Cadmium (Cd) exposure through Hyphantria cunea pupae reduces the parasitic fitness of Chouioia cunea: A potential risk to its biocontrol efficiency
YAN Shan-chun ,WU Hong-fei ,ZHENG Lin ,TAN Ming-tao,JlANG Dun#
1 School of Forestry,Northeast Forestry University,Harbin 150040,P.R.China
2 Key Laboratory of Sustainable Forest Ecosystem Management,Ministry of Education,Northeast Forestry University,Harbin 150040,P.R.China
Abstract Heavy metal contamination has been regarded as an environmental variable that affects the efficiency of pest biological control,but the parasitic fitness of parasitoids under heavy metal stress is poorly understood. Herein,the effect of Cd exposure through the host pupa of Hyphantria cunea on the parasitic fitness of Chouioia cunea was investigated,and the mechanism by which Cd exposure affects the interaction between H.cunea and C.cunea from the perspective of innate immunity in host insect and the oxidative status in the parasitoid offspring was explored. Our results indicated that Cd can be transferred from the H.cunea pupae to the parasitoid offspring,and the transfer coefficient reflected biological amplification. There were no significant differences in the rates of parasitism success and offspring emergence between the untreated and Cd-treated groups. However,after parasitizing Cd-accumulated pupae,the parasitic fitness of offspring wasps (e.g.,the number,individual size and life span) decreased significantly. Under Cd exposure,the cellular and humoral immunity of H.cunea pupae decreased significantly. Compared with the untreated group,the H2O2 content of parasitoid offspring in the Cd-treated group was significantly increased. Cd exposure significantly inhibited superoxide dismutase activity in parasitoid offspring,but the contents of ascorbic acid and glutathione were significantly increased by Cd stress. Taken together,these results indicate that Cd exposure reduces the cyclic utilization efficiency of C.cunea on H.cunea pupae. The oxidative status of parasitoid offspring triggered by Cd exposure could be responsible for the reduced parasitic fitness of C.cunea on Cd-accumulated H.cunea pupae.
Keywords: heavy metal,Hyphantria cunea,parasitic fitness,Chouioia cunea,oxidative status,innate immunity
1.Introduction
Heavy metal contamination is a severe environmental problem that has aroused widespread global concern(Hanet al.2022). The emission of heavy metals into the environment occurs through various agricultural and industrial activities,such as sewage irrigation,chemical fuel combustion,and mining (Qinet al.2021). Heavy metals pose a serious threat to aquatic and terrestrial ecosystems because of their hysteresis,irreversibility,accumulation,and toxicity (Maoet al.2021;Zhang H Let al.2021). The five common pollutants in heavy metal contamination are Hg,Pb,Cd,Cr,and metalloid As(Rahman and Singh 2019). Among these,Cd is the most wide-spread pollution metal in China,with a total overstandard rate as high as 7.0% (MEP and MLR 2014). Cd itself does not directly generate free radicals,but it can cause the overproduction of reactive oxygen species by some indirect mechanisms,such as the replacement of iron and copper in various cytoplasmic and membrane proteins,the depletion of antioxidants and the inhibition of antioxidant enzymes (Valkoet al.2005). In recent decades,many studies have shown the ecotoxicological effects of Cd contamination,and have established Cd as a non-essential element that can be toxic to living organisms even at low concentrations (Cuiet al.2022;Kibriaet al.2022). It is worth mentioning that heavy metals (including Cd) enriched in the soil can be transported along the root system to the aboveground parts of plants (Chen Jet al.2021;Nogueiraet al.2022). This transfer ability distinguishes heavy metal contamination from organic contamination,and extends the toxicity of heavy metals into the food chain (Shiet al.2020).
As the link between the primary producers of the food chain and higher-order consumers,herbivorous insects have a higher risk of exposure to heavy metals (Daret al.2019;Siet al.2021). Studies have demonstrated that herbivorous insects can transfer accumulated heavy metals to higher trophic levels (e.g.,insect natural enemies) (Wanget al.2017;Shiet al.2020;Naikooet al.2021a). Studies have also shown that exposure to heavy metals can cause chronic bio-toxic effects on the growth and survival of both herbivorous insects and insect natural enemies (Shiet al.2020;Naikooet al.2021b). Natural enemies are economically important for the biological control of herbivorous insects,but toxic metals can put natural enemies at risk (Sanget al.2018). In a food chain that is under heavy metal stress,the transfer potential of heavy metal toxicity may affect the interactions between herbivorous insects and insect natural enemies and introduce a new environmental variable into the biocontrol efficiency of herbivorous insects. Considering the serious implications of heavy metal contamination and the importance of biological control in pest management (Hajeket al.2020;Maoet al.2021),there is an urgent need to explore the effects of heavy metals on insect natural enemies. Several recent studies have investigated the adverse effects of heavy metals on insect natural enemies through the food chain,but they focus on food chains composed of plants,herbivorous insects,and insect predators (Sanget al.2018;Naikooet al.2021b).The interactions between herbivorous insects and their parasitic wasps under heavy metal exposure have not been widely investigated. In addition,the regulatory mechanism by which heavy metal exposure affects the interactions between herbivorous insects and their natural enemies is also poorly understood.
