Removal Effect of Coagulating Sedimentation Method on Polyethylene Microplastics in Water
Shasha LIU, Qiongru ZHUANG, Hongji HUANG, Xiaodan LIN, Yue YANG, Jinghua WU
Guangdong Provincial Key Laboratory of Environmental Health and Land Resource, School of Environmental and Chemical Engineering, Zhaoqing University, Zhaoqing 526061, China
Abstract Microplastic is a new kind of pollutant. It exists widely in the aquatic environment and seriously endangers the aquatic ecosystem. In this study, the coagulating sedimentation method was used to remove microplastics in water. Polyethylene (PE) was selected as the representative of microplastics, polyferric sulfate (PFS), polyaluminum chloride (PAC) and aluminum sulfate (AS) were used as coagulant, and polyacrylamide (PAM) was used as coagulant aid to study the effects of pH, coagulant concentration and sedimentation time on the removal of PE by single and composite coagulant. The results showed that when the dosage of PFS was 0.5 g/L and pH was 5.0, the removal rate could reach 82.14%, which was better than PAC and AS, indicating that PFS had better coagulation and sedimentation performance for PE; the composite coagulant of PFS+PAC+AS (1 g/L+0.2 g/L+0.2 g/L, pH was 5.0) had the highest removal rate of PE, reaching 96.06%; the removal rate of PE increased with the increase in sedimentation time, but considering that the longer sedimentation time has less contribution to the improvement of removal rate, it is recommended that 4 h is appropriate.
Key words Microplastics, Coagulating sedimentation, Polyethylene (PE), Removal
1 Introduction
Because of low price, light weight and durability, plastics and their products are widely used in production and life, resulting in a large number of waste plastics into the water environment[1]. Under long-term weathering, erosion, wear, ultraviolet radiation, biological and human action, they will be broken into plastic particles with a particle size of less than 5 mm, which is called microplastics[2]. In addition, microparticles and beads in industrial raw materials, drug delivery, and personal care products are also sources of microplastics in the environment[3-4]. At present, microplastics have been detected in the global oceans[5-6], rivers[7-8], lakes[9]and other water environments, which can cause toxic effects on aquatic organisms[10]and pose a potential threat to human health through the food chain[11]. Therefore, the removal of microplastics in water environment is an urgent problem to be solved.
Coagulation has become one of the most effective methods to remove microplastics in water because of its high efficiency, simple operation and low cost[12-14]. Compared with rapid sand filtration, membrane bioreactor and disc filtration processes, the removal rate of microplastics by coagulation is higher, reaching 90%[15]. In this study, we used the coagulating sedimentation method to remove microplastics in water, selected polyethylene (PE) as the representative of microplastics, polyferric sulfate (PFS), polyaluminum chloride (PAC) and aluminum sulfate (AS) as coagulant, and polyacrylamide (PAM) as coagulant aid to study the effects of pH, coagulant concentration and sedimentation time on the removal of PE by single and composite coagulant, and determine the optimal removal conditions of PE, so as to provide a scientific basis for the remediation of micro-plastic pollution in water environment.
2 Materials and methods
2.1 Preparation of water sampleIn this experiment, microplastics polluted wastewater was artificially prepared to simulate the microplastic pollution. We weighed 50 mg of PE and put it into a beaker, added deionized water, fully mixed it, transferred it to a 1 L volumetric flask, and made the PE evenly distributed by ultrasound after constant volume, and finally obtained a PE solution with a concentration of 50 mg/L.
2.2 Coagulating sedimentation experimentFirst, we poured the well-mixed PE solution (400 mL) into a beaker, and added coagulant (including PAC, PFS and AS). Then, we used 0.1 mol/L HCl and 0.1 mol/L NaOH to adjust pH of the water sample, and added 250 mg/L polyacrylamide (PAM) to the water sample, and the water sample was stirred by a stirrer for coagulation experiment. The specific steps were as follows:stirred at high speed for 15 min (400 r/min), stirring at moderate speed for 10 min (200 r/min), and stirred at low speed for 5 min (100 r/min) in turn, and then placed for later use. Dried the filter paper in an oven (60 ℃) until constant weight, denoted as M1(g). Took the supernatant, added HCl to remove impurities, filtered and dried, and obtained the weight M2(g). The removal rate of PE was calculated using the following formula:
2.2.1Single coagulant. In the PE polluted water sample, we separately added single PFS (0.5 g/L), single PAC (0.5 g/L) and single AS (0.5 g/L) to investigate the influence of different factors (pH, coagulant concentration and sedimentation time) on the PE coagulation effect, and determine the optimal conditions. Set the pH at 5.0, 6.0, 7.0, 8.0, and 9.0. The concentration of coagulant (PAC, PFS, and AS) was 0.0, 0.2, 0.5, and 1 g/L, respectively. The sedimentation time was 1, 4, and 12 h.
