus formation,starting with pericycle cell division,and mainly affected auxin distribution and accumulation in the regulation process. Moreover,MdNLP7 upregulated the expression of genes related to auxin biosynthesis and transport in the callus formation stage.The results demonstrated that MdNLP7 may play a role in the nitrate-modulated regeneration of root explants. Moreover,the results revealed that nitrate–auxin crosstalk is required for de novo callus initiation and clarified the mechanisms of organogenesis.
Keywords: MdNLP7,callus initiation,auxin,nitrate,pericycle,shoot regeneration
1.Introduction
Plant cells can regenerate organs from differentiated somatic tissuesviadenovoorganogenesis under appropriate culture conditions (De Klerket al.1997). For many plants,exosomes can be regenerated from isolated cultures. This is useful for germplasm preservation,the industrial propagation of elite plants,and molecular plant analysis techniques such as gene editing (Thorpe 2007;Sengar 2010;Michael 2013;Shahzadet al.2017).De novoshoot organogenesis (DNSO) depends on the totipotency of somatic cells (i.e.,single somatic cells can be used to regenerate whole plantsinvitro),and it is the most common method for regenerating plantsinvitro(De Klerket al.1997;Sangwan and Sangwan-Norreel 1997;Curtis 2010). In the last century,Skoog and Miller(1957) developed tissue culture techniques that led to a rapid understanding and control of plant tissue and organ regeneration. The specific culture conditions utilize a higher auxin/cytokinin ratio on a callus-inducing medium(CIM) to induce the initial pericycle cell of the root explants to form a callusviaextensive cell division;subsequently,the callus differentiated into shoots was incubated in a shoot-inducing medium (SIM) under a higher cytokinin/auxin ratio (Skoog and Miller 1957;Valvekenset al.1988).
The molecular mechanism of the “organogenesis capacity of callus” has been one of the central scientific questions in the field of plant regeneration. It has been proposed that the callus is similar to the root tip meristem(Sugimotoet al.2010),and the mechanism by which the callus regenerates new organs has been revealed.First,the callus possesses an undifferentiated state of stem cells. Second,the mid-layer cells of the callus are characterized by a double hormonal signaling peak;that is the cells feature both high auxin accumulation and cytokinin hypersensitivity. These two characteristics enable callus to regenerate both roots and shoots (Zhai and Xu 2021).
Recently,molecular mechanisms related to auxin transportation,distribution,gradients,and regulation have been extensively studied,elucidating the molecular control of DNSO (Benková and Ivanchenko 2003;Petrásek and Friml 2009;Vanneste 2009;Bohn-Courseau 2010;Friml 2010;Overvoordeet al.2010;Tsugekiet al.2010;Zhanget al.2010). The synthetic auxin-responsive promoterDR5(DR5 consists of a direct repeat element TGTCTC repeated 7 times)is employed to visualize auxin,but its expression decreases over time,and it becomes undetectable within large calli (Gordonet al.2007). PIN-FORMED1(PIN1) has been observed to display a similar pattern,suggesting thatPIN1regulates the formation of auxin granules within the callus,which is essential for the formation of competent cells (Gordonet al.2007;Pernisovaet al.2009;Su and Zhang 2009).The inhibition of theYUCCA(YUC)gene preventsWUSCHEL-RELATEDHOMEOBOX11(WOX11)expression and affects the transformation of competent cells (Chenet al.2016). Under the influence of early wound signaling,auxin biosynthesis begins and accumulates in converter cells. The biosynthesis process involves numerous genes,including those of theYUCfamily (Liuet al.2014;Chenet al.2016).Long-term wound signaling activates the expression ofYUCCA4(YUC4),a regeneration-competent cell near the wound site,probably to maintain auxin levels(Chenet al.2016). Endogenous cytokinin controls auxin-induced DNSO by regulating efflux-dependent intracellular auxin distribution (Pernisovaet al.2009).In various tissues,the expression of theArabidopsis responseregulators(ARR5) gene is correlated with cytokinin levels (Pernisovaet al.2009). The transcript ofARR5is upregulated in thedenovoshoot meristem initiation zone and developing shoot meristems but is downregulated in organ primordia (Valvekenset al.1988;Gordonet al.2007;Attaet al.2009).
A recent study indicated that nitrate (NO3-) is an essential nutritional element and a plant-signaling molecule (Stitt 1999). NIN-like proteins have been identified to play central roles in primary nitrate response inArabidopsis. Among the proteins,AtNLP7functions as a transcriptional activator and an intracellular nitrate sensor,and its function has been extensively studied(Konishi and Yanagisawa 2019;Liuet al.2022).AtNLP7is phosphorylated by Ca2+-sensor protein kinases (CPKs,i.e.,CPK10/30/32),which mediates nitrate-induced gene expression by retainingAtNLP7in the nucleus (Liuet al.2017). The direct binding of nitrate toAtNLP7triggers conformational changes inAtNLP7,leading to the deregulation of transcriptional repression of downstream genes (Liuet al.2022).