Unlike vertebrates,insects lack acquired immunity but develop innate immunity,including cellular immunity and humoral immunity,to defend against foreign invasion(Bitencourtet al.2021). The immune response mediated by hemocytes is called cellular immunity,which includes phagocytosis,melanization,and encapsulation (Wojda 2017). Humoral immunity induces the production of antimicrobial peptides through the IMD,Toll,or JAK/STAT signaling pathways to kill invading organisms(Ozakman and Eleftherianos 2020). The interaction between host insects and insect natural enemies is known to be inseparable from the innate immunity of the host insects (Yanget al.2020,2021). However,several studies have shown that heavy metal exposure triggers immunotoxic effects on the cellular and humoral immunity of herbivorous insects (Wu and Yi 2015;Wuet al.2022). Therefore,heavy metals may indirectly affect the interactions between insect pests (host) and their parasites by modulating the innate immunity of the host insects. In addition,the direct adverse effects of heavy metals accumulated by herbivorous insects on their insect natural enemies should also be noted (Gardiner and Harwood 2017;Duet al.2019). Oxidative status is the most direct manifestation of this adverse effect (Gardiner and Harwood 2017). According to the trade-off effect of resource allocation,insect natural enemies under heavy metal stress may mainly use their energy for combating reactive oxygen species (ROS) and its effects,rather than for growth (Monaghanet al.2009;Metcalfe and Alonso-Alvarez 2010). This will reduce the fitness of the insect natural enemies and may be another mechanism by which heavy metal exposure affects the interaction between host insects and insect natural enemies.
Hyphantriacunea(Drury) is a quarantine pest worldwide that is characterized by a high reproductive capacity,long damage cycle,and strong adaptability to the environment (Edosaet al.2019).Hyphantriacuneaoccurs mainly in street or green trees along highways and railways. These areas are also the main pollution sites of heavy metals (Fanget al.2021),soH.cuneahas a high risk of heavy metal exposure.ChouioiacuneaYang is a pupal parasitic wasp of lepidopteran pests,which has a high parasitism rate,strong fecundity and female-biased progeny production (Yanget al.2006). Biological control using the parasitic waspC.cuneais an important strategy in controlling the spread ofH.cunea(Xinet al.2017).
In this study,we investigated the effect of Cd exposure through contaminated pupae ofH.cuneaon the parasitic fitness ofC.cunea. Furthermore,we also explored the mechanism by which Cd exposure affects the interaction betweenH.cuneaandC.cuneafrom the perspectives of innate immunity in the host insect and oxidative status in the parasitoid offspring. Our findings may clarify the potential impacts of Cd exposure on the efficiency of biological control using parasitic wasps as a carrier,and provide the theoretical basis for optimizing pest control strategies in heavy metal-polluted areas.
2.Materials and methods
2.1.lnsect rearing
Egg masses ofH.cuneaand their artificial diet were purchased from the Chinese Academy of Forestry (Beijing,China). The eggs were sterilized with 10% formaldehyde solution and then incubated in a thermostatic incubator under a 14 h L:10 h D photoperiod,25°C temperature,and 60% relative humidity. All the newly hatched larvae were fed an artificial diet and cultured up to the 3rd instar. The newly molted 3rd instar larvae were divided into two groups and fed either Cd-treated or untreated artificial diets until pupation. There were 200 larvae in in each Cd-treated group and untreated group. The treatment concentration of Cd (46.515 mg kg–1) was referenced to a body weight inhibitory concentration of 40% in the larval stage,as determined in our preliminary experiment. Briefly,artificial diets containing six different concentrations of Cd (0,5,15,30,60 and 120 mg kg–1)were prepared. The 3rd instar larvae ofH.cuneawere divided into six groups,with each group fed one of the six diets from the 3rd to the 6th instar stage. Larval body weight was recorded (Appendix A) and a 40% body weight inhibitory concentration (46.515 mg kg–1;95% confidence interval: 21.596–134.35 mg kg–1) was obtained by Probit regression analysis.
2.2.Evaluation of parasitic fitness
The parasitic waspC.cuneaused in this experiment was purchased from Keyun Biological Company (Beijing,China). Two newly emerged wasps were transplanted into a glass finger-shaped tube containing an early stage pupa (pupation for 1 day) ofH.cunea. After 48 h,when parasitism was established,the parasitic wasps were removed. The pupae ofH.cuneacontinued to be cultured in the incubator until either the emergence of the parasitoid offspring or the emergence ofH.cuneaadults.During the experiment,the life history parameters of parasitic wasps were recorded and calculated,including the rates of parasitism success and offspring emergence,the number of parasitoid offspring,the duration of embryonic development,as well as the individual size measurements (body weight,body length,abdomen length,thorax width and head width) and life span of the parasitoid offspring. Among these,the rates of parasitism success and offspring emergence were set to four replications,with 25 pupae in each. The number of parasitoid offspring was set to 20 replications,with one pupa per replicate. The other parameters were set at 50 replications,each with one parasitoid offspring.
