A Systematic Review of Patient-Reported Outcome Measurement for Psoriasis in Chinese Population
Li Kexin,Zhang Fang,Huang Zhe
(1.School of Business Administration, Shenyang Pharmaceutical University, Shenyang 110016, China;2. ZTE Foundation, Shenzhen 518055, China)
Abstract Objective To review the development of patient-reported outcome measurement (PROM) for patients with psoriasis in China,and to analyze the main results and methodology.Methods The systematic review method of COSMIN (consensus-based standards for the selection of health measurement instruments) was adopted,and the domestic and foreign databases were searched to find the patient-reported outcome scales based on Chinese psoriasis patients.Then,the included studies were evaluated by using COSMIN risk of bias checklist.Results and Conclusion A total of 3 studies were included,involving 3 scales.We found that the quality evaluation of the development process of the 3 scales was not high,and there were large methodological loopholes in the whole cycle of scale development and verification.The included studies have many problems such as low extrapolation,poor quality,and lack of validation,which can provide more insights for the quality control requirements of the whole cycle of scale development in the future.
Keywords: psoriasis;health related quality of life (HRQOL);patient-reported outcome;reliability;validity
1 Background
1.1 Overview of psoriasis
Psoriasis is a chronic,non-infectious,painful,disfiguring and disabling disease for which there is no cure[1].The etiology of psoriasis is unknown,but there is evidence of a genetic predisposition and a role for the immune system.In addition,exogenous and endogenous triggers can also cause psoriasis,including minor trauma,sunburn,infection,systemic medications,and stress[2].The most common symptoms of psoriasis are scaly skin,itching,erythema,fatigue,swelling,burning,and bleeding[3].In addition to skin symptoms,psoriasis is accompanied by an inflammatory arthritis called psoriasis arthritis (PSA),which damages the spine and other joints.Clinically,psoriasis is dominated by psoriasis vulgaris (85%-90%).Other types of psoriasis account for about 10%,including pustular psoriasis,erythrodermic psoriasis,and arthritic psoriasis[1].The published prevalence rates for psoriasis in various countries range from 0.09% to 11.4%.In most developed countries the prevalence rate is 1.5%-5%.In 2012,the standardized prevalence of psoriasis in China was 0.47%[2].Psoriasis patients not only have a huge economic burden,but also face disfigurement,disability and loss of the workforce.In addition,social exclusion,discrimination and stigma can be devastating to psoriasis sufferers and their families[4].Therefore,psoriasis seriously affects the quality of patient life.
1.2 Health-related quality of life (HRQOL) and patient-reported outcome (PRO)
HRQOL,which clinicians and basic science researchers focus on,is built around the biomedical model and focuses on etiology,pathological processes,biological,physiological,and clinical outcomes.Its knowledge is rooted in biology,biochemistry and physiology[5].However,in terms of HRQOL,social scientists pay attention to the dimension of function and overall well-being,and its knowledge is rooted in sociology,psychology and economics[5].The two different HRQOL models were built based on different roles,which did not include all HRQOL variables.Therefore,they lacked links between variables and logical explanations.
After a lot of research,Ferrans,et al.[6]summarized and formed the more popular and applicable HRQOL model conceptual framework (Fig.1).This framework emphasizes that HRQOL starts from biological factors such as heredity and genes,which is manifested in biological representations as symptoms.Such symptoms may be physical manifestations or psychological manifestations.Therefore,they affect the ability of biological individuals to perform basic functions,and the external environment will also influence the interaction of the biological itself,emphasizing the logical relationship of various influencing factors of HRQOL and forming a selfpreference for patients HRQOL cognition.It is a combination of subjective and objective products,which truly reflects the patient’s situation.
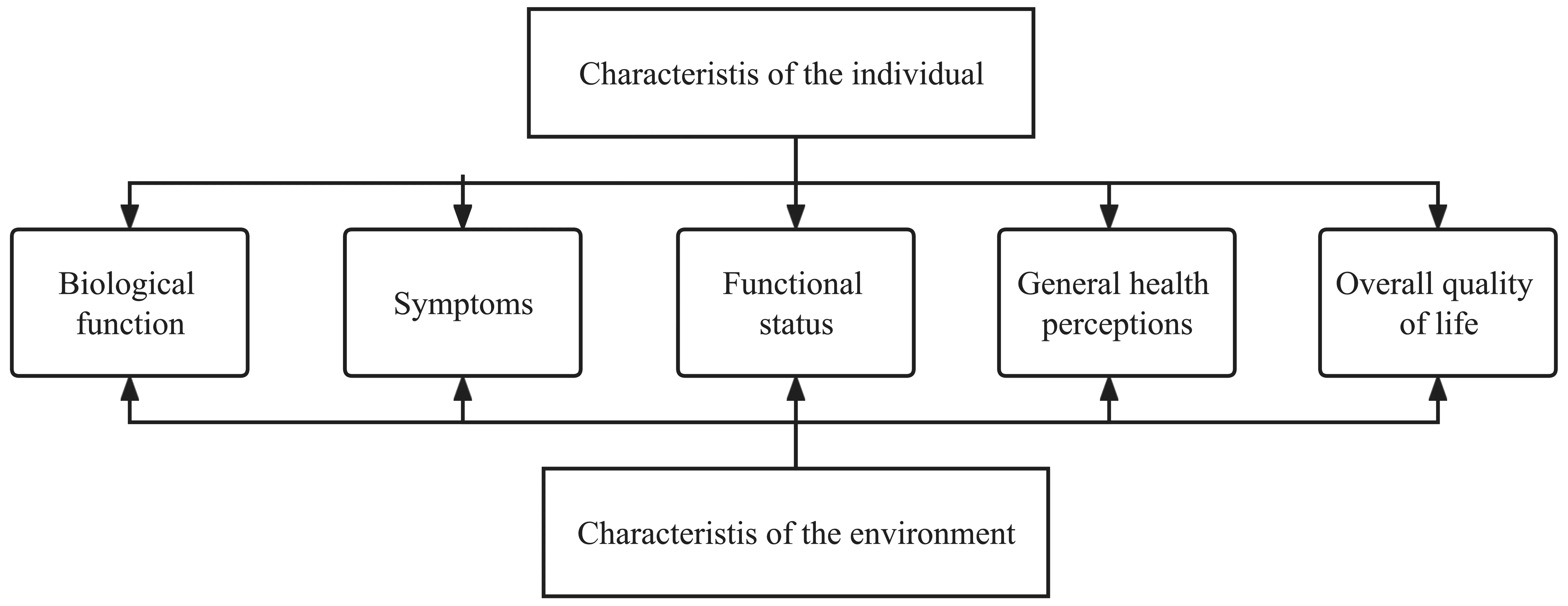
Fig.1 Ferrans’ HRQOL model framework
PRO measurement instruments have become one of the important tools to measure patients’ HRQOL,and it is the most widely used one[7].There are many types of quality-of-life assessment scales for psoriasis patients at home and abroad.Therefore,this study aims to review the research based on the assessment scale of quality of life for patients with psoriasis,identify the current development of the health scale,and put forward some suggestions.