One study found that crosstalk occurred between the nitrate and auxin signaling pathways (Averyet al.1937). Studies have also found that nitrate supplied to roots transcriptionally regulated auxin biosynthesis and transport (Tianet al.2009;Krouket al.2010;Maet al.2014). A transcriptomic study demonstrated thatAtPIN1,AtPIN2,AtPIN4,andAtPIN7were specifically regulated by nitrate (Gutiérrezet al.2007). Thus,only a few transcription factors that contribute to nitrate-dependent auxin efflux have been studied.AtNLP7can influence the expression levels ofauxincarriersPIN-LIKES3(AtPILS3) to optimize plant development in response to external nitrate cues (Kumaret al.2023).AtNLP7-induced auxin efflux activates theAtPIN7transcription factor and regulates local auxin fluctuations to build up and sustain root primordia,which determines the number of lateral roots (LRs) (Overvoordeet al.2010).Auxin is an upstream target of theAtTCP20-AtNLP6/7-mediatedROP2-TOR-E2Fa/bsignaling pathway (Guan 2017). In auxin synthesis,tryptophan aminotransferase related 2 (TAR2) plays a significant role (Taoet al.2008).AtNLP7directly binds to theAtTAR2promoter and upregulates its expression to promote nitrate-mediated LR development,thereby maintaining auxin distribution in the LR primordia (Zhanget al.2021).
Studies have shown that theMdNLP7transcription factor in apples is involved in nitrate uptake and transport through the regulation of transcript levels ofMdNIA2andMdNRT1.1.MdNLP7may also regulate plant biomass through root development and enhanced nutrient absorption (Fenget al.2022). Moreover,MdNLP7overexpression can regulate LR development in the TAR2-dependent pathway (Zhanget al.2021).
Auxin biosynthesis,distribution,and LRs capacity developments are essential for the regeneration of root explants inArabidopsis (Laskowskiet al.1995).MdNLP7has been involved in nitrate signaling during LRs development,and it also regulates the expression of auxin factors (Zhanget al.2021;Fenget al.2022).These findings suggest a possible role forMdNLP7in callus formation and shoot regeneration. In addition,the mechanisms by whichMdNLP7regulates this process are not yet fully understood. In this study,we demonstrated that ectopic expression ofMdNLP7could accelerate the regeneration process from root explants ofArabidopsis. Specifically,MdNLP7was mainly involved in regulating the process of callus formation that began with cell division in the pericycle cell,and this regulation was due to the distribution and accumulation of auxin. Furthermore,the transcript levels of genes related to auxin biosynthesis and transportation were upregulated byMdNLP7during callus formation from root explants. Finally,our findings confirmed thatMdNLP7plays an important role in the nitrate-modulated regeneration process of root explants.
2.Materials and methods
2.1.Plant materials and growth conditions
The Columbia ecotype ofArabidopsisplants was used in this study. Transgenic lines were derived from:MdNLP7-OX(Zhanget al.2021),proMdNLP7::GUS(Fenget al.2022),DR5::GUS(Yuet al.2016),andARR5::GUS,these were obtained from our laboratory’s preserved stocks.The homozygous transgenic materials ofMdNLP7-OX/DR5::GUSandMdNLP7-OX/ARR5::GUSwere obtained by hybridization.
TheArabidopsisseeds were sterilized with 75%absolute ethanol for 60 s,1.3% sodium hypochlorite solution for 8 min,sterile water rinsed 5 times and germinated on half-strength Murashige and Skoog medium(1/2 MS medium;Coolaber),1% sucrose,0.8% agar (pH 5.8) to overcome dormancy at 4°C for 3 d. Plates were placed in a 16/8-h light/dark photoperiod,with (22±2)°C.
It was found that ‘Orin’ apple calli grew best on MS medium with 0.45 mg L-16-benzylaminopurine (6-BA),1.6 mg L-12,4-dichlorophenoxyacetic acid (2,4-D),3%sucrose,and 0.8% agar,adjusted to pH 5.8 with 1.0 mol L-1sodium hydroxide. The calli were subcultured every 18 d at 26°C in the dark (Fenget al.2022).
2.2.Genetic transformation of apple callus using the MdNLP7 expression vector
Through PCR,full-length fragments ofMdNLP7were amplified from ‘Gala’ apples. The primers used were MdNLP7-F 5´-ATGGAACAAAAGTTGATTTCTGAAGA-3´;MdNLP7-R 5´-CGCACTCCCGCAAGAGCT-3´. In order to produce the MdNLP7 overexpression vector,the correctly sequenced fragment was enzyme digested and ligated into expression vector pRI 105-AN. As a next step,heat-shock was used to transform pRI 105-AN plasmid and overexpression vector intoAgrobacteriumGV3101 afterwards. TheAgrobacterium-mediated transformation method was used to obtain transgenic apple calli (MdNLP7-OX) that overexpressMdNLP7(Zhaoet al.2016). Use 250 mg L-1cephalosporin and 50 mg L-1basta for transgene selection.