2.3.Determination of Cd accumulation in H.cunea pupae and C.cunea
Hyphantriacuneapupae that pupated within 24 h and parasitoid offspring that emerged within 12 h were collected for Cd determination. Each group had four replicates,and each replicate consisted of 10 pupae or 1 000 parasitoid offspring. Subsequently,all samples were dried at 75°C,weighed,digested with a mixture of HNO3-HClO4(at 5:1;v/v) in a Graphite-digestion device,and analyzed by inductively-coupled plasma mass spectrometry (NexION 2000,PerkinElmer,USA).
2.4.Determination of oxidative status-related parameters in parasitoid offspring
Parasitoid offspring that emerged within 12 h were collected. This experiment included four replicates in the Cd-treated or untreated groups,each consisting of 400 parasitoid offspring. Using a mortar,the collected offspring were homogenized in pre-cooled saline and centrifuged at 3 000×g for 10 min (at 4°C). The supernatant was collected,and the activities or levels of superoxide dismutase (SOD),peroxidase (POD),ascorbic acid (ASA),glutathione (GSH) and hydrogen peroxide (H2O2) were detected using the corresponding kits (Nanjing Jiancheng Biology Co.,Ltd.,China). Briefly,SOD activity was determined using the xanthine oxidase method at 550 nm. The enzyme-activity unit (U) was defined as the amount of enzyme causing 50% inhibition in each mL of the reaction solution,and SOD activity was expressed as U mg–1protein. Based on the principle of catalyzing the hydrogen peroxide reaction by POD,the POD activity was obtained by measuring the change in absorbance at 420 nm,and was also expressed as U mg–1protein. In the ASA assay protocol,Fe3+reacts rapidly with ASA to produce Fe2+,which is then reacted with 4,7-diphenyl-1,10-phenanthroline in a chromogenic reaction. The absorbance of the reaction system was assayed spectrophotometrically at 536 nm,and the content of ASA was expressed as µg mg–1tissue weight with reference to the corresponding standard curve. In the GSH assay,the GSH in the supernatant is reacted with dithiodinitrobenzoic acid to form a yellow compound.The absorbance was assayed spectrophotometrically at 420 nm,and the content of GSH was expressed as mg g–1tissue weight with reference to the corresponding standard curve. H2O2was reacted with ammonium molybdate solution to form a complex,and the amount of H2O2was calculated by measuring the absorbance at 405 nm. The content of H2O2was expressed as mmol g–1tissue weight with reference to the corresponding standard curve. The protein content in samples was measured using Coomassie blue staining,and bovine serum albumin was used as the standard.
2.5.Humoral immunoassay in H. cunea pupae
Hyphantriacuneapupae that pupated within 24 h were collected. This experiment included four replicates in the Cd-treated or untreated groups,each consisting of five pupae. With the help of the RNAeasy™ Animal RNA Isolation Kit with Spin Column (Beyotime Biotechnology,China),the total RNA of the samples was extracted.After checking for completeness and purity,the RNA was reverse transcribed into cDNA using the PrimeScript™ RT reagent Kit (with gDNA Eraser) (TaKaRa,Dalian,China).Then,the levels of immunorecognition genes (PGRP-LC,PGRP-LD,PGRP-SA-1,PGRP-SA-2,GNBP1,GNBP3-1,andGNBP3-2),signal transduction genes (Relish,Spz-1,Spz-2,Tak,Toll,Tube,IMD,Myd88,DreddandDI/DIF)and antimicrobial peptide genes (Attacin-1,Attacin-2,Cecropin,Lebocin,Moricin,Gloverin-1,andGloverin-2) of the Toll and Imd signaling pathways were detected by RTPCR,as previously described (Wuet al.2022). Briefly,each RT-PCR reaction mixture of 20 µL contained 10 µL of KOD SYBR®qPCR Mix (Toyobo Co.,Ltd.,Osaka,Japan),0.4 µL of each primer,1 µL of cDNA,and 8.2 µL of ddH2O. The amplification program was run at 98°C for 2 min,followed by 40 cycles of denaturation at 98°C for 10 s,annealing at 50°C for 10 s,and elongation at 68°C for 30 s. Gene expression levels were normalized to the levels of theRPS16gene,and the relative expression levels for all target genes were determined using the 2–∆∆Ctmethod. All gene primer sequences are presented in Appendix A.
