Experimental study of Al agglomeration on solid propellant burning surface and condensed combustion products
Cheng-yin Tu,Xiong Chen,Ying-kun Li,Bei-chen Zhang,Chang-sheng Zhou
School of Mechanical Engineering,Nanjing University of Science and Technology,Nanjing,210094,PR China
Keywords:Solid propellant Al particles Condensed combustion products Agglomeration Microscopic morphology
ABSTRACT Aluminum (Al) particles are commonly added to energetic materials including propellants,explosives and pyrotechnics to increase the overall energy density of the composite,but aluminum agglomeration on the combustion surface may lower the combustion efficiency of propellants,resulting in a loss in twophase flow.Therefore,it is necessary to understand the agglomeration mechanism of aluminum particles on the combustion surface.In this paper,a high-pressure sealed combustion chamber is constructed,and high-speed camera is used to capture the whole process of aluminum accumulation,aggregation and agglomeration on the combustion surface,and the secondary agglomeration process near the combustion surface.The microscopic morphology and chemical composition of the condensed combustion products(CCPs)are then studied by using scanning electron microscopy coupled with energy dispersive(SEM-EDS) method.Results show that there are three main types of condensed combustion products:small smoke oxide particles oxidized by aluminum vapor,usually less than 1 μm;typical agglomerates formed by the combustion of aluminum agglomerates;carbonized agglomerates that are widely distributed,usually formed by irregular movements of aluminum agglomerates.The particle size of condensed combustion products is measured by laser particle size meter.As the pressure increases from 0.5 MPa to 1.0 MPa in nitrogen,the mass average particle size of aluminum agglomerates decreases by 49.7%.As the ambient gas is changed from 0.5 MPa nitrogen to 0.5 MPa air,the mass average particle size of aluminum agglomerates decreases by 67.3%.Results show that as the ambient pressure increases,the higher oxygen content can improve combustion efficiency and reduce the average agglomeration size of aluminum particles.
1.Introduction
Aluminum powder (5-20 μm) is usually added to solid propellants to increase the engine’s specific impulse for its high energy density,low cost and safety [1].However,since aluminum agglomerates are formed,during the combustion of the solid propellant,a small amount of aluminum may fail to burn in the combustion chamber.It has been reported that for every 10% of unburned aluminum,there is 1% loss in specific impulse(Isp)[2].In addition,aluminum agglomerates will increase the size of condensed combustion products (CCPs) [3],resulting in the accumulation of slag,nozzle erosion,and loss in two-phase flow in the nozzle,etc.[4].Moreover,particle phase distribution combustion may cause acoustic instability in the engine [5].Therefore,it is necessary to fully understand the mechanism and influencing factors of aluminum agglomeration.
The reaction process of aluminum in solid rocket motors can be divided into two stages(Fig.1).The first stage is the escape process of aluminum particles on the combustion surface,when part of the aluminum particles agglomerate.During the combustion process of the propellant,as the burning surface progresses,the aluminum particles are gradually exposed to the burning surface.Part of the particles stay on the combustion surface and aggregate with neighboring ones particles to form larger agglomerates,which then leave the combustion surface and enter the high-temperature combustion chamber under the effect of airflow.Aluminum particles that do not form agglomerates are directly separated from the combustion surface and enter the combustion chamber.The second stage is the combustion process of aluminum in the combustion chamber.In the chamber,gas-phase combustion products will be condensed into liquid aluminum oxide,which will later be deposited on the surface of the particles and form an oxidation cap,causing changes in the distribution of the vaporization rate,temperature and other parameters of the aluminum surface.The burned aluminum particles eventually become CCPs,which can be divided into two categories,agglomerates and smoke oxide particles(SOPs)[6].The former are large-sized alumina particles formed during the combustion of agglomerated aluminum.The latter are small-sized alumina particles formed from the oxidation of aluminum vapor.

Fig.1.Combustion of aluminum particles in solid rocket motors.