2.3.Callus formation and shoot regeneration
It was used the two-step protocol described by Valvekenset al.(1988) for regeneration experiments. Firstly,root explants of~1 cm length were incubated for 0–20 d on CIM (consisting of Gamborg’s B5 medium,2.2 µmol L-12,4-D (Sigma,USA),0.22 µmol L-1kinetin (KT) (Sigma),2% glucose,0.05% MES and 0.8% agar,pH 5.8) under 16/8-h light/dark photoperiod with (22±2)°C,transferred to new medium every 7 d. In the next step,root explants with calluses were transferred to SIM (consisting of Gamborg’s B5 medium,0.9 µmol L-1indole acetic acid(IAA) (Sigma) and 5 µmol L-12-isopentenyladenine (2-iP)(Sigma),2% glucose,0.05% MES,0.8% agar,pH 5.8) for 0–35 d under 16/8-h light/dark photoperiod with (22±2)°C,transferred to new medium every 7 d. To examine the effect of nitrate on regeneration,the root explants were cultured on CIM/SIM which prepared with nitrogendeficient Gamborg’s B5 medium and 0.1 mmol L-1NH4+plus 0.5 mmol L-1NO3–/5 mmol L-1NO3–(LN/HN).
2.4.Explant imaging and analysis
As part of the regeneration procedure,explants were viewed with an Olympus SZX-16 stereoscopic microscope(Olympus,Japan). LEICA inverted microscope (Model Dmi8 manual,Leica Microsystems GmbH,Germany) was used to examinate of root explants reveals their fine structure and GUS staining. A photograph of the developed calli was taken and the area of the callus was quantified using ImageJ (Shanget al.2016). Photographs of regenerate shoots were taken and the efficiency of shoot regeneration was determined.At least three biological replicates of each treatment and genotype were done with 20 independent plants each.
2.5.GUS staining assay
Root explants stored in GUS staining fluid (containing 0.5 mmol L-1K3[Fe(CN)6],0.5 mmol L-1K4[Fe(CN)6]·H2O,100 mmol L-1sodium phosphate (pH 7.2),10 mmol L-1EDTA·Na2·H2O,1 mmol L-1X-gluc (Solarbio,Beijing,China)) at 37°C for 2 h in dark. After being immersed in 70% v/v ethanol for several hours,the explants were subsequently viewed under a LEICA inverted microscope.
2.6.Apple callus growth under the 2,4-D and 6-BA treatments
The 18-d-old WT andMdNLP7-OXtransgenic apple callus were subcultured on a solid medium containing MS,MS+1.6 mg L-12,4-D,MS+0.45 mg L-16-BA,and MS+1.6 mg L-12,4-D+0.45 mg L-16-BA,respectively,for 21 d in the dark. Growth was monitored using a fresh weight assay.
2.7.Detection of phytohormones in apple callus
Sample preparation and plant hormone extractionAfter harvested fresh apple callus samples,immediately frozen them in liquid nitrogen,ground them into powder and store them at–80°C until needed. Weighed 50 mg of plant sample into 2 mL plastic microtubule under the condition of keeping liquid nitrogen frozen,and dissolved it with 1 mL of methanol/water/formic acid (15:4:1,v/v).The extraction solution was mixed with 10 µL of an internal standard solution (100 ng mL–1) for quantification,as the internal standard (IS). Next,the vortex mixture was centrifuged for 5 min (12 000 r min–1,4°C) after 10 min. The supernatant after centrifugation was taken into clean plastic microtubes and evaporated to dry,and then dissolved in 100 µL of 80% methanol (v/v). Finally,LC-MS/MS analysis was performed after filtration through a 0.22-µm membrane filter.
Detection of phytohormonesBased on the QTRAP 6500 LC-MS/MS platform from AB Sciex,MetWare (http://www.metware.cn/) detected phytohormone contents .
2.8.RNA extraction and gene expression
Root explants incubated on CIM for 7 d and freezed with liquid nitrogen. Apple callus were subcultured on a solid medium containing MS,MS+1.6 mg L-12,4-D,MS+0.45 mg L-16-BA,and MS+1.6 mg L-12,4-D+0.45 mg L-16-BA for 21 d and freezed with liquid nitrogen. CWBIO’s OminiPlant RNA Kit (CWBIO,Beijing,China) was used for RNA isolation and extraction. The extracted RNA was stored in RNAfollow (tissue RNA preservation solution),and as directed by the manufacturer’s instructions,reversetranscription was conducted using a PrimeScript™ RT reagent Kit (TaKaRa,Dalian,China). The quantitative real-time PCR reactions were performed on each cDNA dilution using UltraSYBR Mixture (with ROX) withActinas as an internal reference to detect the expression levels of selected genes. The reaction system and procedures performed were conducted as previously described (Zhanget al.2021). Each genotype was represented by three biological replicates. Normalized transcript abundances were calculated using the 2–ΔΔCTmethod and all primers used were given in Appendix A.
2.9.Statistical analysis
All experiments were repeated independently three times.The significant difference analysis and column chart were performed with DPS and GraphPad Prism 7 Software (La Jolla,CA,USA). Data were analyzed by one-way ANOVA with Tukey’s multiple comparison test,presented as means±SD,applying aP-value threshold of 0.05.
