Fused-benzotriazole Based p-Type Polymers:Fine-tuning on Absorption Band-width and Bandgap via Backbone Thiophene and Selenophene Strategies
TIAN Mei, ZHANG Zhiyang, ZHAN Chuanlang
Fused-benzotriazole Based p-Type Polymers:Fine-tuning on Absorption Band-width and BandgapBackbone Thiophene and Selenophene Strategies
TIANMei, ZHANGZhiyang, ZHANChuanlang*
(,,,010022,)
Four fused-benzotriazole based p-type polymers(BDT-TT, BDT-Se, BDD-TT, and BDD-Se) were designed and synthesized, and the fine-tuning on absorption band-widths and bandgapsthe backbone selenophene and thiophene strategies were reported. First, we introduced dithienothiophen[3,2-b]pyrrolobenzotriazole to co-polymerize with BDT-2F and synthesized BDT-TT. Then, we used selenophene to replace the thienothiophene units on the dithienothiophen[3,2-b]pyrrolobenzotriazole and synthesized BDT-Se. Compared to BDT-TT, BDT-Se showed a reduced bandgap from 2.0 eV to 1.89 eV. After that, we used BDD to replace BDT-2F and synthesized BDD-TT by co-polymerizing with dithienothiophen[3,2-b]pyrrolobenzotriazole. In comparison to BDT-TT, BDD-TT showed extended absorption band-width with the full-width-at-the-half-maximum(FWHM) increased from 138 nm to 229 nm and reduced bandgap from 2.0 eV to 1.71 eV. At last, we combined BDD and diselenophen[3,2-b]pyrrolobenzotriazole and synthesized BDD-Se, which achieved extended absorption and further reduced bandgap(1.61 eV). Using PC71BM as the electron acceptor material, the organic solar cells fabricated by the four polymers gave the efficiencies of 1%—2%.
Benzotriazole; Fused-ring; Polymer; Organic solar cell; Bandgap
1 Introduction
As a renewable energy technology, organic solar cells(OSCs) have attracted considerable attention due to their advantages of low-cost, light-weight, flexibility, and large-area fabrication[1—7]. Recently, owing to the rapid development of photovoltaic materials and device engineering, the power conversion efficiencies(PCEs) of OSCs have exceeded 19%[8—18].
In general, the active layer of an OSC consists of a p-type conjugated polymer donor material and an n-type organic electron acceptor material. Compared with the rapid development of non-fullerene acceptor materials, the development of donor materials has been relatively slow, and there are yet only a few donor materials that can be used for preparing high-efficiency OSCs. At present, most of the high-performance polymer donor materials are constructed using the 4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b∶4,5-b']dithiophene(BDT) unit as the electron-donating(D) unit[19—25]. This is due to its symmetrical structure of rigid coplanar backbone tending to form highly ordered-stacking for efficient charge transport[26—28]. In all, synthesizing donor polymers has been a central task for developing high-performance OSCs.
Since the invention of the A-DA'D-A type small-molecule non-fullerene acceptors,., the Y-series[29], big progress in the field of OSCs has been made. The DA'D type fused-ring has the following advantages: (1) introduction of the electron-deficient core unit into the fused-ring unit not only fine-tunes the energy levels and molecular packing but also modulates the D-A(donor-acceptor) interaction and inter- and intramolecular interactions, which are beneficial for enhancing the charge separation and electron mobility. (2) With the inclusion of the fused-pyrrolo ring, its electron-donating ability is beneficial to upshifting the high occupied molecular orbital(HOMO) energy level and then narrow the bandgap. (3) The decoration of the side chains on the fused-pyrrolo-N positions greatly reduces the aggregation because of the steric hindrance resulting from the orthogonal orientation to the backbone plane[30—32].
In this paper, we report four fused-benzotriazole(BTA) based polymers that are designed with the DA'D type fused-BTA as the co-polymerized units(Fig.1). We first co-polymerize dithienothiophene[3,2-b]pyrrolobenzotriazole with 4,8-bis[5-(2-ethylhexyl)-4-fluorothiophen-2-yl]benzo[1,2-b∶4,5-b']dithiophene(BDT-2F) to afford BDT-TT, which shows a bandgap of 2.0 eV. We then replace the thienothiophene unit with selenophene and couple the diselenophen[3,2-b]pyrrolobenzotriazole with BDT-2F, giving BDT-Se. The inclusion of selenophene enhances the quinoidal character[33—35], leading to a reduction in bandgap, down to 1.89 eV. Interestingly, the replacement of BDT-2F with 1,3-bis(2-ethylhexyl)-5,7-di(thiophen-2-yl)-4H, 8H-benzo[1,2-c∶4,5-c']dithiophene-4, 8-dione(BDD), the film absorption band-width is extended, with the full-width-at-the-half-maximum(FWHM) increased from 138 nm to 229 nm. Again, the bandgap is reduced to 1.71 eV. The extension of band-width and reduction of bandgap can be due to the enhanced backbone quinoidal character with the involvement of the BDD unit. The combination of the above two strategies,., co-polymerization of diselenophen[3,2-b]pyrrolobenzotriazole with BDD leads to BDD-Se, which shows extended absorption and again reduced bandgap. These results demonstrate that including the selenophene(., the backbone selenophene strategy) and replacement of BDT with BDD(., the backbone thiophene strategy), especially, the synergetic effect from the two strategies is a straightforward approach to fine-tune the polymer absorption.