2.6.Cellular immunity in H. cunea pupae
Hyphantriacuneapupae that pupated within 24 h were dissected on ice,and hemolymph was collected to determine the cellular immune parameters,including the total hemocyte count,melanization,and phagocytic activity. This experiment included four replicates in the Cd-treated or untreated groups,each consisting of the hemolymph from six pupae. To determine the total hemocyte count,the hemolymph was diluted twice with a pre-cooled anticoagulant solution (supersaturated PTURinger’s saline),and hemocytes were counted under an electron microscope using a hemocytometer. For the melanization analysis,a 2 µL aliquot of newly extracted hemolymph was dropped onto a white plastic sheet and the degree of melanization was observed after 20 min.Fluorescein isothiocyanate (FITC)-labeledEscherichia coliwas established to determine the phagocytic activity,and the number of hemocytes that phagocytosed the FITC-labeledEscherichcoliwas counted using a fluorescence microscope. Phagocytic activity was defined as the ratio of the number of fluorescent hemocytes to the total hemocytes (Wuet al.2022). In addition,the expression levels of seven genes related to phagocytosis (DSCAM,Hemolin-1,andHemolin-2)and melanization (Galectin-1,Galectin-2,PPO-1,andPPO-2) inH.cuneapupae were determined by RT-PCR,as described above. All gene primer sequences are presented in Appendix A.
2.7.Statistical analysis
For percentage data (the rate of parasitism success and the rate of offspring emergence),square root transformation was performed to ensure data normality.All the parameters were compared between the Cdtreated and untreated groups by an independent samplet-test atα=0.05. The principal component analysis (PCA),the values of variable importance in projection (VIP),Pearson’s correlation coefficients and TOPSIS analysis were calculated or performedviathe Spssau website(http://www.spssau.com).
3.Results
3.1.Cd accumulation in H.cunea pupae and C.cunea
Inductively-coupled plasma mass spectrometry revealed that Cd can be transferred to theH.cuneapupae through the artificial diet. The Cd content of pupae in the Cd treatment group was the same as the treatment concentration in the artificial diet,while the control group showed no Cd accumulation (Appendix A).In contrast,the Cd content of offspring wasps increased significantly after parasitizing the Cd-accumulated pupae,and the transfer coefficient of Cd between pupae and offspring wasps represented biological amplification (Appendix A).
3.2.Parasitical fitness of C.cunea
To intuitively determine whether Cd exposure affects the interaction between host insects and insect parasitoids,the parasitic fitness ofC.cuneaon Cd-accumulated or untreatedH.cuneapupae was investigated (Table 1).The rates of parasitism success and offspring emergence were over 87% in both the Cd-accumulated and untreated pupae,and were not significantly different between the two environments for parasitism. However,after parasitizing Cd-accumulated pupae,the number of parasitoid offspring decreased significantly,the duration of embryonic development was significantly prolonged,the individual size (body weight,head width,thorax width,body length,and abdomen length) of parasitoid offspring decreased significantly,and the life span of offspring wasps decreased significantly.
3.3.Oxidative status evaluation in parasitoid offspring
To analyze the mechanism of offspring dysplasia,any change in the oxidative status induced by Cd exposure was systematically analyzed in the parasitoid offspring by measuring the reactive oxygen species and antioxidantdefense levels. Compared with the control,the H2O2content of parasitoid offspring in the Cd-treated group was significantly increased (Fig.1-A). Although there was no significant difference in POD activity between the treatment and control groups,Cd exposure significantly inhibited SOD activity in the parasitoid offspring (Fig.1-B and C). The levels of non-enzymatic antioxidants varied consistently in response to Cd exposure,and the ASA and GSH levels in parasitoid offspring were significantly increased by Cd stress (Fig.1-D and E).
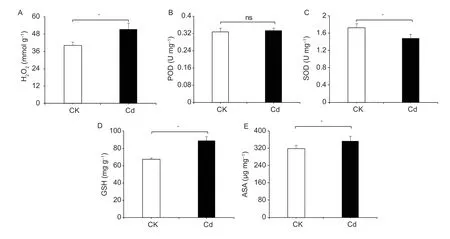
Fig.1 Effects of Cd exposure on the contents or activities of H2O2 (A),peroxidase (POD) (B),superoxide dismutase (SOD) (C),glutathione (GSH) (D) and ascorbic acid (ASA) (E) in parasitoid offspring. CK,untreated group;Cd,Cd-treated group. Data are expressed as mean±standard deviation (n=4). *,P<0.05;ns,no significance.