2 Methods
2.1 Literature search and screening
2.1.1 COSMIN(consensus-based standards for the selection of health measurement instruments)search filter
(1) Background of the strategy.We know that many HRQOL measurement tools have certain similarities.To retrieve related research on databases such as PubMed and Embase quickly and accurately,the international health measurement instrument COSMIN has summarized a set of efficient retrieval strategies[8].This set of search terms came from 10 000 PubMed research records[8].The search terms were selected and combined from these studies,and their retrieval sensitivity was calculated,which was extensively validated.Finally,an efficient,accurate and standardized PROM (patient-reported outcome measurement)search filter was obtained.
(2) Structure of search strategy.It mainly includes four parts: Construct search is to find the main measurement content,such as “HRQOL”.Population search is to find the target population of the study,such as “psoriasis patients”.Instrument search is the tool that the study focuses on,such as “PROM”.Measurement properties filter is a concept such as scale test,such as “reliability” “validity”“psychometrics” “internal consistency” and “Cronbach alpha”.Logical connectives are used to combine the above keywords and exclude irrelevant records.The structure of the search strategy recommended by COSMIN is formed (Table 1)[8].
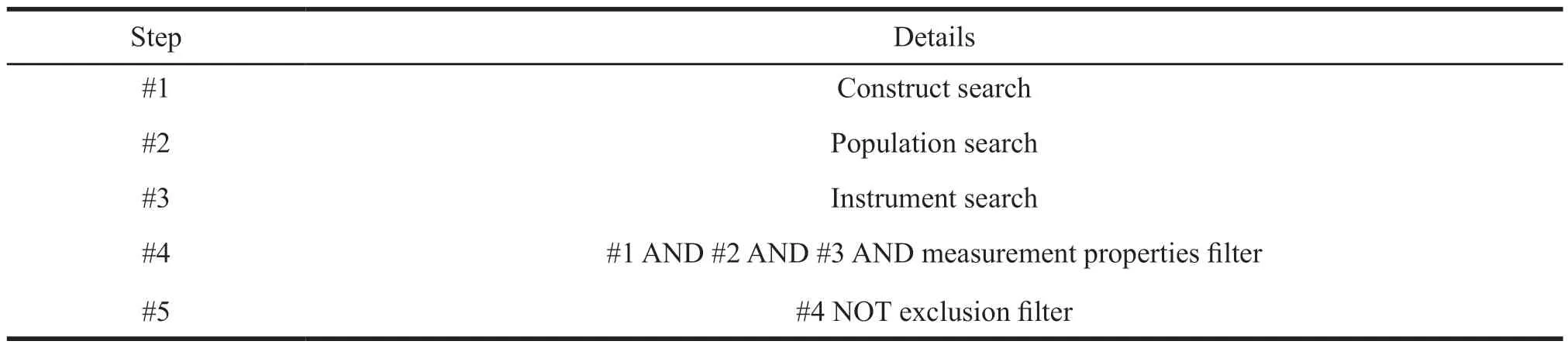
Table 1 Structure of search strategy
2.1.2 Search strategies and results
(1) Searching in English databases.In this study,three English databases including PubMed,the Cochrane Library,and Embase were retrieved in the form of subject headings combined with free words.Keywords such as “psoriasis” “HRQOL” “PRO”“validation study” “correlation” and “reliability” were connected by logical operations.
(2) Searching in Chinese databases.In this study,three Chinese databases including CNKI,VIP,and Wanfang were retrieved,and the search term strategy was combined with keywords such as “psoriasis”“quality of life” and “scale”.
(3) Search results.A total of 2 317 relevant studies were retrieved in this study,including 293 Chinese database retrieval studies and 2 024 English database retrieval studies.A total of 14 additional studies were included in this preliminary screening.All were imported into the Ranyan document manager for preliminary screening.
2.1.3 Screening strategies and results
(1) Screening method.Two investigators screened the materials back-to-back.A third person was asked to make a decision on controversial studies.A preliminary screening was performed based on the title and abstract,all possible studies were imported into endnote for full-text download,and a second screening was performed after reading the full text.
(2) Screening criteria.Based on the research objectives,we set the corresponding inclusion and exclusion criteria (Table 2).

Table 2 Literature screening criteria
(3) Screening results.After primary screening and full-text screening (Fig.2),a total of 80 psoriasis scale studies (Table 3) were found,including 34 original scale development studies and 46 scale validation studies.A total of 35 scales were involved,of which 3 were developed based on patients with psoriasis in China.This study conducted a systematic review of three patient-reported scales developed based on the Chinese population.
Table 3 Classification of scales

Fig.2 Literature selection process

Fig.3 Guideline for systematic reviews of patient-reported outcome measures (PROMS)
2.2 COSMIN systematic literature review methodology
COSMIN has developed a comprehensive methodological guide for systematic reviews of PROM[12],which aims to provide a comprehensive overview of the research characteristics of measurement tools and to form recommendations based on the evidence from the review.A total of 10 steps are included to guide the systematic review of PROM.The 10 steps are subdivided into 3 parts(Fig 3).