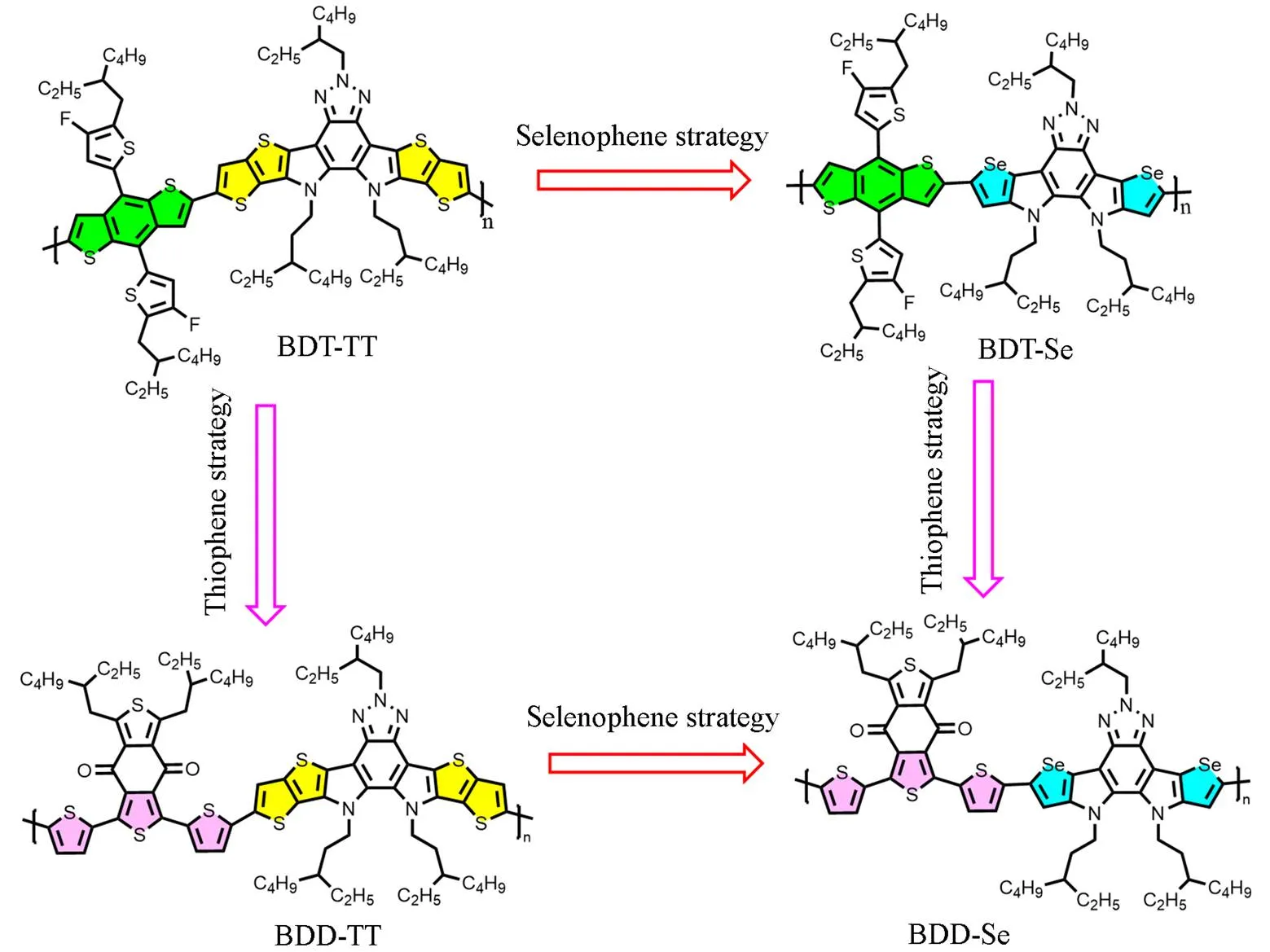
Fig.1 Chemical structures of the fused⁃BTA⁃based polymers: BDT⁃TT, BDT⁃Se, BDD⁃TT, and BDD⁃Se
2 Experimental
2.1 Materials and Measurements
Tri(-tolyl)phosphine[P(-tol)3, 97%], tris(dibenzylideneacetone)dipalladium(0)[Pd2(dba)3, 97%], Sigma-Aldrich(Shanghai) Trading Co., Ltd.; tributyl(thieno[3,2-b]thiophen-2-yl)stannane(98%), Derthon Optoelectronic Materials Science Technology Co., Ltd.; tributyl(selenophen-2-yl)stannane(99%), Suna Tech Inc.; 2,6-bis(trimethyltin)-4,8-bis(5-(2-ethylhexyl)-4-fluorothiophene-2-)benzodithiophene(BDT-2F-Sn, 97%), 1,3-bis(2-ethylhexyl)-5,7-bis(5-thiophene-trimethyltin-2-yl)-benzo[1,2-c∶4,5-c'] dithiophene-4,8-dione(BDD-Sn, 98%), Organtecsolar Materials Inc.; tetra--butylammonium hexafluorophosphate(99%),,-dimethylformamide(DMF, 99.9%), 1,2-dichlorobenzene(-DCB, 98%),-bromosuccinimide(NBS, 99%), potassium carbonate(K2CO3, 99%), potassium iodide(KI, 99%), chloroform-d with 0.03% TMS, Beijing InnoChem Science & Technology Co., Ltd.; anhydrous sodium sulfate, A. R., Tianjin Zhiyuan Chemical Reagent Co., Ltd.; dichloromethane, petroleum ether, ethyl acetate,-hexane, methanol, ethanol, isopropanol, A. R., Tianjin Zhiyuan Chemical Reagent Co., Ltd.
Avanse Ⅲ 400 and Ascend 600 MHz nuclear magnetic resonance(NMR) spectrometer, Switzerland Bruker; CH1660E electrochemical workstation, CH Instruments, Inc.; UV-2600 spectrometer, Japan Shimadzu.
2.2 Synthesis and Characterizations
2.2.1Synthesis and Characterizations of MaterialsScheme 1 exhibits the synthetic routes for the four polymers.
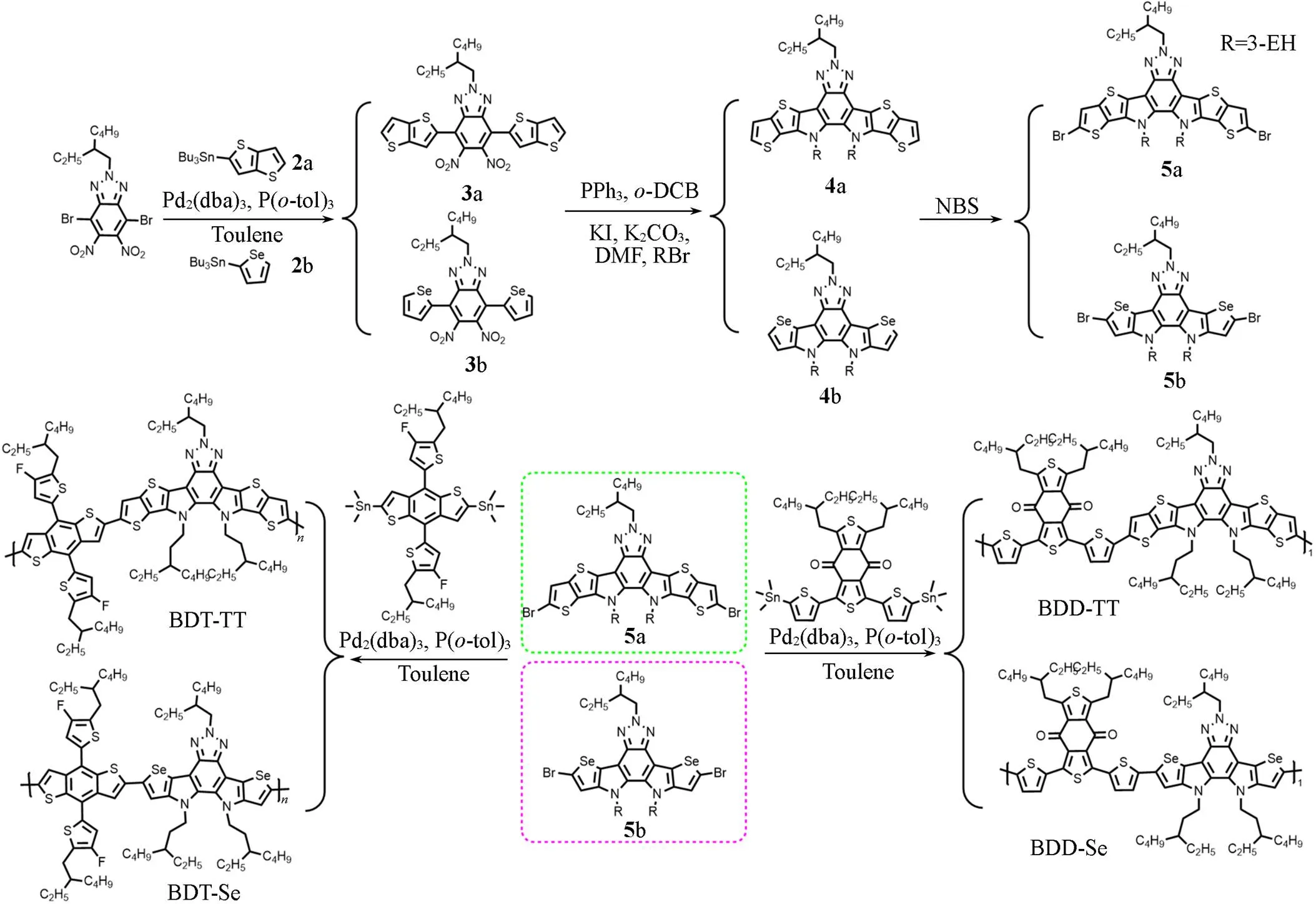
Scheme 1Synthetic routes for the polymers of BDT⁃TT, BDD⁃TT, BDT⁃Se and BDD⁃Se
Compound 3a: 4,7-dibromo-2-(2-ethylhexyl)-5,6-dinitro-2H-benzo[d]-[1,2,3]-triazole(200 mg, 0.417 mmol), Pd2(dba)3(7.6 mg, 0.008 mmol), and P(-tol)3(10.2 mg, 0.033 mmol) were dissolved in toluene after degassing for 10 min, and then tributyl(thiophene-[3,2-b]thiophene-2-yl)tin(394.2 mg, 0.918 mmol) was added. The mixture was refluxed under nitrogen for 4 h, cooled down to room temperature, and then concentrated under reduced pressure. Using petroleum ether/dichloromethane(4∶1, volume ratio) as eluent, the crude product was chromatographically purified to obtain an orange-red solid(166.2 mg, yield 66.7%).1H NMR(600 MHz, CDCl3),: 7.75(s, 2H), 7.54(d,=5.2 Hz, 2H), 7.32(d,=5.3 Hz, 2H), 4.76(d,=6.9 Hz, 2H), 2.28—2.24(m, 1H), 1.37—1.36(m, 2H), 1.34(dd,=7.5, 2.5 Hz, 2H), 1.33—1.29(m, 4H), 0.97—0.95(m, 3H), 0.91—0.89(m, 3H).13C NMR(151 MHz, CDCl3),: 140.17, 136.35, 134.88, 129.59, 123.37, 123.09, 121.53, 120.37, 109.23, 58.30, 48.21, 39.34, 35.81, 32.57, 31.64, 29.37, 27.65, 27.35, 24.93, 22.88, 21.95, 21.84, 13.05, 12.91, 9.69, 9.54.
Compound 3b: ginger solid(10∶1, volume ratio) with a yield of 66.1%.1H NMR(600 MHz, CDCl3),: 8.40(d,=5.6 Hz, 2H), 7.69(d,=4.7 Hz, 2H), 7.43(dd,=5.6, 3.9 Hz, 2H), 4.74(d,=6.7 Hz, 2H), 2.24—2.20(m, 1H), 1.37—1.30(m, 8H), 0.97(t,=7.4 Hz, 3H), 0.90(d,=7.2 Hz, 3H).13C NMR(126 MHz, CDCl3),: 140.39, 138.01, 136.07, 133.26, 131.55, 131.33, 129.36, 129.15, 120.04, 59.82, 40.03, 38.97, 29.50, 27.35, 22.90, 21.88, 13.02, 9.56.
