Hypersensitivity Reaction Caused by Intravenous Gadolinium-based MRI Contrast Agents
Lai Jing,Qin Liangyi,Qin Yane,Lan Xiaobu,Zhang Qi
(1. Pharmacy Department of The First People’s Hospital of Nanning, Nanning 530021, China;2. 923rd Hospital of PLA, Guangxi 530021, China)
Abstract Objective To present a rare case of skin allergic reaction to gadobutrol,a magnetic resonance imaging (MRI)contrast agent,in a 37-year-old man.Methods The adverse reactions of gadobutrol were analyzed combined with the instructions and related literatures.Results and Conclusion The presence of this patient is consistent with the adverse reactions in the instructions of gadobutrol.The incidence of ADR in gadobutrol is considered to be low,although sometimes patients report a hypersensitivity reaction when undergoing MRI.There are only a few cases of immediate adverse reactions to gadobutrol.However,we should improve the ability of medical staff to use drugs safely and take preventive measures.
Keywords: gadobutrol;magnetic resonance imaging (MRI);hypersensitivity reaction;allergy;safety
MRI technology has been widely used as an important imaging diagnostic method in clinic since the 1980s.In 1987,the first gadolinium-based contrast agent (GBCA) was authorized for the inspection of MRI enhancement by the United States Food and Drug Administration (FDA)[1].To date,nine GBCAs have been marketed worldwide,which are generally considered to be safe with low incidence of adverse reactions in clinic.The types of GBCAs’ adverse reactions can be classified as acute,delayed and extremely delayed[2].A meta-analysis by Behzadi,et al.[3]included 716 978 patients injected with GBCA found that the total rate of acute adverse reactions was 9.2/100,with 9.2/1000 of serious adverse effects.According to the results of meta-analysis,81% of acute adverse reactions were mild,13% were moderate,and 6% were severe.In addition,the rate of acute adverse reactions in macrocyclic contrast agents was higher than that in linear contrast agents,which was related to the large ring structure,protein binding,and ion status.There were some reports of negative effects caused by GBCAs in China,and most of them were GGD glucomamide.Fatal allergic reactions caused by gadobutrol have been reported abroad[4],but allergic reactions of it have not been reported in China.
The chemical name of gadobutrol is 10-(2,3-dihydroxy-1-hydroxymethylpropyl) -1,4,7,10-tetraazacyclic dodecane -1,4,7-triacetic acid with the molecular formula C18H31GdN4O9and the relative molecular mass of 604.72.Its chemical structure is shown as Fig 1.
1 Clinical materials
A 37-year-old man with repeated abdominal pain for more than 4 months came to the hospital.The patient reported abdominal pain 4 months ago with no obvious inducement,which was confined to the right abdomen,presented with paroxymorial dull pain,lasted for several seconds,and became worse when the body position changed.The patient was undiagnosed but had recurrent abdominal pain.His body temperature was 36.4 ℃,the pulse at 8 times/min,respiration was 20 times/min,blood pressure at 129/80 mHg,and the heart rate was 8 beats/min.Admission diagnoses were:(1) Abdominal pain (cholecystitis or peptic ulcer?);(2)Blood in the stool (internal hemorrhoids?);(3) Fatty liver.In terms of treatment,some relevant examinations had to be made.As a result,the patient underwent a hepatobiliary magnetic resonance examination to determine the presence of biliary calculi.
The patient underwent MRI plain scan +enhancement+biliary hydrographic imaging (MRCP)of the liver and biliary+pancreas+spleen.Before the examination,8.5 mL of gadobutrol was injected (the recommended dose for adults in the instructions was 0.1 mol/kg,equivalent to 0.1 mL/kg) according to the patient’s weight of 65 kg.Approximately 10 min after receiving gadobutrol,the patient experienced facial and neck rashes,as well as nausea (Fig.2).It was believed that the patient had an allergic reaction to the gadobutrol.