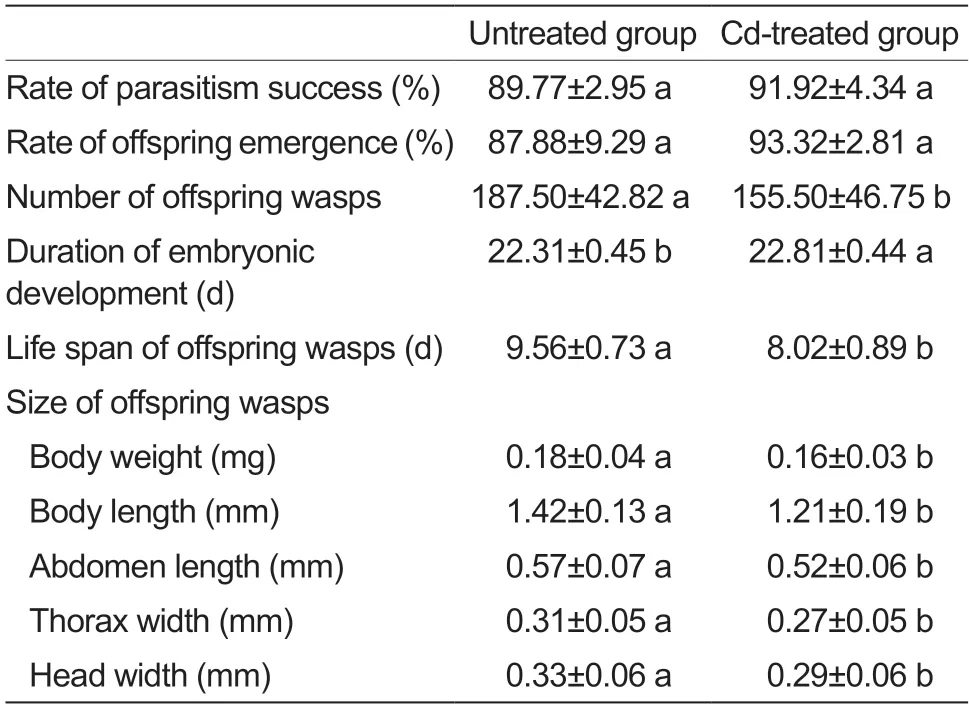
Table 1 The parasitic fitness of Chouioia cunea to Cdaccumulated Hyphantria cunea pupa
3.4.Cellular immunity in H.cunea pupae
For evaluating the defensive ability of Cd-accumulated pupae againstC.cunea,cellular immunity was analyzed in the pupae,including the total hemocyte count,melanization,and phagocytic activity. Cd exposure significantly reduced the total hemocyte count inH.cuneapupae compared to untreated pupae (Fig.2-A–C).Untreated pupae showed stronger melanization than Cdtreated pupae;and the haemolymph of the Cd-treated pupae still showed light blue after 20 min of exposure to room temperature (Fig.2-G–I). The trend of phagocytic activity was consistent with the total hemocyte count and melanization,and it was significantly reduced in the Cdtreated pupae (Fig.2-D-F). Gene expression analysis revealed that the expression levels of three genes(Galectin-1,PPO-1,andPPO-2) involved in melanization and one gene (Hemolin-2) involved in phagocytosis were significantly inhibited by Cd-treated group compared with the untreated group (Fig.3).
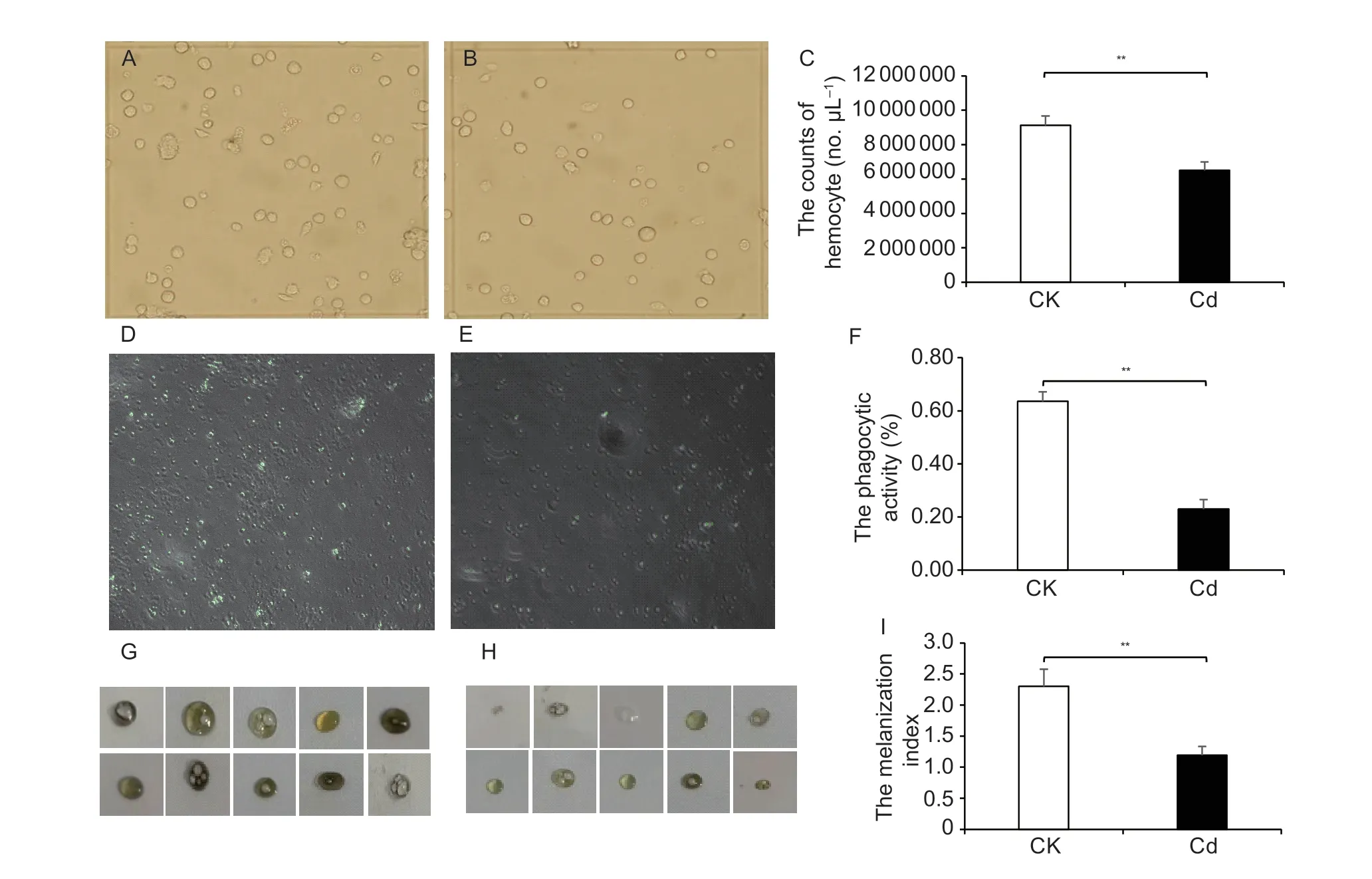
Fig.2 Cellular immunity analysis in Cd-treated Hyphantria cunea pupae. A–C,the count of hemocytes. D–F,phagocytic activity.G–I,melanization activity. CK,untreated group;Cd,Cd-treated group. Data are expressed as mean±standard deviation (n=4).**,P<0.01.
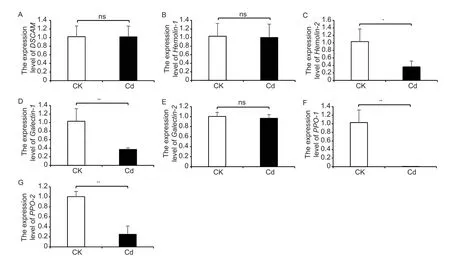
Fig.3 The expression of genes related to phagocytosis (A–C) and melanization(D–G) in Cd-treated Hyphantria cunea pupae. CK,untreated group;Cd,Cdtreated group. Data are expressed as mean±standard deviation (n=4). *,P<0.05;**,P<0.01;ns,no significance.
3.5.Humoral immunity in H.cunea pupae
To analyze the effect of Cd-accumulated pupae on the fitness ofC.cuneafrom the perspective of host insect immunity,the humoral immunity responses ofH.cuneapupae were determined,namely,immunorecognition,signal transduction,and the expression of antimicrobial peptide genes. Of the seven immunorecognition genes analyzed,the levels ofPGRP-LC,PGRP-LD,andGNBP1were not significantly different between the untreated and Cd-treated groups,while those ofPGRP-SA-1,PGRP-SA-2,GNBP3-1,andGNBP3-2were significantly lower in the Cd-treated group than the untreated group(Fig.4-A). The levels of signal transduction genesRelish,Tak,IMD,andDI/DIFwere significantly lower in the Cd-treated group than the untreated group,while the levels of the remaining