Compound 4a: compound 3(166.2 mg, 0.278 mmol) and triphenylphosphine(365.1 mg, 1.392 mmol) were dissolved in-DCB(5 mL). The reaction was cooled to room temperature under nitrogen at 180 ℃ overnight. The solvent was evaporated, leaving the precipitate. The precipitate, potassium iodide(23.1 mg, 0.139 mmol) and potassium carbonate(575.9 mg, 4.176 mmol) were dissolved in 5 mL of DMF under the protection of nitrogen, to which gradually added 1-bromo-3-ethylhexane(161.1 mg, 0.835 mmol). The mixture was heated to 140 ℃ in the dark for 6 h. The mixture was cooled to room temperature, extracted with ethyl acetate and washed with water, and the organic phase was dried on magnesium sulfate. The crude product was purified by thin layer chromatography using petroleum ether/dichloromethane(5∶1, volume ratio) as eluent to obtain yellow oil(123.6 mg, yield 58.6%).1H NMR(600 MHz, CDCl3),: 7.42(d,=5.1 Hz, 2H), 7.37(d,=5.1 Hz, 2H), 4.74(d,=7.1 Hz, 2H), 4.68(t,=7.7 Hz, 4H), 2.38—2.33(m, 1H), 1.61—1.56(m,=13.7, 6.5 Hz, 4H), 1.43—1.39(m,=13.1, 6.8 Hz, 3H), 1.36—1.26(m, 4H), 1.19—1.14(m, 4H), 1.13—1.08(m,=14.9, 7.2 Hz, 4H), 1.04—0.98(m, 6H), 0.95—0.83(m, 10H), 0.66(t,=7.3 Hz, 6H), 0.58(t,=7.4 Hz, 6H).13C NMR(151 MHz, CDCl3),: 138.33, 136.33, 134.78, 129.65, 128.68, 127.58, 123.05, 120.94, 109.31, 58.33, 48.30, 39.31, 35.78, 32.58,31.59, 29.39, 27.65, 27.35, 24.93, 22.89, 21.94, 21.84, 13.03, 12.91, 9.70, 9.52.
Compound 4b: ginger oil(10∶1, volume ratio) with a yield of 46.6 %.1H NMR(600 MHz, CDCl3),: 7.97(d,=5.6 Hz, 2H), 7.50(d,=5.6 Hz, 2H), 4.72(d,=7.1 Hz, 2H), 4.56(t,=9.3 Hz, 4H), 2.38—2.31(m, 1H), 1.68—1.54(m, 6H), 1.44—1.38(m, 6H), 1.36—1.23(m, 6H), 1.19—1.07(m, 12H), 0.98(t, 3H), 0.90(t, 3H), 0.74(dd,=8.3, 6.2 Hz, 6H), 0.66(t,=7.4 Hz, 6H).13C NMR(126 MHz, CDCl3),: 145.61, 134.94, 129.52, 127.37, 117.66, 114.32, 114.04, 110.65, 57.99, 47.49, 46.80, 39.44, 38.78, 35.61, 35.52, 32.00, 31.47, 27.52, 24.68, 21.71, 12.87, 12.78, 9.48.
Compound 5a: compound 4(123.6 mg, 0.163 mmol) was dissolved with NBS(72.5 mg, 0.407 mmol) in 4 mL of DMF in the dark at room temperature for 24 h. After completion of the reaction, the solvent was evaporated and the crude product was purified on a thin-layer column using petroleum ether as the eluent to give a pale-yellow oil(109 mg, yield 73%).1H NMR(600 MHz, CDCl3),: 7.41(s,2H), 4.72(d,=6.9 Hz, 2H), 4.51(s, 4H), 2.37—2.33(m, 1H), 1.91—1.86(m, 2H), 1.61(d,=5.7 Hz, 4H), 1.41 (d,=19.1 Hz, 6H), 1.35—1.27(m, 6H), 1.25(s, 2H), 0.98(t,=7.2 Hz, 8H), 0.90—0.85(m, 8H), 0.65—0.59(m, 6H), 0.57—0.52(m, 6H).13C NMR(151 MHz, CDCl3),: 138.33, 136.33, 134.78, 129.65, 128.68, 127.58, 123.05, 120.94, 109.31, 58.33, 48.30, 39.31, 35.78, 32.58, 31.59, 29.39, 27.65, 27.35, 24.93, 22.89, 21.94, 21.84, 13.03, 12.91, 9.70, 9.52.