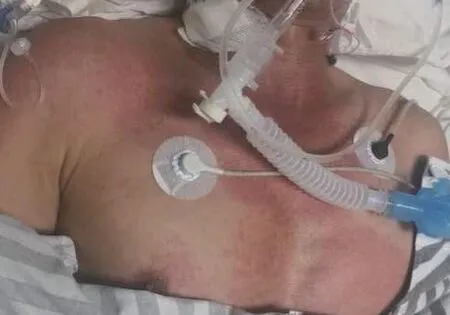
Fig.2 Photography of the skin allergic reaction to gadobutrol
His symptoms improved and the rash disappeared after receiving prompt therapy with dexamethasone 5 mg injection and loratadine 5 mg orally.The patient was released from the ward after receiving treatment and gastric care,which greatly reduced the patient’s abdominal pain and stabilized his vital signs.
2 Discussion
For imaging examinations,the second-generation nonionic macrocyclic GBCA known as gadobutrol is frequently utilized.Smaller doses can be tested because it has a higher ionic concentration than other MRI contrast agents[5].According to reports,gadobutrol has a good imaging effect,is well tolerated,and has a low level of toxicity.Clinical trials have shown a low incidence of adverse drug reactions.
Although GBCAs are generally associated with fewer adverse reactions than other types of contrast agents,there has been a rise in reports of these side effects due to the widespread use of this agent.
The most common adverse reactions occur within the first 5 min after administration when a patient takes gadobutrol.These reactions include nausea,vomiting,and urticaria.Other common side effects include dizziness,dyspnea,and tachycardia[6].
In our case,the patient began exhibiting onset symptoms roughly 10 min after gadobutrol delivery.Dexamethasone and loratadine were used to treat his acute adverse effects.He underwent a smooth hospital stay and was successfully extubated before being released.
Hypersensitivity reactions can be classified as immediate reactions which occur within 1h after contrast media (CM) administration,and nonimmediate reactions which become apparent more than 1 h after CM exposure[7].Research has shown that severe immediate reactions may be mediated by IgE.Skin tests had been used to diagnose immediate hypersensitivity reactions in CM for many years,but positive tests were rare and only reported in patients with severe reactions.
Currently,European Society of Urogenital Radiology’s “Guidelines for the Prevention of Generalized Contrast Medium Reactions” recommends that high-risk patients should receive oral prednisolone (30 mg) or methylprednisolone(32 mg) orally 12 h and 2 h before CM exposure[8].However,severe CM-induced anaphylactic reactions have occurred in previous reactors despite such prophylactic use of corticosteroids[9].In spite of using sufficient premedication with corticosteroids,a 2019 systematic review and meta-analysis found that in patients with a history of an immediate reaction to GBCA,39% of patients may experience repeated hypersensitivity reactions to the same GBCA[10].
Therefore,all patients taking gadobutrol should be prepared for acute adverse reactions.Take antianaphylactic shock and cardiopulmonary resuscitation drugs and equipment (e.g.,oxygen,epinephrine,antihistamines,atropine,dexamethasone,β2agonist aerosols,saline,anticonvulsants,blood pressure monitors,suction devices,simple respirators,etc.).Before gadobutrol injection,doctors should ask patients whether they have a history of allergy to contrast agents,bronchial asthma,or other allergies,because these patients are at higher risk of developing allergies.It should still be used with caution,and patients should be informed that there might be delayed allergic symptoms after use.
3 Conclusion
Gadobutrol hypersensitivity reactions are extremely uncommon side effects[11,12],but when they do happen,they can be lethal within minutes.Therefore,it is crucial for clinicians to be aware that deadly mishaps might happen even during MRI exams,and to make sure that medical facilities and appropriately qualified employees are on standby at any time[13].
- 亚洲社会药学杂志的其它文章
- Effect of the Policies to Prevent Drug Shortage and Stabilize Drug Prices in Medical lnstitutions
- Research on the Countermeasures for the Development of Biopharmaceutical Industrial Parks in China
- Research Progress in FDA’s Focus Areas of Regulatory Science for Drugs and Suggestions for China
- Cost-effectiveness Analysis of lnsulin Degludec and Liraglutide lnjection in the Treatment of Type 2 Diabetes
- A Systematic Review of Patient-Reported Outcome Measurement for Psoriasis in Chinese Population
- Exploration and Research on the Integrated Development of “Internet Plus Medical Treatment”