3.Results
3.1.MdNLP7 is involved in the shoot regeneration from root explants
To verify whetherMdNLP7participated in regeneration from root explants,Col and threeMdNLP7overexpressionArabidopsislines (MdNLP7-OX1,MdNLP7-OX2,andMdNLP7-OX3) were used (Fenget al.2022). They were subjected to a two-step tissue culture technique. Root explants were cultivated in CIM for 7 d,and they were subsequently cultivated in SIM to enable shoot regeneration. Under regeneration conditions,allMdNLP7-OXlines showed a significant enhancement in shoot regeneration capacity compared with Col (Fig.1-A),and this enhancement was unaffected by changes in SIM(Fig.1-B).

Fig.1 Overexpression of MdNLP7 in Arabidopsis promotes shoot regeneration. A,shoot regeneration from root explants of the Col,MdNLP7-OX1,MdNLP7-OX2,and MdNLP7-OX3. Use two-step tissue culture technique: callus formation after cultivated on callus-inducing medium (CIM) for 7 d (7 DAC) from root explants,then shoot regeneration cultivated on shoots-inducing medium(SIM) for 0,7,14,21,and 35 d (0,7,14,21,and 35 DAS). Scale bars,2 mm. B,the frequency of shoot regeneration formed in root explants after 7 DAC+0,7,14,21,and 35 DAS. Data are presented as mean±SD (n=20). Different letters indicate significant differences at P<0.05 determined by one-way ANOVA with Tukey’s multiple comparison test.
3.2.Effect of MdNLP7 on the distribution of cytokinin and auxin in shoot regeneration
The crosstalk between auxin and cytokinin is essential forArabidopsisshoot regeneration (Skoog and Miller 1957;Valvekenset al.1988). To verify whetherMdNLP7regulated cytokinin and auxin distribution during shoot regeneration,MdNLP7-OX/ARR5::GUSand MdNLP7-OX/DR5::GUStransgenicArabidopsiswere generated. GUS staining showed thatMdNLP7overexpression had no effect on the accumulation and distribution of cytokinin and auxin during the root explant regeneration of shoots (Fig.2).
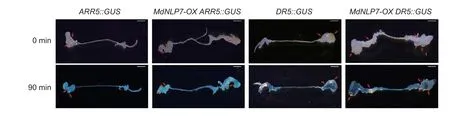
Fig.2 Effect of MdNLP7-OX on the distribution of cytokinin and auxin in shoots. Shoot formation after 7 DAC+12 DAS from the root explants of ARR5::GUS,MdNLP7-OX ARR5::GUS,DR5::GUS,and MdNLP7-OX DR5::GUS. GUS staining for 0 and 90 min for analysis of expression pattern of auxin and cytokinin with the proARR5::GUS and proDR5::GUS reporter gene in MdNLP7-OX.Arrows indicate the position of regenerated shoots. DAC,days after CIM;DAS,days after SIM. Scale bars,100 µm.
3.3.Transcript of AtNLP7 and MdNLP7 during callus formation from root explants
Considering thatMdNLP7-OX-enhanced shoot regeneration was not affected by SIM (Fig.1) and thatMdNLP7did not affect the distribution of cytokinin and auxin during shoot regeneration (Fig.2),the higher concentration of cytokinin in SIM may not be the limiting factor in the shoot regeneration capacity ofMdNLP7-OXand Col. One study showed thatAtNLP7had a higher expression in Col cultured in CIM for 6 d (Appendix B-a;Zhai and Xu 2021),which suggested thatMdNLP7may promote root explant regeneration during callus formation. To prove this hypothesis,proMdNLP7::GUSArabidopsisroot explants were obtained,which showed thatMdNLP7exhibited a higher tissuespecific expression pattern during both the CIM-induced callus formation phase and the early stages of SIM-induced regenerative shoot development (Appendix B-c). These results suggest thatMdNLP7plays a role in the earlyde novoregeneration ofArabidopsisroot explants.
3.4.MdNLP7 promotes the distribution of auxin during callus formation
Furthermore,we investigated the potential role ofMdNLP7in auxin and cytokinin distribution during callus formation. Over the four different stages (I–IV) of callus formation in the pericycle,the cytokinin gradually increased and peaked in the LR primordia instead of the callus from pericycle cells (Fig.3,left two panels),while auxin distribution accumulated strongly at the site of callus formation in pericycle cells (Fig.3,right two panels).Furthermore,MdNLP7overexpression promoted auxin distribution in the callus;however,the overexpression had no apparent effect on cytokinin distribution in the callus (Fig.3). These results suggest thatMdNLP7was involved in auxin distribution during callus formation.