six genes (Spz-1,Spz-2,Toll,Tube,Myd88,andDredd) were not significantly different between the two groups (Fig.4-B). Of the seven effector genes investigated,the levels ofAttacin-1,Gloverin-2andCecropinwere not significantly different between the two groups,while those of the other four genes (Attacin-2,Lebocin,Moricin,andGloverin-1) were significantly lower in the Cd-treated group than the untreated group(Fig.4-C).

Fig.4 The expression levels of pathogen recognition(A),signal transduction (B),and effector (C) genes in Cd-treated Hyphantria cunea pupae. CK,untreated group;Cd,Cd-treated group. Data are expressed as mean±standard deviation (n=4). *,P<0.05;**,P<0.01;ns,no significance.
3.6.Comprehensive data analysis
To assess the overall trend of innate immunity in CdaccumulatedH.cuneapupae,a TOPSIS analysis of immune-related parameters was conducted (Appendix A).Compared with the control group,the evaluation objects in the Cd-treated group were further away from the optimal scheme. According to the relative proximity between the evaluation object and the optimal scheme,the overallsituation of cellular immunity,humoral immunity and their synthesis inH.cuneapupae under Cd stress was weaker than that of the untreated pupae. To visualize the results of this study,the common data visualization method of PCA analysis was performed. The Cd-treated group was significantly separated from the untreated group,with 88.6 and 4.2% of the variation explained by the first two principal components (PC1 and PC2),respectively(Fig.5-A). Based on the VIP values,which is a way to identify the contributions of parameters to the differences between untreated and Cd-treated groups,the Cd content in pupae made the greatest contribution to the inter-group separation,followed byPPO-1,PPO-2and Cd content in offspring wasps (Fig.5-B). Correlation clustering analysis showed that all parameters affecting the parasitic fitness ofC.cuneawere clustered into two categories(Fig.5-C). Among them,the first category mainly consists of oxidative status and Cd content parameters,and the second category mainly involves immune-related parameters. All the parameters of cellular immunity and humoral immunity that were significantly correlated with parasitic fitness were positively correlated. The Cd content in pupae,Cd content in offspring wasps and H2O2content in offspring wasps were negatively correlated with most of the parasitic fitness parameters (Appendix A).