2.2.2Composite coagulant. In the PE polluted water sample, PFS+PAC (0.5 g/L+0.2 g/L), PFS (0.5 g/L)+AS (0.2 g/L), PAC (0.2 g/L)+AS (0.2 g/L) and PFS (0.5 g/L)+PAC (0.2 g/L)+AS (0.2 g/L), pH was set as 5.0, 6.0, 7.0, 8.0 and 9.0, respectively. The sedimentation time was 1, 4 and 12 h, respectively, and the effects of different pH and sedimentation time on the coagulation effect of PE were investigated. PFS, PAC and AS with concentrations of 0.05, 0.1, 0.2, 0.5 and 1 g/L were combined in pairs or three combinations, and the removal rates of PE under different concentrations were analyzed.
3 Results and analysis
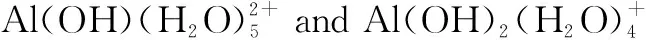
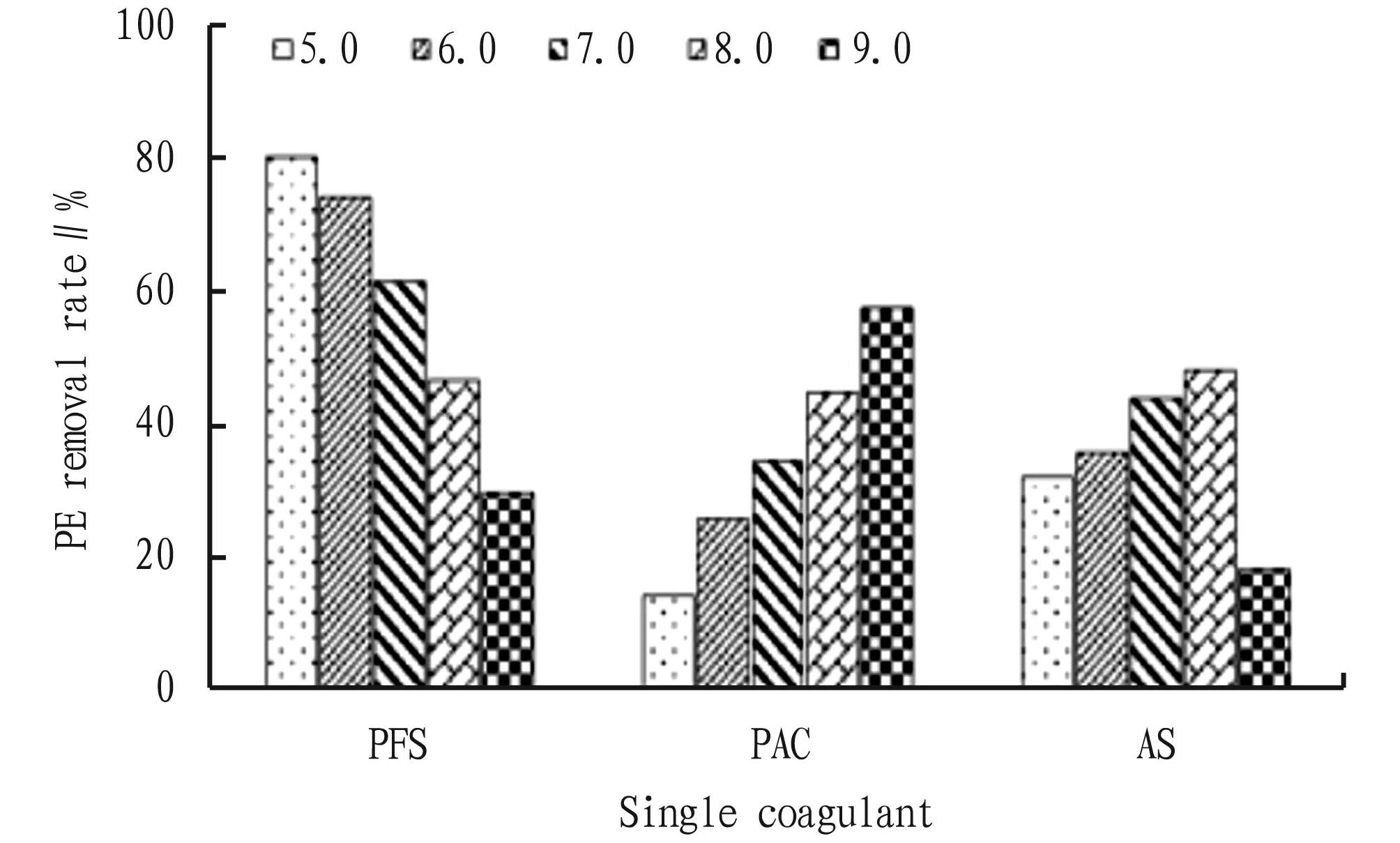
Fig.1 Removal effect of single coagulant on PE under different pH conditions
3.2 Effects of coagulant concentration on the removal effect of PEThe removal effects of PFS, PAC and AS with different concentrations on PE are shown in Fig.3. In the concentration range of 0.05-1.0 g/L, the removal rate of PE by PFS was significantly higher than