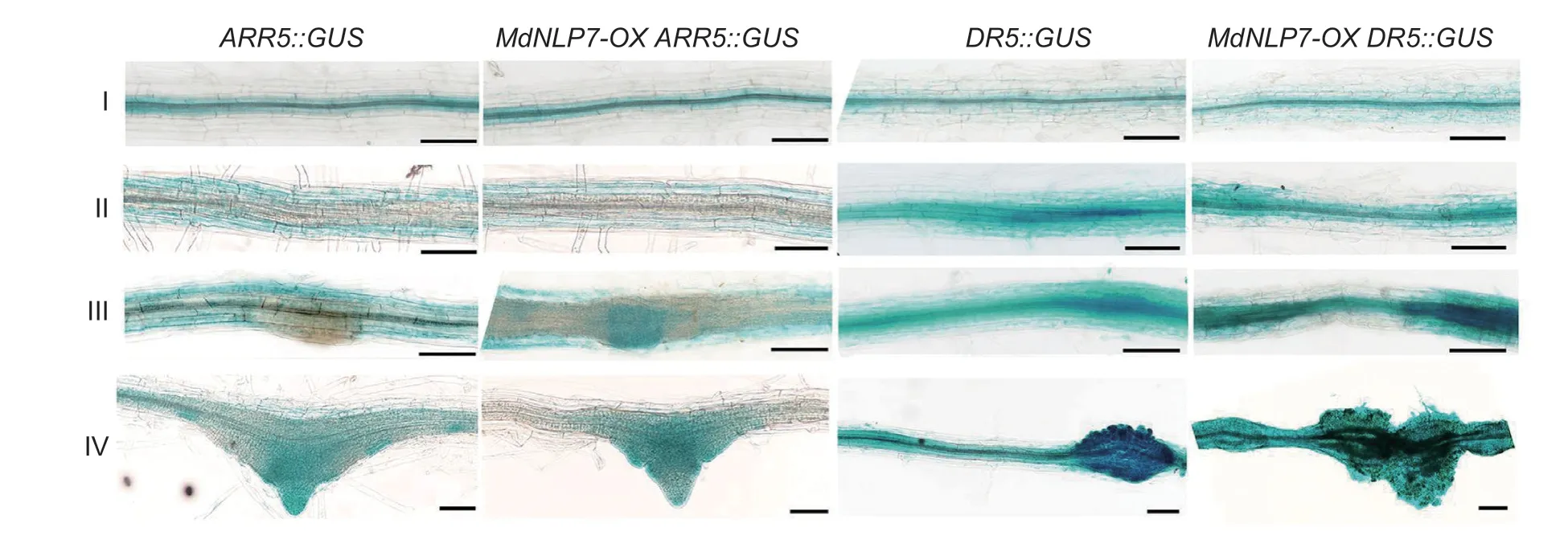
Fig.3 Effect of MdNLP7-OX on the distribution of cytokinin and auxin during callus formation. Callus formation after cultivated on callus-inducing medium (CIM) for several days from the root explants of ARR5::GUS,MdNLP7-OX ARR5::GUS,DR5::GUS,and MdNLP7-OX DR5::GUS. GUS staining analysis of expression pattern with the proARR5::GUS and proDR5::GUS reporter gene in MdNLP7-OX. The four pictures show the four different stages of callus formation and are represented as I–IV. Scale bars,100 µm.
In addition,fourMdNLP7transgenic apple calli were obtained,with higher transcript levels ofMdNLP7inMdNLP7-OX-L3/MdNLP7-OX-L4thanMdNLP7-OX-L1/MdNLP7-OX-L2(Appendix C-b). After the apple callus on optimal medium MS+2,4-D+6-BA was incubated for 21 d,it was found thatMdNLP7-OX-L1/MdNLP7-OX-L2grew faster than WT,whileMdNLP7-OX-L3/MdNLP7-OX-L4grew much slower and smaller compared with WT(Appendix C-a–c),which had the negative correlation with the transcript levels ofMdNLP7. To further investigate the regulatory mechanism,2,4-D or 6-BA was added separately to the MS medium,and it was found that the growth trend of MS+2,4-D treatment was consistent with that of MS+2,4-D+6-BA,while the MS+6-BA treatment showed a growth trend consistent with the MS. This phenomenon revealed that the mechanism ofMdNLP7response to cytokinin or auxin during callus formation is different (Appendix C-a–c). Generally,auxin promotes plant growth at low concentrations and inhibits growth at high concentrations (Yuanet al.2005).Interestingly,MdNLP7-OX-L1/MdNLP7-OX-L2,with lower expression ofMdNLP7in the apple callus,exhibited a higher growth rate thanMdNLP7-OX-L3/MdNLP7-OX-L4,which had higher expression ofMdNLP7,suggesting that the auxin regulated byMdNLP7was vital for callus formation. To further confirm the hypothesis,WT,MdNLP7-OX-L2,andMdNLP7-OX-L3apple calli were selected and cultured on MS+2,4-D+6-BA for 21 d.An ultra-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) approach was used to quantify the levels of endogenous auxin and cytokinin.By comparison,the levels of auxin and cytokinin were increased inMdNLP7-OXcompared with those in WT(Appendix C-f). Meanwhile,the increase of total auxin levels was significantly higher than cytokinin levels in the 21-d-old apple callus ofMdNLP7-OXcompared with WT (Appendix C-g–h). In summary,the results demonstrated thatMdNLP7increased the distribution and accumulation of auxin during callus formation both inArabidopsisand apple.