Researchers have carried out a lot of research on aluminum agglomeration and aluminum combustion in solid propellants.As a rule,the agglomerates are affected by the size of the parent aluminum particles and their percentages [7-14],the size of AP particles [9,12,15],fraction of coarse and fine AP particles[7,11,13,16],type of the oxidizer [9,10,17],properties of the binder[10],burning atmosphere [18],acceleration effect [19],chamber pressure[7,10,11,15,16,20-22],burning rate of the propellant[9,13]and the use of other additives,such as other binders and ballistic modifiers [23].
Many researchers have studied aluminum agglomerates through CCPs and proved that the propellant composition and environmental conditions can affect the distribution of agglomerate sizes [24,25].Control parameters for the agglomeration of aluminum particles include the size and percentage of the precursor particles [7,8,26-30],size of AP particles [27,31],ratios of coarse AP particles and fine AP particles [25,28,30],type of the oxidizer[16,29,31],and properties of the binder[29],distance from the combustion surface [15],acceleration effect [32],pressure[33,34]and propellant combustion rate[8,27].The studies of nanoaluminum [35,36],porous aluminum [37],and substitutes for aluminum powder in propellants revealed different shapes of agglomerates and agglomeration mechanisms.By coating the surface of aluminum particles,the time of molten aluminum particles staying on the combustion surface is shorter,which can decrease the probability of agglomeration.Current research focuses on the use of metal [38]or polymer [39,40]coatings to adjust the agglomeration and combustion properties of micron aluminum particles.Ao[24,41]considered replacing aluminum particles with metal alloys to reduce agglomeration in aluminum-containing propellants.Five different aluminum-based alloys were used:Al-Mg,Al-Ni,Al-Si,Al-B,and Al-Zn.By analyzing the combustion products of the five different aluminum alloy propellants,results showed that Al-Ni produced the least amount of agglomeration,with an average agglomerate size of about 30% smaller than the baseline value.The agglomerates produced by Al-Zn were large,with an average size of about 15% larger than the baseline.Liu [42]established an online detection system for condensed products based on laser scattering technology,detected the size of plume condensed products in solid rocket motors,and conducted numerical simulation under the same conditions.The results showed that the measurement system could be used to obtain the particle size distribution of the condensed products in the plume.Yuan[43]found three forms of the combustion products of Al/AP/HTPB propellant: SOPs (<1 μm),medium-sized particles(1-60 μm),and agglomerates (>60 μm).As the environmental pressure increases,the size and number of agglomerates decrease,while the number of small particles increases.Liu[44]used a new home-made CCP collection device to study the effect of RDX content on the combustion and agglomeration of aluminized HTPB propellants.RDX greatly aggravated aluminum agglomeration on the burning surface.The 6 wt% and 12 wt% RDX propellants increased the average size of CCPs size from 46.3 to 86.7 μm and 96.6 μm,respectively.The combustion efficiency of aluminum in propellants was reduced by 15% when the RDX content was increased from 0 to 12%.Li[45]used sampling method to study the minimized fuel-rich propellants in solid fuel ramjets.Based on the classical Pocket theory and particle size analysis experiments,a new agglomeration size prediction model was established,which could be used to predict the agglomeration size on the burning surface.
Another way to study aluminum agglomeration is to capture the image of agglomerations on the combustion surface through optical measurement method such as high-speed and high-resolution cameras.The accumulation,aggregation and agglomeration of aluminum particles can be observed,and the agglomeration mechanism revealed.In a previous study,the diameter of the agglomerates was measured through the images taken by high-speed cameras,and the mass average particle size of the agglomeratesD43on the burning surface,was obtained through calculation[7].It was proved that the environmental pressure [9,10]and propellant combustion [8]had an effect on the mass average particle size of the agglomerates.However,most of the agglomeration images were captured at low pressures below 1 MPa [46,47].Liu [11,48]used an optical photography method combined with a telephoto microscope lens and a high-speed camera to study the dynamic combustion process of aluminum particles in an aluminumcontaining propellant at different pressures.Both particles on the propellant’s combustion surface and those that had left the surface were studied.By adding a neutral density filter with different transmittances in front of the lens,overexposure of the camera’s imaging due to excessive burning of aluminum droplets was solved.By measuring different positions of the aluminum droplets of the same size in two adjacent pictures,the flow velocity of aluminum droplets of different sizes was calculated.The experimental results showed that by increasing the RDX content in the propellant,the particle size of the agglomerates increased.As the pressure increases,the agglomeration time of agglomerates of the same size was shortened.With a constant pressure,the agglomeration time was shortened as the particles size decreased.