Compound 5b: ginger oil(6∶1, volume ratio) with a yield of 43.7%.1H NMR(600 MHz, CDCl3),: 7.49(s, 2H), 4.66(d,=7.1 Hz, 2H), 4.47(d,=5.5 Hz, 4H), 2.29(d,=5.5 Hz, 1H), 1.63—1.52(m, 6H), 1.41—1.34(m, 6H), 1.33—1.24(m,=6.3 Hz, 6H), 1.17—1.05(m, 12H), 0.98—0.95(m, 3H), 0.91—0.87(m, 3H), 0.76—0.72(m, 6H), 0.62(t,=7.3 Hz, 6H).13C NMR(126 MHz, CDCl3),: 143.03, 134.72, 128.76, 118.73, 118.06, 117.77, 112.34, 110.74, 58.24, 47.76, 39.64, 35.71, 35.67, 32.23, 31.65, 31.35, 27.78, 24.87, 21.99, 21.92, 13.12, 9.71, 9.59.
Polymerization of BDT⁃TT: compound 5a(109 mg, 0.119 mmol), BDT-2F-Sn(111.8 mg, 0.119 mmol), Pd2(dba)3(3.3 mg, 0.004 mmol), and P(-tol)3(7.2 mg, 0.024 mmol) were degassed for 10 min and then dissolved in toluene, and the reaction was conducted at 110 ℃ for 24 h. After the reaction was cooled to room temperature, it was precipitated into methanol and extracted by Soxhlet. The polymers were extracted with methanol, acetone,-hexane, and chloroform, respectively. Then, the chloroform solution was concentrated and the polymer was precipitated into methanol. Finally, a dark brown solid was obtained (58.6 mg),n=154000, PDI=1.45.
Polymerization of BDT⁃Se: compound 5b(103.7 mg, 0.112 mmol), BDT-2F-Sn(105.2 mg, 0.112 mmol), Pd2(dba)3(3.1 mg, 0.003 mmol), and P(-tol)3(6.8 mg, 0.024 mmol) were degassed for 10 min and then dissolved in toluene, and the reaction was conducted at 110 ℃ for 24 h. Following a similar process to that of BDT-TT obtained a dark brown solid(113.8 mg),n=213000, PDI=5.26.
Polymerization of BDD⁃TT: compound 5a(105 mg, 0.111 mmol), BDD-Sn(105.9 mg, 0.111 mmol), Pd2(dba)3(5.1 mg, 0.0033 mmol), and P(-tol)3(8.5 mg, 0.0223 mmol) were degassed for 10 min and then dissolved in toluene, and the reaction was conducted at 110 ℃ for 24 h. Following a similar process to that of BDT-TT obtained a dark solid(51 mg),n=38000, PDI=2.05.
Polymerization of BDD⁃Se: compound 5b(154.2 mg, 0.166 mmol), BDD-Sn(155.4 mg, 0.166 mmol), Pd2(dba)3(4.6 mg, 0.005 mmol), and P(-tol)3(10.1 mg, 0.033 mmol) were degassed for 10 min and then dissolved in toluene, and the reaction was conducted at 110 ℃ for 24 h. Following a similar process to that of BDT-TT obtained a dark brown solid(52.7 mg),n=153000, PDI=2.22.
2.2.2Preparations and Measurements of OSC DevicesIn order to investigate the photovoltaic performance of the polymers, indium tin oxide(ITO)/poly(3,4-ethylenedioxythiophene)∶poly-(styrenesulfonate)(PEDOT∶PSS)/active layer/3,3'-(1,3,8,10-tetraoxoanthra[2,1,9-def∶6,5,10-d'e'f']diisoquinoline-2,9(1H, 3H, 8H, 10H)-diyl)bis(,-dimethylpropan-1-amineoxide)(PDINO)/Al devices were fabricated. The fabrication process was as follows: the ITO glass was sequentially washed with detergent, deionized water, acetone, and isopropanol and then treated in ultraviolet ozone for 25 min. The PEDOT∶PSS was spin-coated onto the treated ITO glass at 4500 r/min for 30 s, then annealed at 150 ℃ for 20 min, and transferred to a glove box after completion. The weight ratio of D∶A was 1∶1.5, the concentration was 20 mg/mL after dissolution in chloroform, and the whole active layer solution was heated and stirred at 30 ℃ for 4.5 h using 0.5%(volume fraction) 1-chloronaphthalene(1-CN) used as an additive. For the use of Y6 as the non-fullerene acceptor, the D∶A weight ratio was 1∶1.2, and the concentration was 16 mg/mL in chloroform, 1-CN(0, 0.5%, and 0.75%) was used as the solvent additive. The active layer solution was spin-coated onto the PEDOT∶PSS layer at 3000 r/min for 30 s, then 1 mg/mL of the PDINO methanol solution was spin-coated atop the active layer at 3000 r/min for 30 s as the electron transporting layer ETL. Finally, Al(80 nm) was deposited on the ETL layer. The effective area of the device was 0.04 cm2. The current-voltage() characteristic of the device was measured by a Keithley 2400 test system under the simulated solar illumination at AM 1.5 G, 100 mW/cm2. The carrier mobility of the blend film was tested by the space charge-limited current(SCLC) model, characterized by the device structures of ITO/PEDOT∶PSS/active layer/Au(the hole-only device)and ITO/active layer/PDINO/Al(the electron-only device), respectively. The mobilities were then calculated according to the Mott-Gurney formula:=90r2/(83), where ε0is the dielectric constant of the vacuum (8.85419×10-12F/m), εris the relative dielectric constant of the transport medium component,is the carrier mobility andis the thickness of the active layer.
3 Results and Discussion
3.1 Synthesis
The synthetic routes for the polymers of BDT-TT, BDT-Se, BDD-TT, and BDD-Se are shown in Scheme 1. The monomers of 5a and 5b were synthesized by following the published methods[36,37]. These polymers were synthesized from the monomer 5a or 5b and BDT-2F or BDD unit by following the Stille- coupling polymerization with Pd2(dba)3and P(-tol)3as the catalyst and toluene as the solvent. All compounds were characterized by1H NMR and13C NMR, the corresponding spectra are provided in the Electronic Supplementary Information of this paper. The molecular weights of the four polymers were measured by high-temperature gel permeation chromatography(GPC). The number-average molecular weights(n) of the polymers BDT-TT, BDT-Se, BDD-TT, and BDD-Se were 154000, 213000, 38000, and 153000, and corresponding polydispersity index(PDI) were 1.45, 2.56, 2.05 and 2.22, respectively.
3.2 Optical Properties
The absorption spectra of the four polymers in chloroform solutions and films are shown in Fig.2. The absorption bands occurring at 300—400 nm[Fig.2(A)] or 350—480 nm[Fig.2(B)] correspond to the-* transition of the polymer backbone, whereas the bands at 400—650 nm[Fig.2(A)] or 480—780 nm [Fig.2(B)] are correlated to the intramolecular charge transfer(ICT) absorption between the D and A units[38,39].

Fig.2 UV⁃Vis spectra of the polymers in chloroform solutions and films
(A) Polymers of BDT⁃TT and BDT⁃Se; (B) polymers of BDD⁃TT and BDD⁃Se.