Part A focuses on research design,mainly in literature search and screening.Part B is an evaluation of all the measurement characteristics of the systematic review PROM,such as content validity,construct validity,internal consistency,measurement invariance,criterion validity,construct validity and responsiveness.Part C focuses on the feasibility of the PROM and interpretation of the data,producing recommendations based on all the evidence[13].Based on them,COSMIN provides a risk of bias checklist for systematic review of the PROM[14]according to the above three-step approach (Table 4).Each box in the list has its corresponding content of concern.For example,box 1 is used to detect the development of PROM,and box 2 is used to detect content validity,etc.The evaluation is set on four grades,from excellent to poor,which are very good,adequate,doubtful,inadequate,and not applicable(NA).The lowest rating of any standard in the box is taken to determine the overall quality of a study(the worst score counts principle).For example,if for a reliability study one item in a box is rated as “inadequate”,the overall methodological quality of that reliability study is rated as “inadequate”.The response option “NA” should not be considered in the worst score counts[14].In this study,2 researchers read the development research of the three scales “back-toback” and independently completed the “COSMIN’s PROM Bias Risk Evaluation Checklist”.Finally,a third party can review and resolve conflicts.Their opinions are integrated to form the final evaluation result.
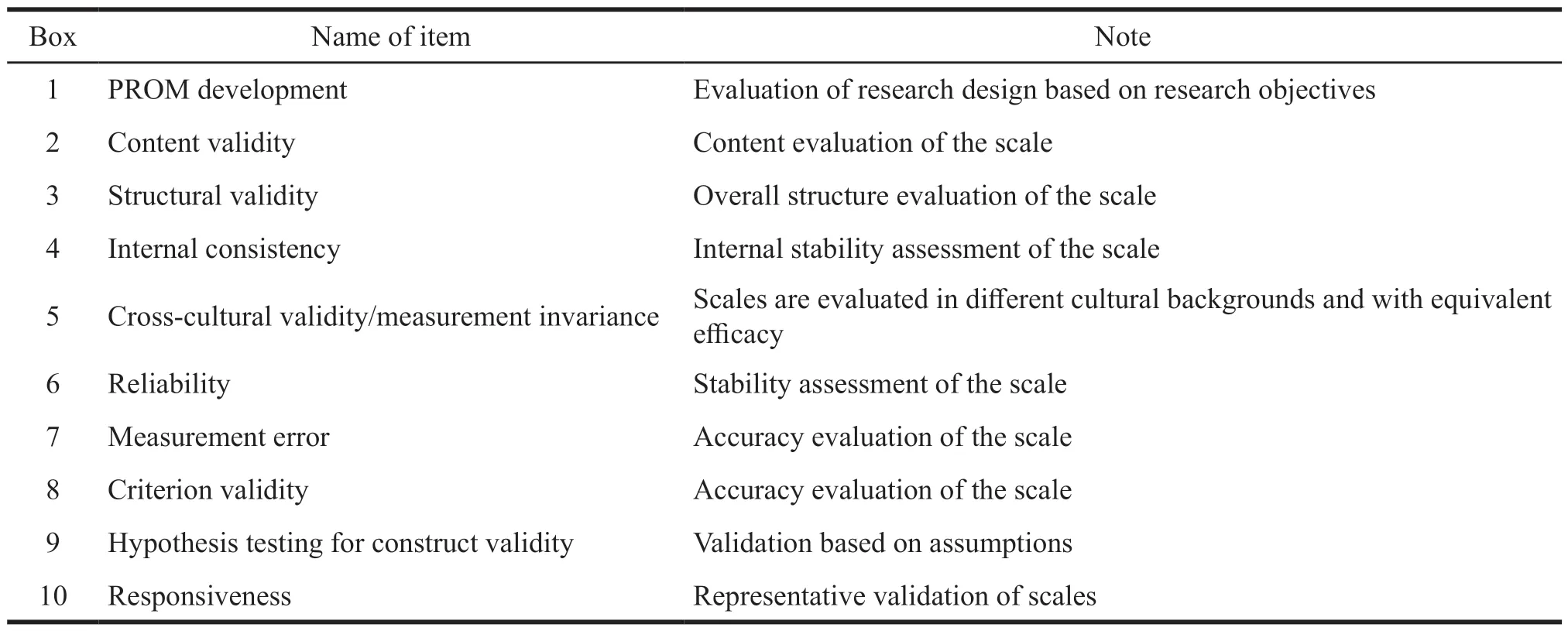
Table 4 COSMIN risk of bias checklist
3 Results
3.1 Basic characteristics of the scale
The three studies were developed based on the scales generated by Chinese psoriasis patients as the target population,one was on the scale framework based on the theory of traditional Chinese medicine,and others were based on the theory of Western medicine.One article focused on psoriasis vulgaris patients with the highest proportion of psoriasis populations.Two studies formed a multi-dimensional scale,mainly including physiology,psychology and society,while one was a single-dimensional scale.The number of items in the final version of the study output was 14,22,25,respectively.The test reference scales for the three studies revolved around two tools,the psoriasis area and severity index(PASI) and the dermatological life quality index(DLQI).Two studies used one of them separately,and one study used two instruments as a control test(Table 5).