3.5.MdNLP7 promotes callus formation
CIM induces root explants to form callus by a high auxin/cytokinin ratio and initiates callus developmentviathe activation of pericycle cell division (Attaet al.2009;Sugimotoet al.2010). Then,the root explants ofMdNLP7-OXArabidopsiscultured in CIM were used to detect the callus-forming ability. As expected,in the early stages of callus formation,cell cluster formation in the pericycle cells ofMdNLP7-OXwas faster and larger than Col (Fig.4-A). After 20 d of cultivation in CIM,macroscopic calli formed in all types of root explants,andMdNLP7-OXgenerated much more calli than the Col(Fig.4-B and C). These results confirm the positive effect ofMdNLP7in the callus induction phase of root explants.In apple calli cultured in the hormone-free MS medium,MdNLP7overexpression helped maintain callus growth to some extent compared with WT,probably becauseMdNLP7maintained the biosynthesis and accumulation of endogenous auxin (Appendix C-a–c).
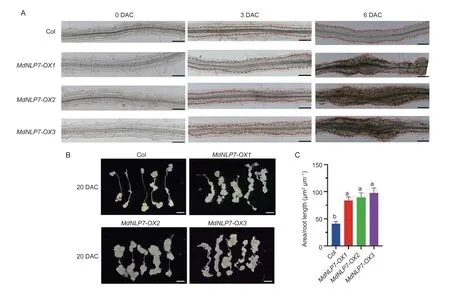
Fig.4 The influence of MdNLP7 on callus formation from root explants. A,callus formation after cultivated on callus-inducing medium (CIM) for 0,3,and 6 d (0,3,and 6 DAC) from root explants of the Col,MdNLP7-OX1,MdNLP7-OX2,and MdNLP7-OX3.Scale bars,100 µm. B,callus formation after cultivated on CIM for 20 d (20 DAC) from root explants of the Col,MdNLP7-OX1,MdNLP7-OX2,and MdNLP7-OX3. Scale bars,2 mm. C,the area of callus formed in root explants after cultivated on CIM for 20 d.Data are presented as mean±SD (n=20). Different letters indicate significant differences at P<0.05 determined by one-way ANOVA with Tukey’s multiple comparison test.
3.6.MdNLP7 promotes the transcript levels of genes related to auxin biosynthesis and transportation in callus
Studies have shown that fourPINand threeYUCgenes are differentially expressed during callus formation in barley,suggesting thatPINs andYUCs are necessary during auxin-induced callus formation (Suoet al.2021). Similarly,screening the result of single-cell RNA sequencing (RNA-seq) in callus from root explants shows that transcript levels of sixPINand fiveYUCgenes were increased during callus formation (Appendix B-a;Zhai and Xu 2021). Then,quantitative real-time PCR (qRTPCR) analysis was performed to explore whether theYUCandPINgenes were regulated byMdNLP7during callus formation. The result showed that transcript levels of most of these genes were increased (Fig.5). Similarly,MdNLP7-OXtransgenic apple callus exhibited a very similar regulation pattern (Appendix C-d and e). These results indicated thatMdNLP7regulated the expression of genes related to auxin biosynthesis and transportation during auxin-induced callus formation.
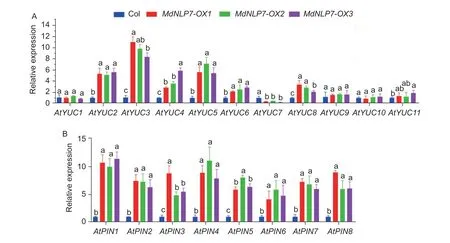
Fig.5 MdNLP7 regulates genes related to auxin biosynthesis and transportation in Arabidopsis. The relative expression levels of YUC family members (A) and PIN family members (B) in callus. Callus formation after cultivated on callus-inducing medium(CIM) for 7 d from root explants of the Col,MdNLP7-OX1,MdNLP7-OX2,and MdNLP7-OX3. AtActin was used as a reference gene. Data are presented as mean±SD (n=20). Different letters indicate significant differences at P<0.05 determined by one-way ANOVA with Tukey’s multiple comparison test.
3.7.MdNLP7 promotes nitrate-modulated regeneration
Nitrogen is an important factor for efficient shoot regeneration (Reinertet al.1967). Recent studies have shown that the nitrate-responsive factor NLP7 protein acts as the primary nitrate sensor (Liuet al.2022).MdNLP7was involved in nitrate uptake and transport in apples (Fenget al.2022). It is important to investigate whetherMdNLP7participated in the nitrate-mediated regeneration process.To test this,we cultivated root explants in CIM and SIM with different concentrations of nitrate to observe the early initiation stages of callus induction,assess callus growth status,and observe statistical shoot regeneration capacity.The results showed that root explants regenerated better under higher nitrate concentrations than those with lower nitrate concentrations,both at the callus induction stage and the shoots induction stage (Fig.6),indicating that the high nitrate with the appropriate concentrations promoted regeneration. AfterMdNLP7-OXwas cultivated in CIM containing high NO3-for 20 d,it generated twice the number of calli generated by Col. Consistently,the frequency of shoots was also significantly higher after 35 d of cultivation in SIM. Thus,the positive effect ofMdNLP7-OXon the regeneration process of root explants was much stronger at higher nitrate (5 mmol L-1NO3-) concentrations in both the callus induction stage and the new shoot induction stage (Fig.6). These results indicated a positive effect ofMdNLP7on the regeneration process of root explants in a nitrate-dependent pathway.