Table 1 Optical and electrochemical properties of the four polymers

From Fig.2(B), the solution absorption of BDD-TT and BDD-Se are seen in the ranges of 450—700 nm and 480—780 nm, respectively. The corresponding FWHM values are 185 and 216 nm, respectively. Shifting from the solution to the film, the maximum absorption peaks are red-shifted, positioning at 590 and 680 nm, respectively. The FWHM values are 229 and 209 nm, respectively. The optical bandgaps were estimated to be 1.71 and 1.61 eV, respectively.
In comparison to BDT-TT, BDD-TT shows extended absorption with FWHM value shifting from 138 nm to 229 nm and again shows reduced bandgap from 2.0 eV to 1.71 eV. This phenomenon can be attributed to the significant increase in backbone quinoidal character with the replacement of BDT with BDD since the BDD unit is constructed with terthiophene, while BDT contains a fused benzene ring. The increased quinoidal character on photovoltaic backbone has been widely proved by replacing benzene with thiophene.
From BDT-TT to BDT-Se and again from BDT-TT to BDD-TT, we can see that the inclusion of the BDD unit in comparison to the BDT unit into the polymer backbone shows more significantly impact to the polymer absorption. When combining the two strategies, the resulting BDD-Se shows a more reduced bandgap(1.61 eV) and again has extended absorption.
Again, it can be seen that the degree of the redshift of the absorption when shifting from the solutions to the films is quite different for the four polymers: by 2 nm for BDT-Se, 23 nm for BDT-TT, 29 nm for BDD-TT and 65 nm for BDD-Se, respectively. This phenomenon indicates that the packing of the polymers can be significantly modulatedthe backbone thiophene and selenophene strategies, again demonstrating the impact of this approach.
3.3 Electrochemical Properties
The HOMO levels and the lowest unoccupied orbital(LUMO) levels of the polymers were measured by cyclic voltammetry(CV). The results are shown in Fig.3. The HOMO/LUMO energy levels of BDT-TT were estimated to be -5.25 eV/-3.58 eV. Compared with the BDT-TT, the LUMO level of BDD-Se remains nearly unchanged(-3.60 eV. -3.58 eV), while the HOMO level is upshifted from -5.25 eV to -5.02 eV. Compared to BDT-TT, the HOMO energy level of BDD-TT is upshifted to -5.06 eV, and the LUMO level is downshifted to -3.71 eV. The HOMO and LUMO energy levels of BDD-Se are -4.99 eV and -3.72 eV, respectively(Table 1).
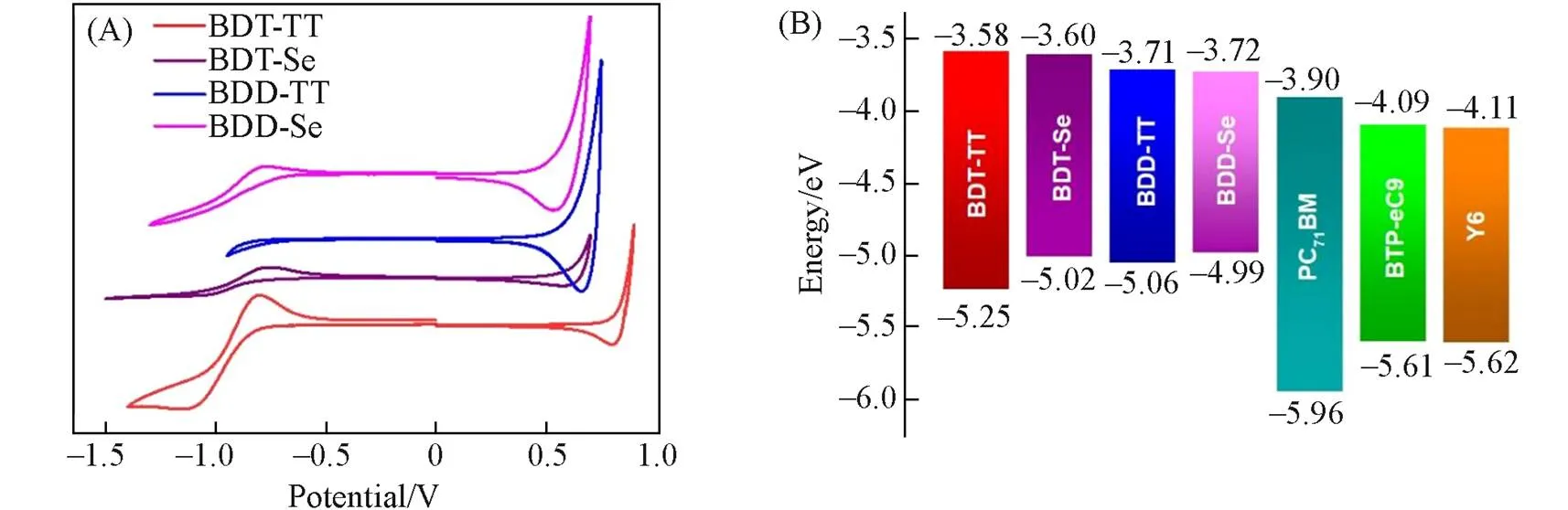
Fig.3 Cyclic voltammograms of the polymers(A) and diagrams of energy levels for the polymers and acceptors used in this work(B)
3.4 Photovoltaic Performance
The photovoltaic properties of polymers were evaluated by using PC71BM as the electron acceptor material and with the device structure of ITO/PEDOT∶PSS/active layer/PDINO/Al. Thecurves of the OSC devices are displayed in Fig.4(A), and the relevant performance parameters are summarized in Table 2. BDT-TT∶PC71BM, BDT-Se∶PC71BM, BDD-TT∶PC71BM, and BDD-Se∶PC71BM devices show efficiencies of 1.65%, 1.19%, 1.86%, and 1.58%, respectively. Compared to BDT-TT and BDT-Se, BDD-TT, and BDD-Se had improvedSC, which was consistent with the red-shifted and extended absorption. Fig.4(B) shows the external quantum efficiency(EQE) spectra. TheSCvalues(Table 2) calculated by integrating the EQE spectra were consistent well with theSCvalues measured from the corresponding-curves.
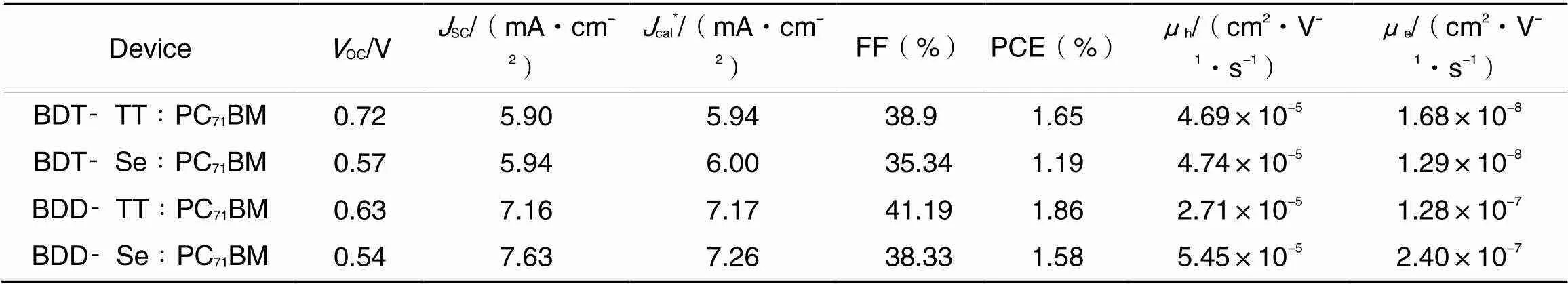
Table 2 OSC device parameters and charge mobilities of the polymer: PC71BM blends
*Obtained from the integration of the EQE spectra.