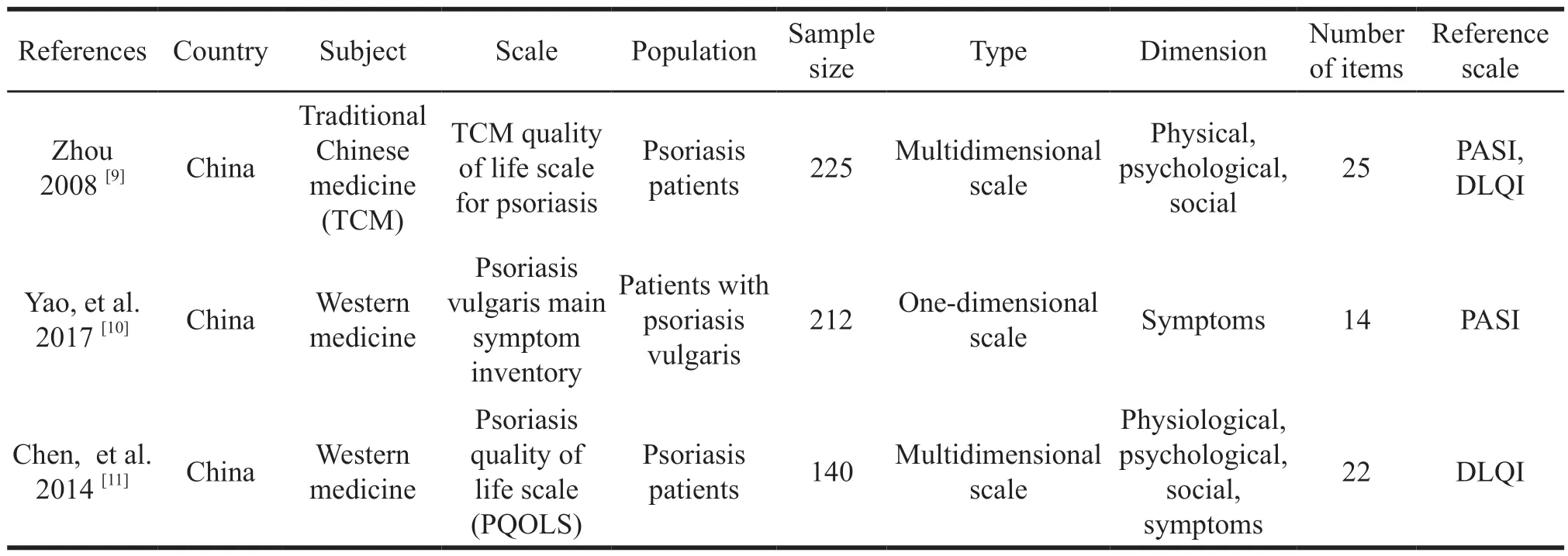
Table 5 Basic characteristics of the included literature
3.2 Development of the scale
3.2.1 Design of the scale
Zhou[9]used the general survival scale of WHOQOL-100 and WHOQOL-BREF and the psoriasis life stress inventory (PLSI) recognized at home and abroad,combined with the basic theory of traditional Chinese medicine and clinical practice experience to build the framework items pool of the basic scale.Yao,et al.[10]used FDA’s PRO scale construction principles to build a framework and item pool.The structure of the three studies is clearly described,the theoretical basis used in constructing the structure is reliable,the scale design focuses on the characteristics of the target population,and the background is clear.Based on the item pool,Chen,et al.[11]selected 20 patients to conduct a conceptual preliminary interview on the relevance and comprehensiveness of the item pool.Then,he deleted and revised inappropriate items.However,neither Yao nor Zhou did preliminary interviews on the relevance and comprehensiveness of the items.Therefore,based on COSMIN’s risk of bias checklist box 1a,the design of these two scales is unqualified.Chen did not explain the data collection method,interview organizer,and interview records during the interview session and concept elicitation session with 20 patients.Therefore,the design of this scale is doubtful.
3.2.2 Cognitive interview and pre-survey
Yao and Zhou did not conduct cognitive interviews for the prototype scale,so the evaluation level in box 1b was inadequate.Chen conducted a pre-survey of 40 people,and only adjusted and revised the items.The rationality of sample selection and the comprehensibility and comprehensiveness of the items were not elaborated.Therefore,the corresponding evaluation grade was unqualified.To sum up,the development stages of the three scales were all inadequate,mainly due to the lack of cognitive interviews and pre-surveys.Cognitive interviews and pre-surveys are the key points to avoid large deviations before the prototype scale is validated on a large scale.A good cognitive interview and presurvey can avoid problems such as missing items and semantic errors.To a certain extent,scales that are validated against large samples cannot have errors in direct identification (Table 6).

Table 6 COSMIN box 1.PROM R&D quality evaluation
3.3 Content validity
Content validity aimed at the relevance,comprehensiveness,and comprehensibility of the prototype scale in the patient population and the relevance and comprehensiveness of the expert interviews for the prototype scale.None of the above three studies carried out content validity validation of patients and expert teams,so the content analysis was unqualified (Table 7).A scale with good content validity is a prerequisite for patients to be able to complete it independently.Patient self-reporting requires patients to self-administer and use without outsider intervention.After a scale that has not been verified for content validity is distributed to patients,there may be cases that the patient cannot complete it independently and needs the help of others.However,the intervention of outsiders will lead to deviations,so it cannot truly reflect the actual situation of the patient,which is contrary to the definition of patient self-report.