Fig.6 The influence of MdNLP7 on regeneration under different nitrate conditions. A,callus formation after cultivated on callusinducing medium (CIM) with low nitrogen (LN) or high nitrogen (HN) for 0,3,and 6 d (0,3,and 6 DAC) from root explants of the Col,MdNLP7-OX1,MdNLP7-OX2,and MdNLP7-OX3. Scale bars,100 µm. B,callus formation after incubation on CIM with LN or HN for 20 d (20 DAC) from root explants of the Col,MdNLP7-OX1,MdNLP7-OX2,and MdNLP7-OX3. Scale bars,2 mm. C,shoot regeneration from root explants of the Col,MdNLP7-OX1,MdNLP7-OX2,and MdNLP7-OX3. Use two-step tissue culture technique: callus formation after incubation on CIM with LN or HN for 7 d (7 DAC) from root explants,then shoot regeneration after incubation on shoot-inducing medium (SIM) with LN or HN for 35 d (35 DAS). Scale bars,2 mm. D,the area of callus formed in root explants after incubation on CIM with LN or HN for 20 d. E,the frequency of shoot regeneration formed in root explants after CIM with LN or HN for 7 d and then SIM with LN or HN for 35 d. Data are presented as mean±SD (n=20). Different letters indicate significant differences at P<0.05 determined by one-way ANOVA with Tukey’s multiple comparison test.
4.Discussion
Plants have a high capacity to regenerate tissue and organs naturally. The regeneration of calli or shoots of plantsinvitromay be mechanistically distinct. Research on plant biotechnology and plant biology relies heavily oninvitroshoot organogenesis and plant regeneration (Yildiz 2012). In this study,the involvement of theMdNLP7gene in regulating plant regeneration was investigated,and the results showed thatMdNLP7played a positive role in confining pericycle competence for callus formation and mainly affected the biosynthesis and distribution of auxin in this process.
4.1.MdNLP7 confers pericycle cells competence for callus formation
In the acquisition of pluripotency by somatic cells,callus formation from the pericycle cell represents a typical cell fate change (Benková and Ivanchenko 2009;Petrásek and Friml 2009). In addition to forming calli,the division of the pericycle cell plays a role in forming LR primordia(Dubrovskyet al.2001). Similar to LR initiation,this process is genetically controlled,and the derived callus is abundantly expressed with key root meristem regulators (Cheet al.2007;Attaet al.2009;Sugimotoet al.2010). The identified regulators of callus formation,such as lateral organ boundary domains,auxin response factors,aberrant root formation protein 4 (ALF4),and v-myb avian myeloblastosis viral oncogene homolog 94/96 (MYB94/96),were mainly involved in LR initiation or auxin signaling (Okushimaet al.2007;Leeet al.2009;Sugimotoet al.2010;Daiet al.2020). In our study,we observed the specific spatiotemporal expression ofMdNLP7during the division ofArabidopsisroot explants from pericycle cells to produce callus (Appendix B). InArabidopsisroot explants overexpressingMdNLP7led to a significant increase in callus formation (Fig.4). Studies have found thatMdNLP7overexpression inArabidopsishad the phenotype of partial restoration of LR growth (Zhanget al.2021). Thus,the increased pericycle division ofMdNLP7-OXappears to be consistent with its LR phenotype.Meanwhile,at the initial stage of root explants cultivated in CIM,the initiation and growth ofMdNLP7-OXcallus were dramatically increased. Hence,this result suggested thatMdNLP7probably played a role in the induction of callus formation during early pericycle cell division.
The apple callus was cultured in the MS medium without any hormones to simulate the callus under hormonal stress (Appendix C-a). It was observed that the transgenic callus ofMdNLP7-OXcould maintain the growth of apple callus to some extent compared with WT(Appendix C-a),and the growth trend of the transgenic callus was consistent with the trend ofMdNLP7gene expressioninvivo(Appendix C-b and c). After the callus was cultivated in the optimal medium (MS+2,4-D+6-BA),a negative feedback relationship occurred between the regulatory effect ofMdNLP7on the apple callus and its gene expression in the presence of auxin and cytokinin.
Thus,MdNLP7might endow pericycle cells with callus formation ability inArabidopsisroot explants by participating in the induction of early peripheral cell division. There may be a negative feedback regulation between the regulatory ability ofMdNLP7on apple callus and its gene expression.