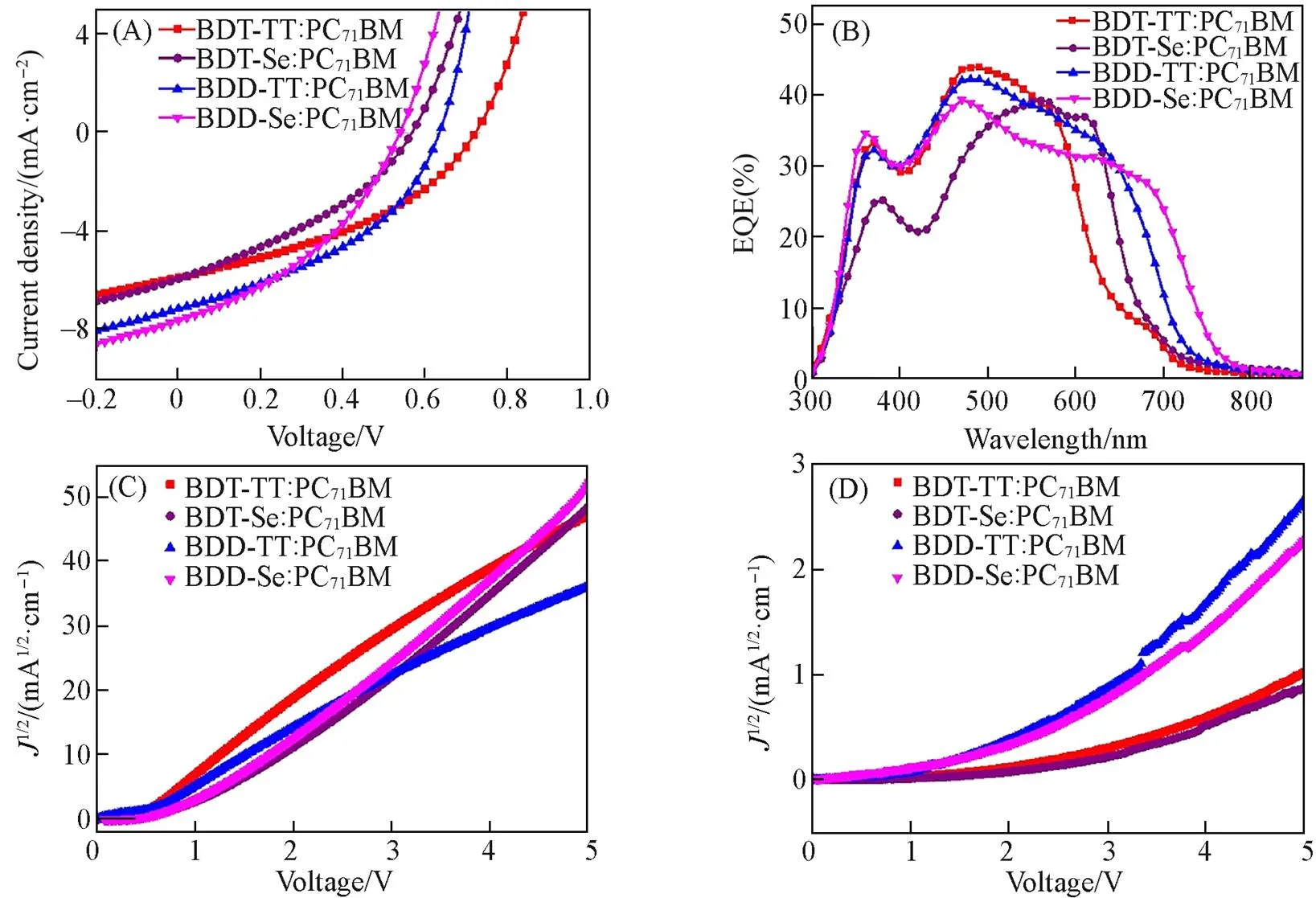
Fig.4 J⁃V curves(A) and EQE spectra(B) of the OSC devices, the dark J⁃V curves for estimating the hole(C) and electron(D) mobilities of the OSC active layers
The hole mobility(h) and electron mobility(e) were estimated using the SCLC method[Fig.4(C) and (D)]. The hole mobilities of BDT-TT∶PC71BM, BDT-Se∶PC71BM, BDD-TT∶PC71BM, and BDD-Se∶PC71BM were of 4.69×10-5, 4.74×10-5, 2.71×10-5, and 5.45×10-5cm2·V-1·s-1, respectively, and their electron mobilities were of 1.68×10-8, 1.29×10-8, 1.28×10-7, and 2.4×10-7cm2·V-1·s-1, respectively(Table 2). The electron mobilities of the BDD-TT and BDD-Se blends were about one order of magnitude higher than those of BDT-TT and BDT-Se polymers, which was again in relation with the increasedSC.
We again tested the photovoltaic properties of four polymers with Y6 as the blended acceptor. Fig.5(A) shows thecurves of the devices and the corresponding photovoltaic parameters are summarized in Table 3. The BDT-TT∶Y6 based device yielded a PCE of 1.19%. When using 0%, 0.5%, and 0.75% of 1-CN as the solvent additive, respectively, the resulting devices all supplied PCEs of around 1.1%—1.2%. For the BDT-Se, BDD-TT, and BDD-Se based devices, 0.55%—0.07% of PCEs were obtained. Fig.5(B) gives the EQE spectra of the four polymers-based devices, which covered the wavelength range of 300—1000 nm. TheSCvalues calculated from the EQE curves were in line well with theSCvalues measured by(Table 3). We also blended BDT-TT with BTP-eC9 to fabricate the OSC devices. PCEs of about 0.9% were obtained when using 0%, 0.25%, and 0.5% of 1,8-diiodooctane(DIO) as the solvent additive, respectively. The best device supplied aOCof 0.62 V, aSCof 4.32 mA/cm2, and an FF of 38.39%.