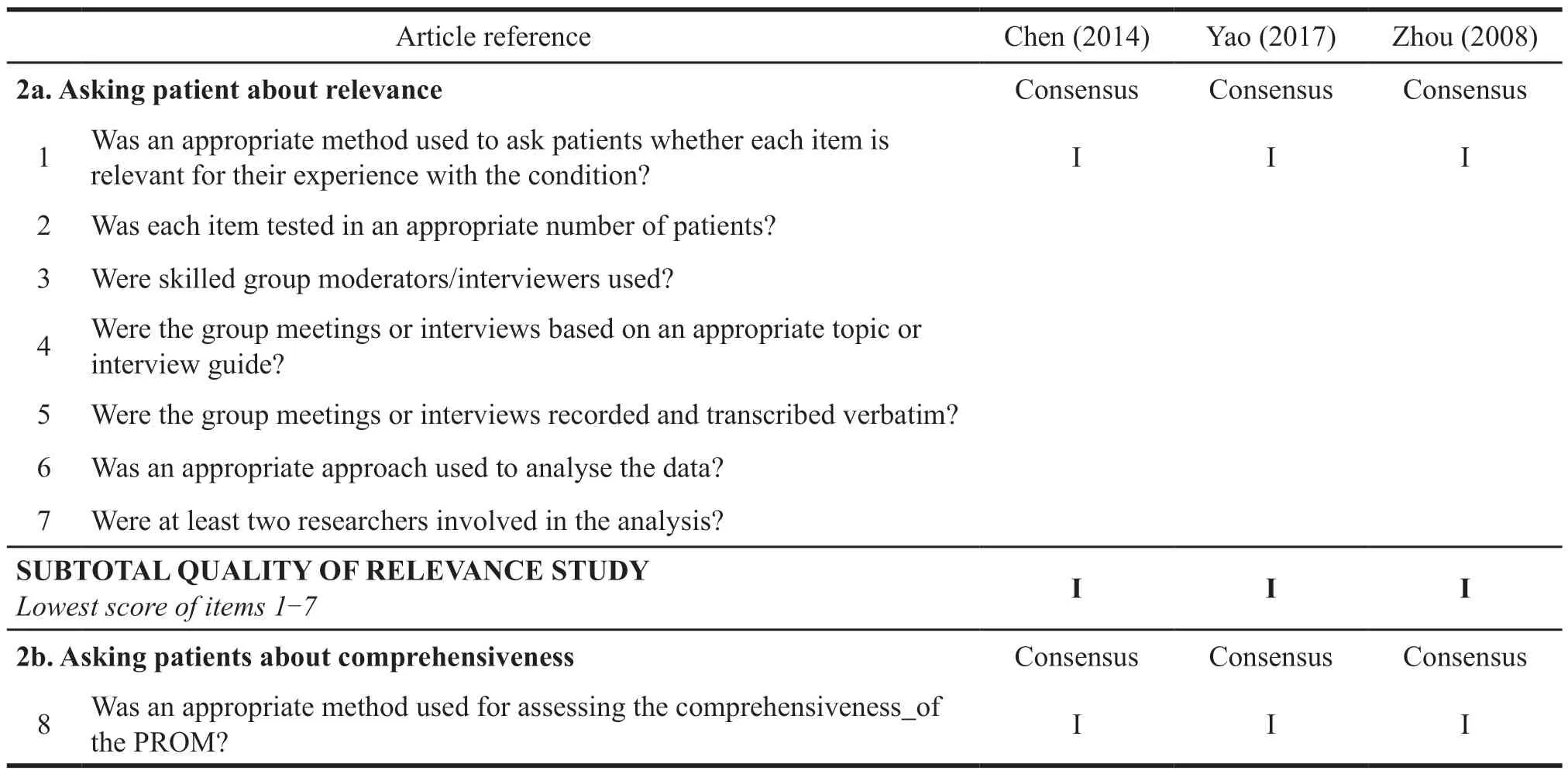
Table 7 COMSIN box 2.PROM content validity evaluation
3.4 Construct validity
Chen used exploratory factor analysis to extract four factors,which were in line with the four dimensions initially set in the scale.When he performed principal component factor analysis,the number of entries was 22,the sample size was 140,so construct validity evaluation based on box 3 was qualified.Yao used exploratory factor analysis to extract two common factors,which were categorized as skin lesions symptoms and pharyngeal symptoms.When he performed principal component factor analysis,the number of entries was 14,and the sample size was 212,the construct validity evaluation was qualified (Table 8).The sample size based on the proportion of the number of items is the basic guarantee to carry out the structural validity,and too few samples will lead to the lack of authenticity of the scale structure verification.In addition,structural validity is an important method to identify ceiling and floor effects.When patients are measured by the scale,the existence of ceiling and floor effects will make patients tend to choose the most serious or the least serious answers to certain questions,which will lead to the bias of high or low scores,which is too different from the real value.
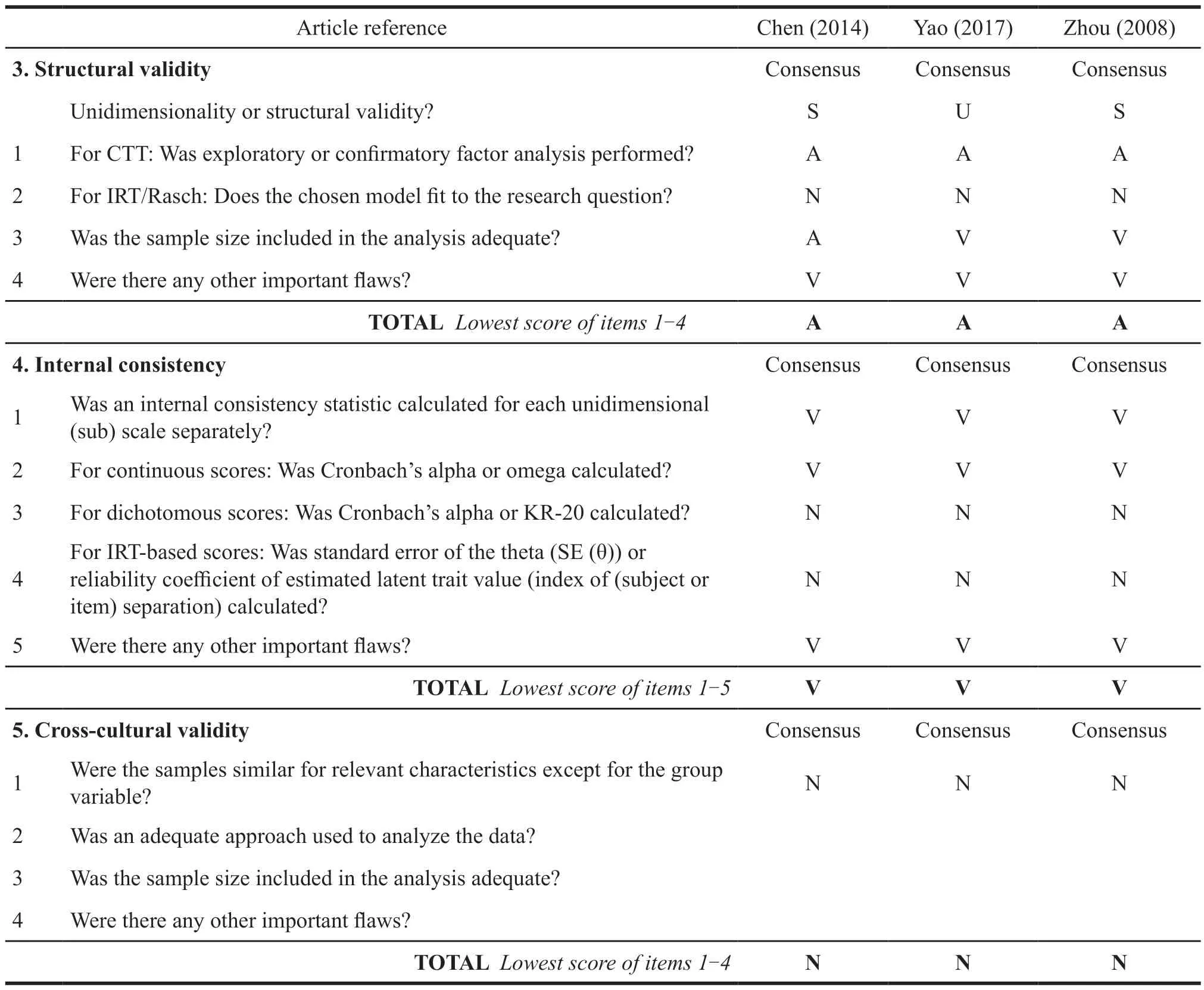
Table 8 COMSIN box 3-10.PROM other measurement characteristics evaluation
3.5 Internal consistency
The two studies calculated the internal consistency reliability of the scale and each dimension.The Cronbach alpha of the scale in Chen’s study was greater than 0.8.The Cronbach alpha coefficient of the scale studied by Zhou was in the range of 0.714 to 0.840.Yao calculated the internal consistency of the male and female versions of the scales,respectively,with a Cronbach alpha of 0.87.The internal consistency evaluation of the three scales was very good (Table 8).The internal consistency evaluation considers whether the core content of the scale measurement is accurate,that is,whether all items are measured with a focus on one indicator,such as the overall internal consistency of the above research scale.For example,whether each item of the scale focuses on the health-related quality of life of psoriasis patient.The internal consistency of each dimension considers whether each item under this dimension pays attention to this dimension indicator.For example,the internal consistency of the physical dimension is to consider whether all items under this dimension focus on the health-related quality of life.Therefore,the core content measured by the above three scales is in line with the goals set by the scales,and there is no conceptual deviation around the healthrelated quality of life in psoriasis.