4.2.The regenerative ability of MdNLP7 is related to auxin accumulation
Generally,auxin triggers the initiation of root explants’pericycle cell callus cultivated in CIM (Attaet al.2009).Auxin is a multifunctional hormone in plants and has been proposed to be a morphogen in meristem formation and embryogenesis. This study found thatMdNLP7significantly increased auxin content in apple callus,thereby regulating callus formation (Appendix C). Moreover,MdNLP7is involved in callus formation inArabidopsisroot explants(Figs.3 and 4;Appendix B). Consequently,MdNLP7promoted auxin accumulation,and its ability to induce regeneration might depend on the concentration of auxin.Studies have demonstrated that by binding directly to theAtTAR2promoter,AtNLP7maintains auxin signaling in LR primordia (Zhanget al.2021). Studies have also found thatAtPIN7was activated byAtNLP7-initiated auxin efflux (Frimlet al.2003;Overvoordeet al.2010). Therefore,this paper is consistent with the findings of previous studies that have shown the involvement ofAtNLP7in the auxin pathway.
YUC,anArabidopsisgene family,encodes flavin monooxygenase-like enzymes that catalyze the ratelimiting step of tryptophan-dependent auxin synthesis(Zhaoet al.2001). According to a previous study,there is a genetic pathway common todenovoroot organogenesis and callus formation (Liuet al.2014). TheYUCgenes participate in multiple signaling pathways during thedenovoorganogenesis of roots from leaf explants (Chenet al.2016). The function of PIN proteins as polar auxin transporters that establish gradients of IAA concentration in plants (Friml and Palme 2001). PIN1/4/6/7 are important for auxin distribution and patterning of thedenovomeristems (Benkováet al.2003;Gordonet al.2007;Su and Zhang 2009;Bohn-Courseau 2010;Friml 2010;Overvoordeet al.2010;Tsugekiet al.2010;Yadavet al.2010;Zhanget al.2010).Arabidopsisregulates the establishment of an auxin gradient within the callusviaPIN1 (Gordonet al.2007;Pernisovaet al.2009;Su and Zhang 2009). In this study,during callus formation inArabidopsisroot explants,MdNLP7caused an increase in the transcription levels of most of the genes related to auxin biosynthesis and transport (Fig.3),indicating thatMdNLP7regulated the expression of auxin biosynthesis and transport-related genes during callus formation. In the apple callus,the expression increase trends forMdPIN1candMdYUCCA4awere consistent with the gene expression trend ofMdNLP7between different callus lines(Appendix C-d and e),which further proves the function ofNLP7in regulating auxin biosynthesis and accumulation.
4.3.MdNLP7 may regulate nitrate-modulated regeneration
Nitrate is an essential nutrient and signaling molecule for plant growth (Wanget al.2012) andAtNLP7acts as a nitrate sensor in plants (Liuet al.2022). In this study,it was found thatMdNLP7-OXhad a positive effect on the regeneration process of root explants,andMdNLP7may promote nitrate-modulated root explants regeneration(Fig.6). Under NO3-conditions,nitrate signaling pathways have a specific stimulatory effect on the growth of LRs (Zhanget al.1999). These pathways involve molecular complexes that regulate several stages of LR development,as well as auxin biosynthesis and transport.
According to our results,we propose that nitratemodulated auxin distribution plays a crucial role in callus formation duringdenovoSAM formation. Our results also suggest thatMdNLP7plays an important role in callus formation and growth in bothArabidopsisand apple.MdNLP7may affect callus development at an early stage and endow root explants with callus formation ability by regulating auxin accumulation and distribution. Future studies are required to unravel the precise mechanism by whichMdNLP7regulates nitrate-and auxin-modulated callus formation and shoot regeneration.
5.Conclusion
In this study,ectopic expression ofMdNLP7could regulate the regeneration process from root explants ofArabidopsis. Further research results indicate thatMdNLP7mediates the process of callus formation that began with cell division in the pericycle cell. During the process of callus formation,MdNLP7can upregulate the expression of genes related to auxin synthesis and transport,and regulate the formation of root explants by affecting the distribution of auxin. Moreover,the results demonstrated thatMdNLP7may play a role in the nitratemodulated regeneration of root explants.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31972378),the Shandong Province Key R&D Program,China (2021CXGC010802),and the China Agriculture Research System of MOF and MARA(CARS-27). We sincerely thank our team leader Dr.Hao Yujin,who will be remembered for his great achievement and for the support in our work. We also thank Li Xingguo and Duan Qiaohong of Shandong Agricultural University for providing special experimental equipment.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on https://doi.org/10.1016/j.jia.2023.08.007
 Journal of Integrative Agriculture2023年10期
Journal of Integrative Agriculture2023年10期
- Journal of Integrative Agriculture的其它文章
- The association between the risk of diabetes and white rice consumption in China: Existing knowledge and new research directions from the crop perspective
- Linking atmospheric emission and deposition to accumulation of soil cadmium in the Middle-Lower Yangtze Plain,China
- Genome-wide association study for numbers of vertebrae in Dezhou donkey population reveals new candidate genes
- Are vulnerable farmers more easily influenced? Heterogeneous effects of lnternet use on the adoption of integrated pest management
- lnfluences of large-scale farming on carbon emissions from cropping:Evidence from China
- Spatio-temporal variations in trends of vegetation and drought changes in relation to climate variability from 1982 to 2019 based on remote sensing data from East Asia