that by PAC and AS, and the removal efficiency of PE reached the highest (82.14%) when the added concentration of PFS was 0.5 g/L, which indicated that PFS had very good coagulation and sedimentation performance for PE. When the concentration of PAC and AS was 0.02 g/L, the removal efficiency of PE was the best, which was 51.02% and 41.79%, respectively. As shown in Table 1, the coagulation and sedimentation capacity of PE can be improved by compounding a single coagulant. PFS+PAC, PFS+AS and PAC+AS had the highest removal rate of PE when the concentration ratio was 0.5 g/L+0.2 g/L, 0.5 g/L+0.2 g/L and 0.2 g/L+0.5 g/L, reaching 89.50%, 80.00%, and 67.50%, respectively. The optimum concentration of PFS+PAC+AS was 1 g/L+0.2 g/L+0.2 g/L, and the removal rate of PE was 96.06%.
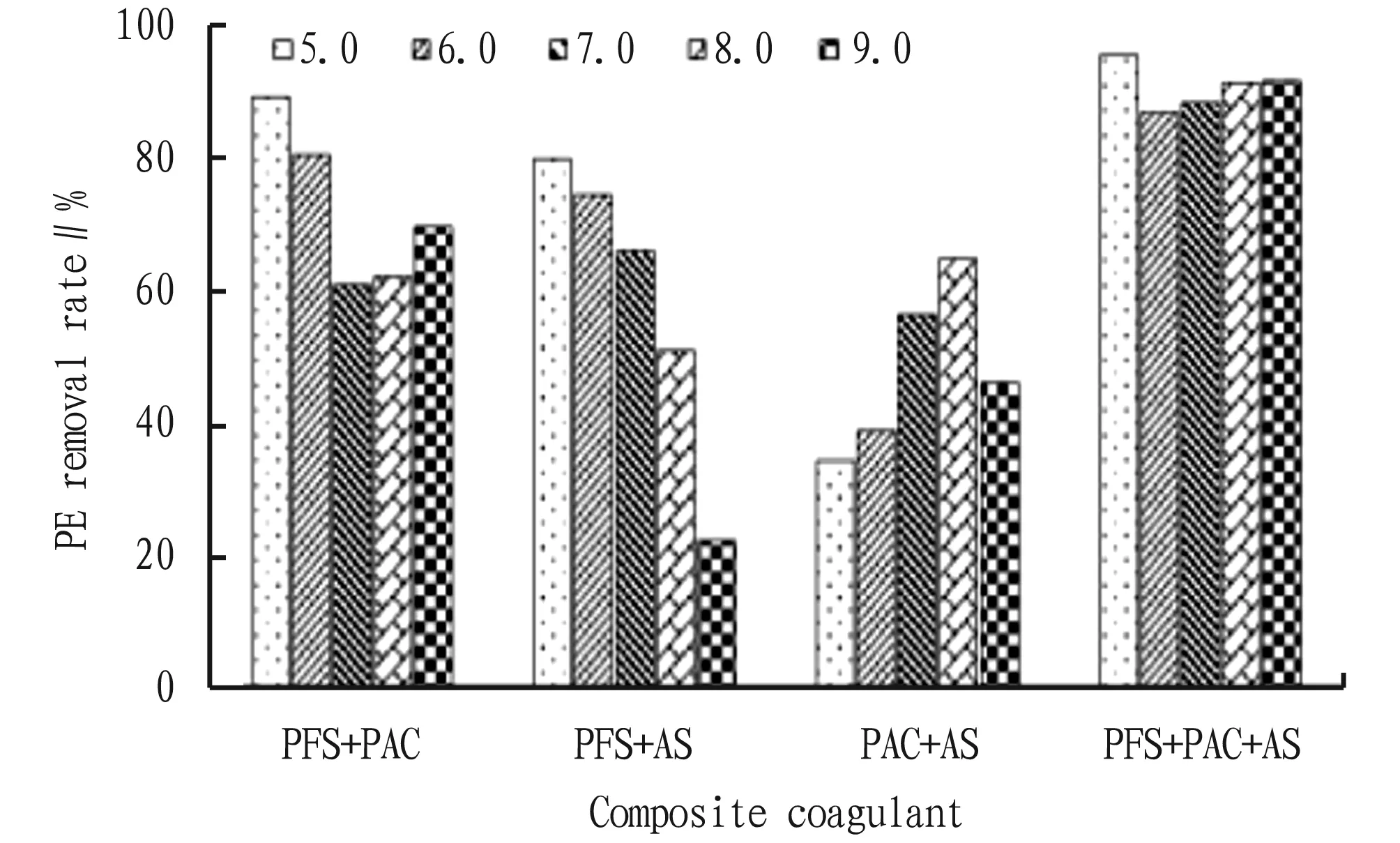
Fig. 2 Removal effect of composite coagulant on PE under different pH conditions
Fig.3 PE removal effect of single coagulant with different concentration
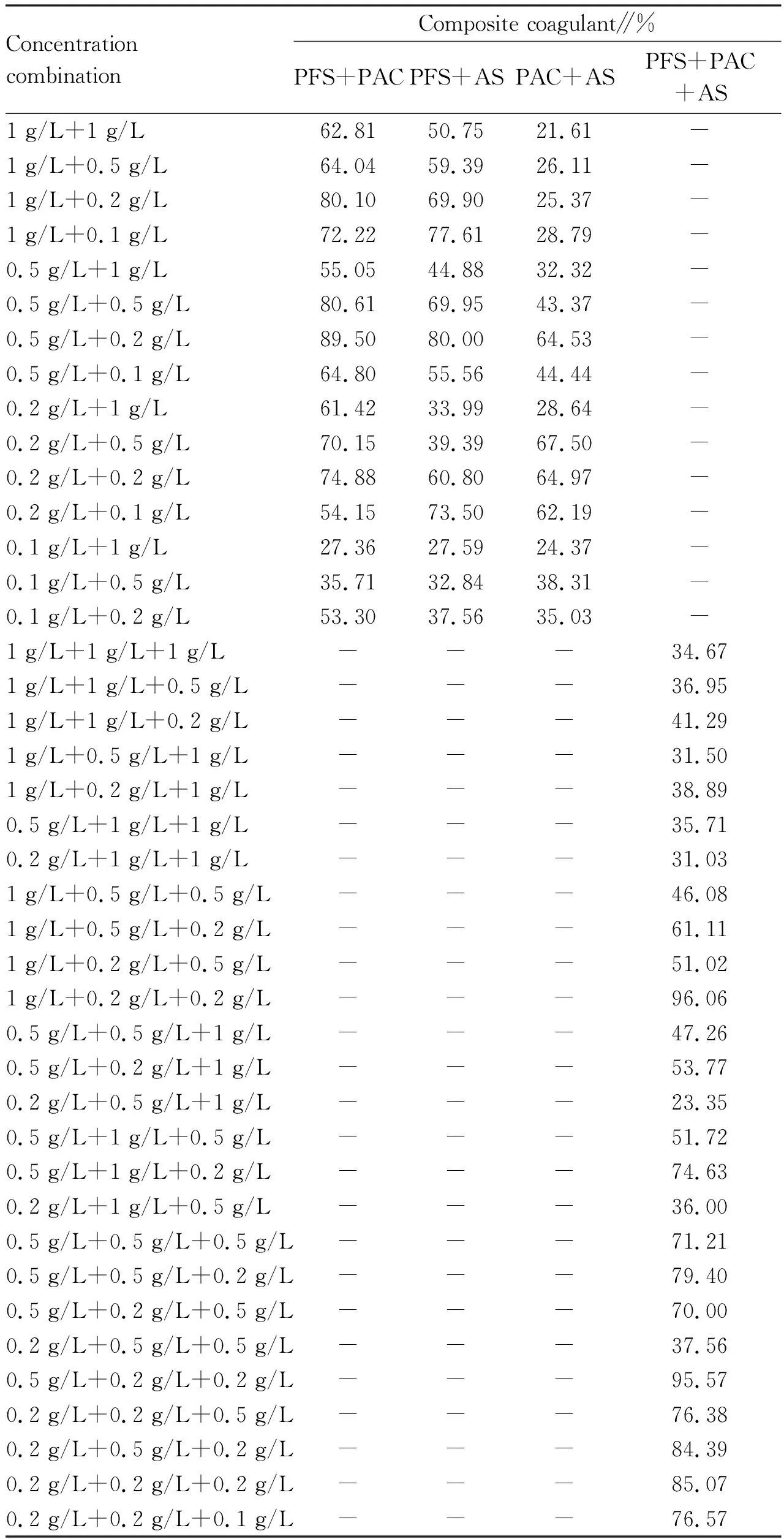