3.6 Cross-cultural validity
None of the three scales are available in other languages,therefore,the cross-cultural validity verification evaluation is not applicable (Table 8).The cross-cultural validity test aims to consider whether the measurement bias is caused by cultural differences when the scale is translated into other languages.Many international well-known scales will carry out this verification when they are translated into other language versions.Through cross-cultural validity verification,the research scale can be used in various countries,increasing the extrapolation possibility of the scale.
3.7 Reliability
Chen used a 2-day time interval to measure the reliability,and the Sperman correlation coefficients were all greater than 0.7.Yao used a 10-hour time interval to measure reliability,and the average Sperman correlation coefficient was 0.88.Zhou used 1 day as the time interval to test the reliability,and the Perason correlation coefficient was 0.969.The three studies did not explain the selection criteria of the time interval and whether the two measurement conditions were similar,thus making the reliability evaluation of the three studies doubtful (Table 8).The reliability evaluation aims to focus on the stability of the scale measurement,assuming that the patient is in a stable state for a short time and the environment has not changed significantly,whether there is a major change in the measurement results.During the reliability verification of the above three studies,the time interval selection was not explained in detail,and the acute symptoms of psoriasis would also have a large deviation in a short period of time.Therefore,there was no explanation on whether the subjects developed acute diseases during the period and whether the measurement environment was stable since they would not be affected by external factors.It was impossible to verify whether the reliability of the scale was good,and it could not be judged only from the parameter results.
3.8 Measurement errors
None of the 3 scales were validated for measurement error,and the corresponding evaluation was not applicable (Table 8).Measurement error is designed to focus on the deviation between the true value and the measured value.Therefore,the measurement error of scale validation tends to use accurate and proven tools in the academic industry as a reference.The PASI score is often used for psoriasis.It is theoretically believed that patients with high PASI scores have a serious impact on the quality of life,and vice versa.None of the scales in the above studies verified whether there were errors in their measurement and whether they could truly reflect the real situation of patients.
3.9 Criterion validity
At present,there is no “gold standard” for the PRO of psoriasis.Since corresponding evaluation has not been carried out in the three scale studies have not carried out,they are not applicable(Table 8).Besides,there is not an internationally recognized “gold standard”,so most studies cannot carry out the verification of criterion validity,which also suggests that a scale can be used as a standard in the future,and it should be taken as the standard in the corresponding validation.
3.10 Hypothesis testing for construct validity
Chen used DLQI as the reference tool to verify the convergent validity,and the Pearson correlation coefficient was 0.821.Yao used PASI as a reference tool for convergent validity verification,and he also verified the discriminant validity of psoriasis subgroup populations with different severity levels.The scale could distinguish patients with different severity levels,so the hypothesis test was good.Convergent validity aims to consider whether the scale as a whole focuses on a central concept.Besides,discriminant validity aims to consider whether each dimension of the scale is different from each other and to distinguish different subgroups of people.Therefore,physiology only pays attention to internal factors.Convergent validity ensures the accuracy of scale measurement concepts,and discriminant validity ensures that all items in the scale are not redundant,and the related items are not repeated.
3.11 Responsiveness
In the verification,there is not a gold standard.Since none of the three studies were compared with other tool constructs,some of them were inapplicable.Yao performed an analysis based on subgroup characteristics,and the results were good.Both Chen and Zhou conducted the patients’responses to the scale before and after treatment,but there was no obvious explanation for the treatment plan,resulting in the doubtful evaluation grade(Table 8).Responsiveness considers two points,one is whether the change of the patient’s state can be accurately measured by the scale after receiving the treatment intervention,another concern should be whether the characteristics of different subgroups of patient population can be displayed by the scale.The former hypothesis considers that after treatment,the improvement in quality of life can be reflected in the scale scores.The latter considers severe patients with lower quality of life evaluation,and vice versa.Therefore,the response verification of the scale also reflects the accuracy of the scale measurement to some extent.
4 Discussion
Three scales of reported outcomes of psoriasis patients in China were systematically reviewed based on COSMIN consensus.Overall,there were large vulnerabilities in the full cycle methodology for the three instruments ranging from scale design to psychometric testing of the final scale.