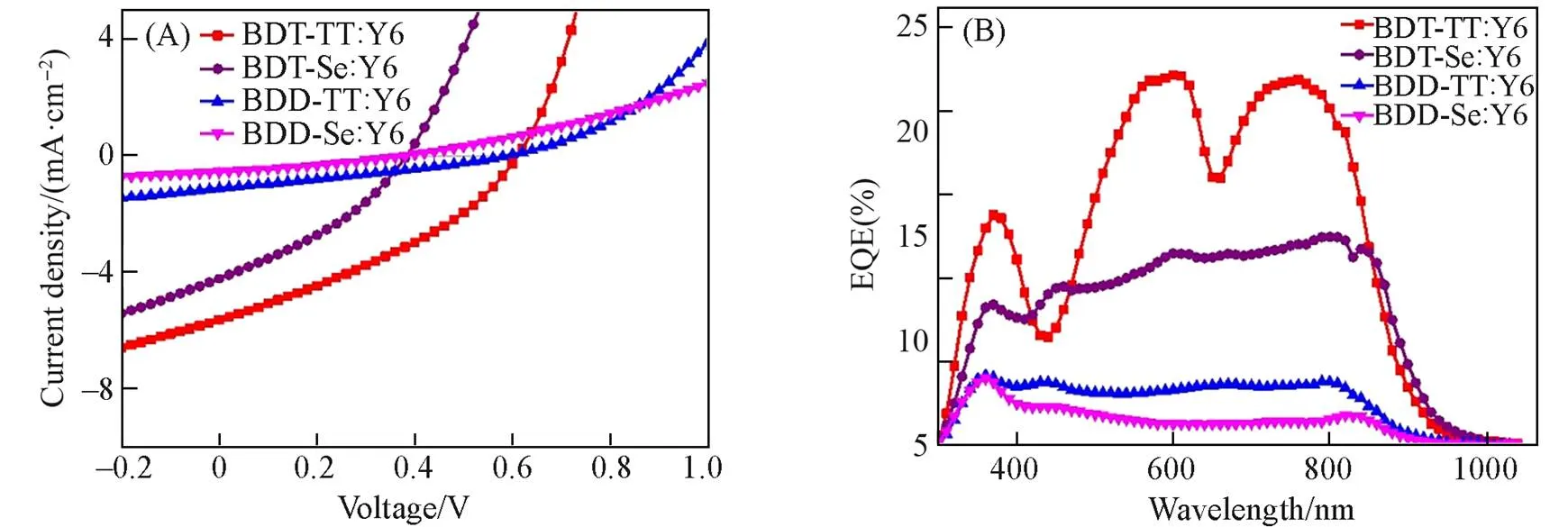
Fig.5 J⁃V curves(A) and EQE spectra(B) of the polymer: Y6 based devices
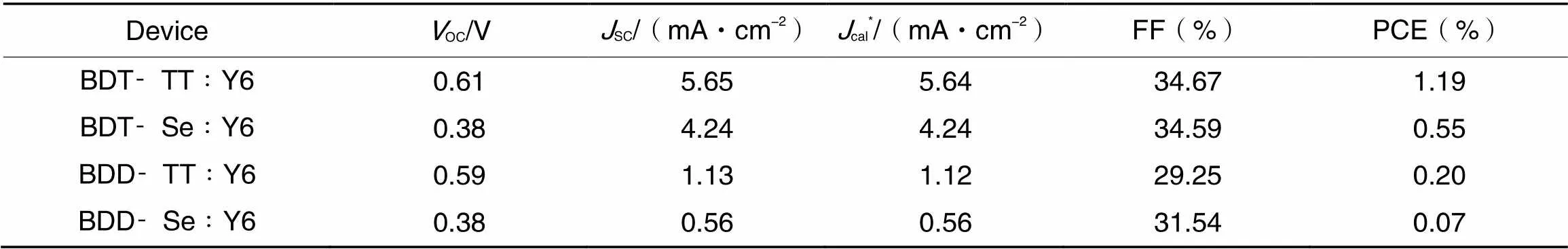
Table 3 Performance parameters of OSCs based on the polymers and Y6
*Obtained from the integration of the EQE spectra.
4 Conclusions
In summary, we used benzotriazole(BTA)-based DA'D fused-ring units to construct fused-BTA-based p-type polymers. By replacement of the fused thienothiophene with selenophene(so called backbone selenophene strategy, herein) and again replacement of BDT with BDD(so called backbone thiophene strategy, herein),., the polymer absorption in both band-width and bandgap can be effectively modulated. The combination of backbone selenophene and thiophene strategies shows a synergetic effect on tuning the polymer absorption. Based on the results, this paper not only presents four new p-type polymers containing DA'D type fused-ring but also demonstrates a molecular approach to fine-tune the absorption of p-type polymers.
The supporting information of this paper see http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20230190.
[1] Tang A. L., Zhan C. L., Yao J. N.,,2015,13), 4719—4730
[2] Li X. F., Pan M. A., Lau T. K., Liu W. R., Li K., Yao N. N., Shen F. G., Huo S. Y., Zhang F. L., Wu Y. S., Li X. M., Lu X. H., Yan H., Zhan C. L.,,2020,(12), 5182—5191
[3] Xu X. P., Lee Y. W., Woo H. Y., Li Y., Peng Q.,,2020,(49), 11241—11249
[4] Zhu C., Yuan J., Cai F. F., Meng L., Zhang H. T., Chen H. G., Li J. L., Qiu B. B., Peng H. J., Chen S. S., Hu Y. B., Yang C., Gao F., Zou Y. P., Li Y. F.,,2020,(8), 2459—2466
[5] Wu J. N., Fan Q. P., Xiong M. H., Wang Q. T., Chen K., Liu H. Q., Gao M. Y., Ye L., Guo X., Fang J., Guo Q., Su W. Y., Ma Z. F., Tang Z., Wang E. G., Ade H., Zhang M. J.,,2021,, 105679
[6] Yang T., Zhan C. L.,,2023,, doi: 10.1007/s11426⁃11023⁃11659⁃11428
[7] Sun H., Zhang P. Y., Zhang Y. N., Zhan C. L.,,2023,(7), 20230076(孙恒,张鹏宇,张英楠,詹传郎. 高等学校化学学报,2023,(7), 20230076)
[8] Liu S., Yuan J., Deng W. Y., Luo M., Xie Y., Liang Q. B., Zou Y. P., He Z. C., Wu H. B., Cao Y.,,2020,(5), 300
[9] Luo Z. H., Liu T., Ma R. J., Xiao Y. Q., Zhan L. L., Zhang G. Y., Sun H. L., Ni F., Chai G. D., Wang J. W., Zhong C., Zou Y., Guo X. G., Lu X. H., Chen H. Z., Yan H., Yang C. L.,,2020,(48), 2005942
[10] Cui Y., Xu Y., Yao H. F., Bi P. Q., Hong L., Zhang J. Q., Zu Y. F., Zhang T., Qin J. Z. , Ren J. Z., Chen Z. H., He C., Hao X. T., Wei Z. X., Hou J. H.,,2021,(41), 2102420
[11] Chong K. E., Xu X. P., Meng H. F., Xue J. W., Yu L. Y., Ma W., Peng Q.,,2022,(13), 2109516
[12] He C. L., Pan Y. W., Ouyang Y. N., Shen Q., Gao Y., Yan K. R., Fang J., Chen Y. Y., Ma C. Q., Min J., Zhang C. F., Zuo L. J., Chen H. Z.,,2022,(6), 2537—2544
[13] Sun R., Wu Y., Yang X. R., Gao Y., Chen Z., Li K., Qiao J. W., Wang T., Guo J., Liu C., Hao X. T., Zhu H. M., Min J.,,2022,(26), 2110147
[14] Wei Y., Chen Z. H., Lu G. Y., Yu N., Li C. Q., Gao J. H., Gu X. B., Hao X. T., Lu G. H., Tang Z., Zhang J. Q., Wei Z. X., Zhang X., Huang H.,,2022,(33), 2204718
[15] Zhu L., Zhang M., Xu J. Q., Li C., Yan J., Zhou G. Q., Zhong W. K., Hao T. Y., Song J. L., Xue X. N., Zhou Z. C., Zeng R., Zhu H. M., Chen C. C., MacKenzie R. C. I., Zou Y. C., Nelson J., Zhang Y. M., Sun Y. M., Liu F.,,2022,(6), 656—663
[16] Fan Q. P., Ma R. J., Bi Z. Z., Liao X. F., Wu B. H., Zhang S., Su W. Y., Fang J., Zhao C., Yan C. Q., Chen K., Li Y. X., Gao C., Li G., Ma W.,,2023,(8), 2211385
[17] Han C. Y., Wang J. X., Zhang S., Chen L. L., Bi F. Z., Wang J. J., Yang C. M., Wang P. C., Li Y. H., Bao X. C.,,2023,(10), 2208986
[18] Pang B., Liao C. T.,Xu X. P., Yu L. Y., Li R. P., Peng Q.,,2023, 2300631
[19] Qian D. P., Ye L., Zhang M. J., Liang Y. R., Li L. J., Huang Y., Guo X., Zhang S. Q., Tan Z., Hou J. H.,,2012,(24), 9611—9617