Table 1 Removal effect of composite coagulant with different concentrations on PE
3.3 Effects of sedimentation time on the removal rate of PE
From Fig. 4, it can be seen that with the sedimentation time increasing from 1 h to 4 h and 12 h, the removal rate of PE by single and composite coagulant increased by 2%-4%. However, the sedimentation time of 4 h and 12 h had little effect on the removal rate of PE. If the sedimentation time is too short, there are still some flocs that have not been completely settled in the supernatant, leading to poor removal of microplastics; by contrast, if the sedimentation time is too long, the formed flocs may be broken again[17], and it has little contribution to further improve the removal rate. Therefore, the sedimentation time of 4 h is recommended.
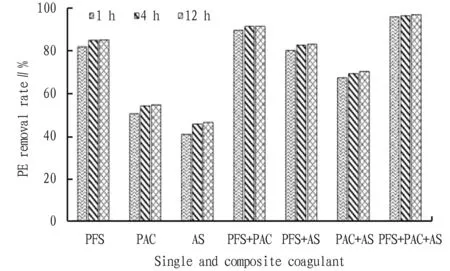
Fig.4 Effect of sedimentation time on the removal of PE by single and composite coagulant
4 Conclusions
After adding single coagulant, the coagulating sedimentation effect of PFS on PE was the best (dosage 0.5 g/L, pH 5.0), followed by PAC (dosage 0.2 g/L, pH 9.0), and the weakest effect of AS (dosage of 0.2 g/L, pH of 8.0). After adding composite coagulant, PFS+PAC+AS (concentration combination of 1 g/L+0.2 g/L+0.2 g/L, pH 5.0) had the highest removal rate of PE. The order of PE removal efficiency of other combinations was PFS+PAC (0.5 g/L+0.2 g/L, pH 5.0) >PFS+AS (0.5 g/L+0.2 g/L, pH 5.0) >PAC+AS (0.2 g/L+0.5 g/L, pH 8.0). The sedimentation time of both the single and composite coagulant was 4 h.
 Asian Agricultural Research2023年9期
Asian Agricultural Research2023年9期
- Asian Agricultural Research的其它文章
- Analysis on the Problems and Strategies of Marketing Channels of Agricultural Products:Taking Liujun Lotus Root as an Example
- Species Composition and Diversity of Fagus longipetiolata in Leigong Mountain, Guizhou Province
- National Protection Zone for Rapeseed Production and Industrial Cluster of Rape in Hubei Province
- Bird Resources and Avifauna of Zijin Mountain in Nanjing
- Effect of Storage Time on Main Quality Indicators of Cotton