4.1 Summary
Issues of bias in the design of scale were caused by inadequate qualitative examination of the target population,especially cognitive interview of the patients.The lack of professional interviewers and detailed records of the interview process contributed to the low-quality assessment (Table 9).In addition,the team of experts should include psychology experts,clinical experts,quality of life experts,humanities experts,and economics experts according to the COMIN consensus.The above three studies only did this with the TCM quality of life scale for psoriasis patients designed by Zhou et al.In the content validation,none of the three scales were designed with appropriate patients as well as an expert sample for examination of the content (Table 10).The face-to-face interviews were conducted in terms of comprehensiveness,relevance,comprehensibility,and comprehensibility.After the inquiry of three categories of characteristics,the scale could be designed more scientific and sound.The examination of those missed entries as well as the revision of entries made the scales easy to understand.In the validation other measurement properties (psychological tests),the factors included in the examination of each scale were also heterogeneous (Table 10).Chen and Zhou examined construct validity,internal consistency,reliability,aggregate validity,and the responsiveness of the scale before and after therapeutic intervention in patients.However,Yao examined construct validity,internal consistency,reliability,aggregate validity,discriminant validity based on patient subgroups,and responsiveness.It can be found that,in the scale validation,not all factors were taken into consideration.And the reference scale chosen in the construct validity test was quite different.Some used the PASI and others used the DLQI.Methodology was more differentiated and the quality was generally low.
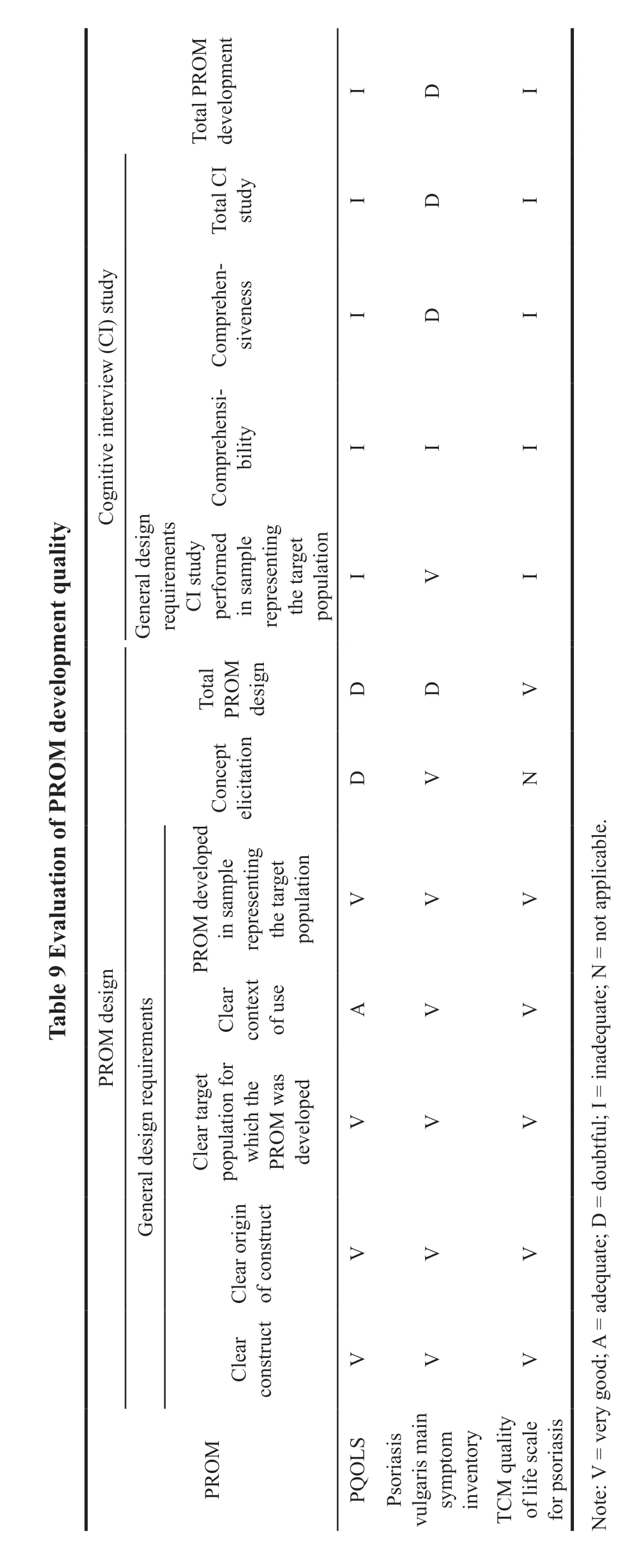
Based on the above studies,it can be seen that there exists a phenomenon of late initiation step,inadequate methodology,and low-quality grade in the design of psoriatic scale in China.There is not enough awareness about the importance of health-related quality of life.Health related quality of life refers to people’s subjective evaluations of their own physical,emotional,and social functions,which emphasize patients’ own reports.Health related quality of life has now become one of the essential evaluation contents in clinical studies of cardiovascular and cerebrovascular diseases,chronic diseases such as rheumatic diseases,malignant diseases of tumors,and rare diseases.There has been a boom internationally in developing qualityof-life scales that patients can report autonomously.While the content formation and validation of scales commonly used internationally are often based on population preferences in European and American countries,such as Canada,Finland,and the United States.Due to the differences in social,cultural,racial,religious,cognitive,and linguistic,the application of these scales often differs from that of China,not only in the use process,but also in the measurement of utility indicators,which leads to potential bias.
4.2 Thinking
The status of Chinese scale research from the included studies can be caused by several reasons.In the future,as to the design of the scale,if these key points are controlled and valued,we can obtain more high-quality scales,which have more possibilities for extrapolation.
4.2.1 Defining measured structure
Unclear measurement constructs can directly lead to the initial design errors of a scale.A clear framework of health-related quality of life can explain the logic among the influencing factors on quality of life in related diseases.However,the generation of such a framework needs to be based on a large number of literature studies and real-life validation.The logical framework of the above scale mainly revolves around the output of existing studies,without the causality of related concepts and further validation in reality.In addition,the international frameworks used by the above studies are all formed based on foreign cultures.There is no evidence that whether they are applicable in China or can be tested by Chinese culture.Therefore,in the future scale design,we should focus on the academic support combined with the social background and cultural background to make adjustments.In this way,a basic principle that conforms to the current environment for the development of the scale is formulated.