[20] Zhang M. J., Guo X., Ma W.,Ade H., Hou J. H.,,2015,31), 4655-4660
[21] Liu Q. S., Jiang Y. F., Jin K., Qin J. Q., Xu J. G., Li W. T., Xiong J., Liu J. F., Xiao Z., Sun K., Yang S. F., Zhang X. T., Ding L. M.,,2020,(4), 272—275
[22] Meng X. Y., Jin K., Xiao Z., Ding L. M.,,2021,(10), 100501
[23] Zeng A. P., Ma X. L., Pan M. G., Chen Y. Z., Ma R. J., Zhao H., Zhang J. Q., Kim H., Shang A., Luo S. W., Angunawela I. C., Chang Y., Qi Z. Y., Sun H. L., Lai J. Y. L., Ade H., Ma W., Zhang F. J., Yan H.,,2021,(33), 2102413
[24] Zhu C., Meng L., Zhang J. Y., Qin S. C., Lai W. B., Qiu B. B., Yuan J., Wan Y., Huang W. C., Li Y. F.,,2021,(23), 2100474
[25] Hu K., Zhu C., Qin S. C., Lai W. B., Du J. Q., Meng L., Zhang Z. J., Li Y. F.,,2022,(20), 2096—2102
[26] Holliday S., Li Y. L., Luscombe C.,,2017,, 34—51
[27] An C. B., Zheng Z., Hou J. H.,,2020,(35), 4750—4760
[28] An C. B., Hou J. H.,,2022,(5), 540-551
[29] Yuan J., Zhang Y. Q., Zhou L. Y., Zhang G. C., Yip H. L., Lau T. K., Lu X. H., Zhu C., Peng H. J., Johnson P. A., Leclerc M., Cao Y., Ulanski J., Li Y. F., Zou Y. P.,,2019,(4), 1140—1151
[30] Li S. X., Li C. Z., Shi M. M., Chen H. Z.,,2020,(5), 1554—1567
[31] Wei Q. Y., Liu W., Leclerc M., Yuan J., Chen H. G., Zou Y. P.,,2020,(10), 1352—1366
[32] Zhao J. J., Yao C., Ali M. U., Miao J. S., Meng H.,,2020,(12), 3487—3504
[33] Yu H., Qi Z. Y., Zhang J. Q., Wang Z., Sun R., Chang Y., Sun H. L., Zhou W. T., Min J., Ade H., Yan H.,,2020,(45), 23756—23765
[34] Zhang Z. Z., Li Y. W., Cai G. L., Zhang Y. H., Lu X. H., Lin Y. Z.,,2020,(44), 18741—18745
[35] Yang C., An Q. S., Bai H. R., Zhi H. F., Ryu H. S., Mahmood A., Zhao X., Zhang S., Woo H. Y., Wang J. L.,,2021,(35), 19241—19252
[36] Yuan J., Huang T. Y., Cheng P., Zou Y. P., Zhang H. T., Yang J. L., Chang S. Y., Zhang Z. Z., Huang W. C., Wang R., Meng D., Gao F., Yang Y.,,2019,(1), 570
[37] Zhang C. J., Yuan J., Chiu K. L., Yin H., Liu W. F., Zheng G. H. J., Ho J. K. W., Huang S. Z., Yu G. X., Gao F., Zou Y. P., So S. K.,,2020,(17), 8566—8574
[38] Raji I. O., Wen S. G., Li Y. H., Huang D., Shi X. Y., Saparbaev A., Gu C. T., Yang C. M., Bao X. C.,,2021,(30), 36071—36079
[39] He K. Q., Kumar P., Yuan Y., Zhang Z. F.,Li X., Liu H. T., Wang J. L., Li Y. N.,,2021,(22), 26441—26450
苯并三氮唑稠环基p-型聚合物:通过骨架噻吩和硒吩策略精细调控吸收与带隙
田梅,张志洋,詹传郎
(内蒙古师范大学化学与环境科学学院, 先进材料化学与器件内蒙古自治区高等学校重点实验室, 呼和浩特 010022)
基于苯并三氮唑DA'D稠环单元,设计合成了4个结构新颖的p-型聚合物(BDT-TT, BDT-Se, BDD-TT和BDD-Se), 通过骨架噻吩和硒吩策略实现了对聚合物吸收及带隙的精细调控. 首先, 将二噻吩并噻吩并吡咯稠合苯并三氮唑应用于设计聚合物, 与BDT-2F单元共聚合成了BDT-TT. 然后, 用硒吩取代二噻吩并噻吩并吡咯稠合苯并三氮唑中的两个噻吩并噻吩单元, 合成了二硒吩并吡咯稠合苯并三氮唑, 并与BDT-2F单元共聚合成了BDT-Se. 骨架硒吩取代策略的应用使聚合物的带隙从BDT-TT的2.0 eV降低到1.89 eV. 而后, 用BDD单元取代BDT-2F, 并与二噻吩并噻吩并吡咯稠合苯并三氮唑共聚, 合成了BDD-TT. 骨架噻吩取代策略的应用使聚合物的吸收半峰宽由BDT-TT的138 nm扩展到BDD-TT的229 nm, 带隙降低为1.71 eV. 最后, 将BDD与二硒吩并吡咯稠合苯并三氮唑共聚合成了BDD-Se, 通过硒吩和噻吩策略协同作用, 实现了吸收峰的展宽和带隙红移. 以PC71BM为电子受体材料, 由该系列聚合物构建的有机太阳电池器件获得了1%~2%的光电转换效率.
苯并三氮唑; 稠环单元; 聚合物; 有机太阳电池; 带隙
O631
A
10.7503/cjcu20230190
网络首发日期: 2023-06-14.
联系人简介: 詹传郎, 男, 博士, 教授, 主要从事激子材料化学与器件领域的研究. E-mail: clzhan@imnu.edu.cn
2023-04-17
内蒙古科技攻关项目(批准号: 2020GG0192)、内蒙古自然科学基金(批准号: 2022ZD04)和内蒙古师范大学项目(批准号: 112/1004031962)资助.
Supported by the Program of the Department of Science and Technology of Inner Mongolia, China(No.2020GG0192), the Natural Science Foundation of Inner Mongolia, China(No.2022ZD04), and the Program of the Inner Mongolia Normal University, China(No.112/1004031962).
(Ed.: N, K)
- 高等学校化学学报的其它文章
- 高性能半透明有机太阳能电池的实现途径
- Beta-alanine as a Dual Modification Additive in Organic Solar Cells
- 半透明有机太阳能电池研究进展
- Aggregation Morphology of Perylene Bisimide Acceptors and the Role on Exciton Processes and Electron Transport in Organic Solar Cells
- 协同富勒烯和非富勒烯受体提高卟啉全小分子三元有机太阳能电池的性能
- 基于受体1-受体2型聚合物给体的高效有机太阳能电池