Fig.5 Bioinformatics and data visualization. A,principal component analysis (PCA) score plots. B,the values of variable importance in projection (VIP). C,heat map of the Pearson’s correlation coefficients. CK,untreated group;Cd,Cd-treated group.
4.Discussion
The transport characteristics of heavy metals along the food chain are the main factor that distinguishes them from other pollutants. Many studies have reported that heavy metals can be translocated along the soil–plant route to primary consumers and then adversely affect their growth and development (Jiang and Yan 2017;Siet al.2021). In contrast,only a few studies have investigated heavy metal accumulation and its impact on secondary consumers,specifically parasitic wasps (Sanget al.2018;Shiet al.2020). In this study,we revealed that Cd exposure toC.cuneathrough the pupa ofH.cuneareduced its parasitic fitness. We also analyzed the mechanisms for the reduction in parasitic fitness from the perspectives of the host insects and the parasitic wasps. To our knowledge,this is the first study to comprehensively evaluate the effects of heavy metal exposure on the interaction between herbivorous insects and their parasitic wasps,and the results may be valuable for guiding pest management decisions in heavy metal polluted regions.
Accumulating evidence suggests that heavy metal pollution can be regarded as an environmental variable that affects the biological control efficiency of pests(Jianget al.2021,2022). In the present study,the rates of parasitism success and offspring emergence were over 87% in both Cd-accumulated and untreated pupae,and not significantly different between the treatment and control groups. These results,together with those of a previous study (Xinet al.2017),highlighted the biocontrol efficiency ofC.cuneaonH.cuneapupae and indicated that the primary control efficiency of the parasitic wasps on host insects was not affected by Cd exposure. In addition to these two parameters,the growth and development of offspring wasps are also important parameters for evaluating the parasitic fitness of parasitoid wasps (Gaoet al.2016). After parasitizingH.cuneaCd-accumulated pupae,the number ofC.cuneaoffspring decreased significantly,suggesting that the secondary control efficiency ofC.cuneaagainst theH.cuneamay be reduced. Moreover,the offspring wasps in the Cd-treated group showed a shorter lifespan,implying that theC.cuneaoffspring were less likely to parasitize new host insects. Furthermore,theC.cuneaoffspring grown in Cd-accumulated pupae also showed prolonged embryonic development and reduced individual size,demonstrating the detrimental effects of Cd exposure on the growth of offspring wasps. These findings suggest that Cd exposure through theH.cuneapupae decreases the parasitic fitness ofC.cunea,which may reduce the cyclic utilization efficiency ofC.cuneaas a biological control agent.
Cd enrichment analysis revealed that the Cd levels in the parasitoid offspring increased significantly after parasitizing Cd-accumulatedH.cuneapupae,indicating that Cd was transferred from theH.cuneapupae toC.cunea. Similar transfers of heavy metal from host insects to insect natural enemies have been reported in several predatory ladybirds,includingCryptolaemus montrouzieri,Coccinellaseptempunctata,andC.transversalis(Daret al.2017;Sanget al.2018;Naikooet al.2021b). The transfer coefficient of Cd betweenH.cuneapupae and parasitoid offspring reflects biological amplification,reinforcing the threat posed by Cd pollution and its subsequent high mobility in the food chain. Insect growth inhibition is the most common consequence of heavy metal toxicity (Jianget al.2020a). The growth retardation of the parasitoid offspring in this study may have been caused by the direct toxic effects of Cd exposure. Therefore,we analyzed the content of H2O2,an important parameter for evaluating the degree of oxidative status (Jianget al.2020b),in parasitoid offspring exposed to Cd. The H2O2content in the parasitoids of the Cdtreated group was significantly increased,indicating the disordering of the ROS or oxidative status in the offspring.Note that the Cd content in pupae and offspring wasps,as well as the H2O2content in offspring wasps,were all negatively correlated with most of the parasitic fitness parameters.
Many studies have reported that insects use an antioxidant defense system to resist the oxidative stress caused by exogenous toxins (e.g.,heavy metals and insecticides),including antioxidant enzymes and nonenzymatic antioxidants (Duet al.2019;Chen Y Zet al.2021). In this study,the activities of antioxidant enzymes in parasitoid offspring exposed to Cd were disturbed,but the response trend to Cd stress was inconsistent.Parasitoid offspring inhabiting Cd-accumulatedH.cuneapupae showed significantly reduced SOD activity and unchanged POD activity. However,the contents of nonenzymatic antioxidants (ASA and GSH) in parasitoid offspring were significantly increased by Cd stress.Combined with the significant increase in H2O2content,these findings suggest that the non-enzymatic antioxidants cannot replace the antioxidant enzymes in alleviating oxidative status. These data reinforce the findings of our previous study (Jianget al.2020b),which indicated that antioxidant enzymes in insects are more vulnerable to the adverse effects of heavy metal exposure compared with the non-enzymatic antioxidants. Antioxidant defense is considered as a likely physiological cost of increased metabolic investment (i.e.,growth,development and reproduction) (Prokićet al.2018;Petrovićet al.2020).Therefore,the alteration of the antioxidant system in parasitoid offspring under Cd stress may consume the energy that would have been used for growth and may be one of the reasons for the decreased parasitic fitness.