4.2.2 Attaching importance to patient demonstration and multidisciplinary expert demonstration
The scale is designed primarily for patient selfassessment,not for others’ interventions.So the scale design is primarily patient-centered,with an emphasis on the patient’s own argument during the initial phase.The above studies,which neglect patients to make changes to their contents,raise doubts as to whether they can be used for patient self-assessment.In addition,expert team arguments emphasize the involvement and discussion of multidisciplinary experts such as medicine,sociology,and economics.The QOL scale focuses not only on indicators of patients’ clinical symptoms,but also on overall wellbeing.Therefore,it involves experts in disciplines such as sociology and economics.This is also in line with Ferrans’ framework for quality of life derived from both biomedical and humanities care.The above studies all reflect consideration in clinical outcome measure evaluation,while ignoring other factors.The explicit scale is generated for the clinical effect evaluation of patients,in which the indicators are certified by multidisciplinary experts.Besides,the resulting scale can accurately find the cut point of measurement in the target population and is guided by objective indicators.
4.2.3 With the help and reference of COSMIN consensus
At present,there is a lack of a well-established methodological standard for the HRQOL scale design in China.The three studies included above have large differences in the methodology.Many current international scale studies refer to the COSMIN consensus,developing and validating a number of good measurement tools.While the academic of scale design in China is not yet immature.With the help of the research consensus of COSMIN,an international organization for standards in health measurement instrument development,the quality control requirements of the entire scale development and validation cycle can be improved.Firstly,it should specify the study objectives of scale design,including the context of use,the study population,and the framework of HRQOL.Secondly,a literature review,qualitative interviews with patients,and multidisciplinary expert consultations should be conducted.Based on the theoretical framework,following the principles of qualitative description research design,the data obtained by observation or interview can be coded.Then,the in-depth understanding and thinking about the data are recorded,similar phrase expression,pattern,topic,sequence and important characteristics in the coded data are identified.Next,commonalities and differences among the data are extracted for further analysis.Finally,the conceptual framework is examined in terms of existing knowledge and theoretical frameworks.During the item generation phase,content analysis[15,16]should be used as the primary method to identify entries and dimensions that qualitatively describe the study.Thus,the basic entry is formed.Based on these,cognitive interviews with patients and experts are conducted to further refine the scale design.This scale is examined multifactorially with standard methodological requirements,statistical parameters as well as appropriate selection of criteria.Controlling the above factors can make the development of health measurement instrument scientific,so that the scale developed in China can be extrapolated to other linguistic cultures.
4.2.4 Paying attention to the special requirements for the development of disease-specific scales
Most of the SF-36,EQ-5D,which are widely used internationally,are common scales.Though they are available for the measurement of health states and the comparison of health outcomes for different diseases,they have low sensitivity for a particular disease,which can affect the decision maker’s tradeoffbetween different interventions for a particular disease.In addition,the current development and application of disease-specific scales are often based on a clinical perspective and are mostly used to assist in disease screening,diagnosis,treatment,rehabilitation,and patient feeling measurement.Their results cannot or are not applicable to cost utility analysis in economic research of health technology assessment due to the lack of utility scoring systems based on the preferences of our patient population.The identification of the characteristics of the target disease,around which the scale research is conducted,is an important breakthrough in the development of the disease-specific scale.
Psoriasis gradually evolves towards a chronic disease that will accompany the patient’s lifetime,not only causing a large economic burden but also severely impairing the patient’s quality of life.And the medical needs of psoriasis remain largely unmet.Clinical guidelines for psoriasis have also been updated.Many new drugs and nonpharmacological treatments are incorporated into the recommendations of clinical guidelines as well.These drugs and treatments require more real-world or pharmacoeconomic evidence to show whether they are cost-effective.Therefore,establishing a disease specific HRQOL and health utility evaluation system for psoriatic patients in China will facilitate the application of health technology assessment (HTA) for skin diseases.Our study found that only a few disease specific HRQOL scales had been validated in Chinese speaking regions.And only a few HRQOL scales had health utility scoring systems,none of the health utility scoring systems were based on the Chinese population.The lack of validated Chinese versions of disease specific health utility scales may hinder the application of HTA for skin diseases.
The construction of person-centered health service system gradually has an important position in China.The “Healthy China 2030 Planning Outline”[17]stated that it is important to put people’s health in a strategic position.National Administration of Traditional Chinese Medicine,National Medical Products Administration (NMPA),National Health Commission of the People’s Republic of China and National Health Security Administration have successively formulated related policies,emphasizing the importance of patients in the construction of the health system.In December 2021,Center for Drug Evaluation,NMPA published the “Guidelines for the Application of Patient Reported Outcomes in the Clinical Development of Medicines (Trial)”[18],emphasizing the importance of the scale in the development of new drugs.In July and December 2021,National Health Commission of the People’s Republic of China issued the “Guidance for the Management of Clinical Comprehensive Evaluation of Pharmaceuticals (Trial 2021)”[19]and the technical guidance for the clinical comprehensive evaluation of cardiovascular and antineoplastic agents and pediatric medicinal products,emphasizing the importance of health-related quality of life based on China’s population and the construction of utility value score system.Meanwhile,since 2019,the adjustment of the “National Drug List of National Health Security Administration”[20]has formally required companies to submit a pharmacoeconomic evaluation model for the development of new drugs,further emphasizing the importance of utility scales in decision making.Therefore,it is imperative to design a population wide health utility measurement scale that is compatible with the characteristics of China’s national conditions.
- 亚洲社会药学杂志的其它文章
- Effect of the Policies to Prevent Drug Shortage and Stabilize Drug Prices in Medical lnstitutions
- Research on the Countermeasures for the Development of Biopharmaceutical Industrial Parks in China
- Research Progress in FDA’s Focus Areas of Regulatory Science for Drugs and Suggestions for China
- Hypersensitivity Reaction Caused by Intravenous Gadolinium-based MRI Contrast Agents
- Cost-effectiveness Analysis of lnsulin Degludec and Liraglutide lnjection in the Treatment of Type 2 Diabetes
- Exploration and Research on the Integrated Development of “Internet Plus Medical Treatment”