Innate immunity,including cellular and humoral immunity,is an important defense mechanism of host insects against parasitic wasp attack (Wanget al.2018).In insects,cellular immunity is based on hemocytes and mainly involves melanization and phagocytosis (Chen and Lu 2018). In the present study,Cd exposure significantly reduced the total hemocyte count inH.cuneapupae,suggesting that Cd exposure disrupts the basis of cellular immunity. Melanization and phagocytic activity showed a similar trend,both of which were significantly reduced in the Cd-accumulated pupae. These results indicate that Cd exposure attenuates the cellular immunity ofH.cuneapupae. Subsequently,the expression of key genes that regulate cellular immunity inH.cuneapupae was assessed. Among the seven genes investigated,three (Galectin-1,PPO-1,andPPO-2) associated with melanization and one (Hemolin-2) associated with phagocytosis were significantly reduced inH.cuneapupae under Cd stress. These results highlight the strong immunotoxic effects of Cd exposure on the cellular immunity ofH.cuneapupae at the molecular level. Humoral immunity involves a cascade mechanism,including recognition,signal transduction,and effector synthesis (Zhang Wet al.2021). The RT-PCR analysis revealed that the expression of pattern recognition receptorsPGRP-SA-1,PGRP-SA-2,GNBP3-1,andGNBP3-2in Cd-accumulated pupae were significantly reduced,indicating that Cd stress interfered with the immune recognition ability ofH.cuneapupae. Cdaccumulated pupae also showed reduced immune signal transduction,as determined by the lower expression levels of transduction genesRelish,Tak,IMD,andDI/DIFof the Toll and Imd signaling pathways under Cd exposure. The level of effector synthesis,such as antimicrobial peptides,determines the degree of humoral immunity defense(Yanget al.2018). We found that among the seven antimicrobial peptide genes investigated,the expression levels ofAttacin-2,Lebocin,Moricin,andGloverin-1were significantly inhibited by Cd treatment. Furthermore,a TOPSIS analysis demonstrated that the overall situation of cellular immunity,humoral immunity and their synthesis inH.cuneapupae under Cd stress was weaker than that of untreated pupae. Therefore,based on the cellular and humoral immunity data,we concluded that under Cd exposure,H.cuneacannot diminish the parasitical fitness ofC.cuneathrough innate immunity. Conversely,a reduced innate immunity in host insects should theoretically promote the parasitic fitness of the parasitoid wasps. These paradoxical findings make it clear that the direct adverse effect of Cd stress on parasitoid offspring is the main reason for their reduced parasitic fitness on CdaccumulatedH.cuneapupae.
5.Conclusion
TheH.cuneaandC.cuneahost-parasite system was successfully employed to analyze the transfer of heavy metals from the host insect to the insect enemy,and its effects on the insect enemy. The primary control efficiency ofC.cuneaonH.cuneapupae was not affected by Cd exposure. However,the parasitic fitness of parasitoid offspring on Cd-accumulatedH.cuneapupae was significantly reduced,which may weaken the cyclic utilization efficiency ofC.cunea. Cd could be transferred from theH.cuneapupae to the parasitoid offspring ofC.cunea,and the transfer coefficient reflected biological amplification. Under Cd exposure,the cellular and humoral immunity ofH.cuneapupae decreased significantly. The direct adverse effect of Cd exposure on parasitoid offspring through oxidative status was the main reason for its reduction in parasitic fitness on CdaccumulatedH.cuneapupae.
Acknowledgements
This research was supported by the project funded by the Natural Science Foundation of Heilongjiang Province,China (YQ2022C006) and the National Natural Science Foundation of China (32101526).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendixassociated with this paper is available on https://doi.org/10.1016/j.jia.2023.04.032
 Journal of Integrative Agriculture2023年10期
Journal of Integrative Agriculture2023年10期
- Journal of Integrative Agriculture的其它文章
- The association between the risk of diabetes and white rice consumption in China: Existing knowledge and new research directions from the crop perspective
- Linking atmospheric emission and deposition to accumulation of soil cadmium in the Middle-Lower Yangtze Plain,China
- Genome-wide association study for numbers of vertebrae in Dezhou donkey population reveals new candidate genes
- Are vulnerable farmers more easily influenced? Heterogeneous effects of lnternet use on the adoption of integrated pest management
- lnfluences of large-scale farming on carbon emissions from cropping:Evidence from China
- Spatio-temporal variations in trends of vegetation and drought changes in relation to climate variability from 1982 to 2019 based on remote sensing data from East Asia