Despite the above-mentioned studies,the existing literature on aluminum combustion and agglomeration in solid propellants is not sufficient for the following reasons.First,there are few studies on the agglomeration process of aluminum particles during the combustion of NEPE propellant,and the specific process of aluminum agglomeration on the burning surface of NEPE propellant has not been fully observed.Second,different collection methods of CCPs will affect the research results,but existing research hasn’t paid attention to this issue.Finally,there are contradictory findings.For example,one previous study [7]showed that as pressure increased,the mass average particle size D(4,3)of CCPs decreased,but Jayaraman [32]claimed that the effect of pressure on the particle size distribution of initial agglomerates is non-monotonic.
In this paper,a high-pressure sealed combustion chamber is built and a method combining quenching and optical measurement is adopted to study the aluminum agglomeration process of a NEPE solid propellant.Through a high-speed camera,the dynamic combustion behaviors of the aluminum agglomerates on the combustion surface of the NEPE solid propellant are captured.A collecting plate is placed in the combustion chamber to collect CCPs,and scanning electron microscope (SEM) and energy dispersion (EDS) are used to study the morphology and chemical composition of the CCPs,the relationship between combustion products and the combustion mechanism.A laser particle size analyzer is used to measure the size of the CCPs and reveal the factors affecting the particle size distribution.The results are helpful for further understanding the combustion of aluminum particles in the propellant.
2.Experimental
2.1.Samples
A Nitrate Ester Plasticized Polyether(NEPE)propellant is used in this study.The composition of the NEPE propellant is shown in Table 1.The basic components include oxidizing agent(ammonium perchlorate,AP,100-130 μm),energetic plasticizers (butanetriol trinitrate,BTTN),high-energy explosive (cyclotrimethylenetrinitramine,RDX),cross-linking agents(cellulose acetate butyrate,CAB),metal fuel (Al,3-7 μm) and a small proportion of other additives.

Table 1Composition of NEPE propellant.
The propellant is prepared according to the following procedures: First,mixed solid materials are added to the prepared liquid material,and then all materials are mixed in a vertical kneader for 1 h.Second,the obtained propellant slurry is degassed and cured in a vacuum oven at 50°C for 7 days.At last,the propellant is cut into samples of the required size to meet the needs of the experiment.
In the experiment,5 × 5 × 5 mm3cube specimen are used.To ensure that burning starts from the ending face,the lateral surface is coated with high-temperature resistant insulating rubber.The SEM image of the NEPE propellant is shown in Fig.2.From Fig.2(a),it can be observed that AP particles are randomly distributed on the surface of the propellant,and aluminum particles and RDX are distributed in the pocket area formed by AP.The SEM image in Fig.2(b) is magnified 2000 times.The aluminum particles in the propellant can be clearly observed,and the adhesive and plasticizer are randomly distributed around the aluminum particles.
2.2.Experimental system
Experiments are carried out in the sealed high-temperature and high-pressure laser ignition system.The schematic diagram of the experiment system is shown in Fig.3.It is mainly composed of the control system,CO2laser optical system,combustion chamber and data acquisition system.The control system is composed of computer software and control card,which is used to adjust the loading time and heat flux density of the CO2laser.The CO2laser has a power of 300 W and a wavelength of 10.6 μm.The optical system can change the horizontal laser beam emitted by the laser into a vertical laser beam,and load it on the surface of the test piece through the laser entrance window at the top of the combustion chamber.The optical system consists of a plane mirror and a focusing mirror at the top of the chamber.The size of the combustion chamber is 150×150×300 mm3.The maximum working pressure it can withstand is 4 MPa.There is an 50 × 100 mm2observation window in the horizontal direction and a laser entrance with a diameter of 20 mm on the top.A square collecting plate with a height of 10 mm and a bottom size of 149×149 mm2is installed at the bottom of the combustion chamber,completely covering the bottom.There is a circular hole of 8 mm at the center of collecting plate,which can allow the cylindrical stand to pass through.The solid combustion products are collected on the plate.
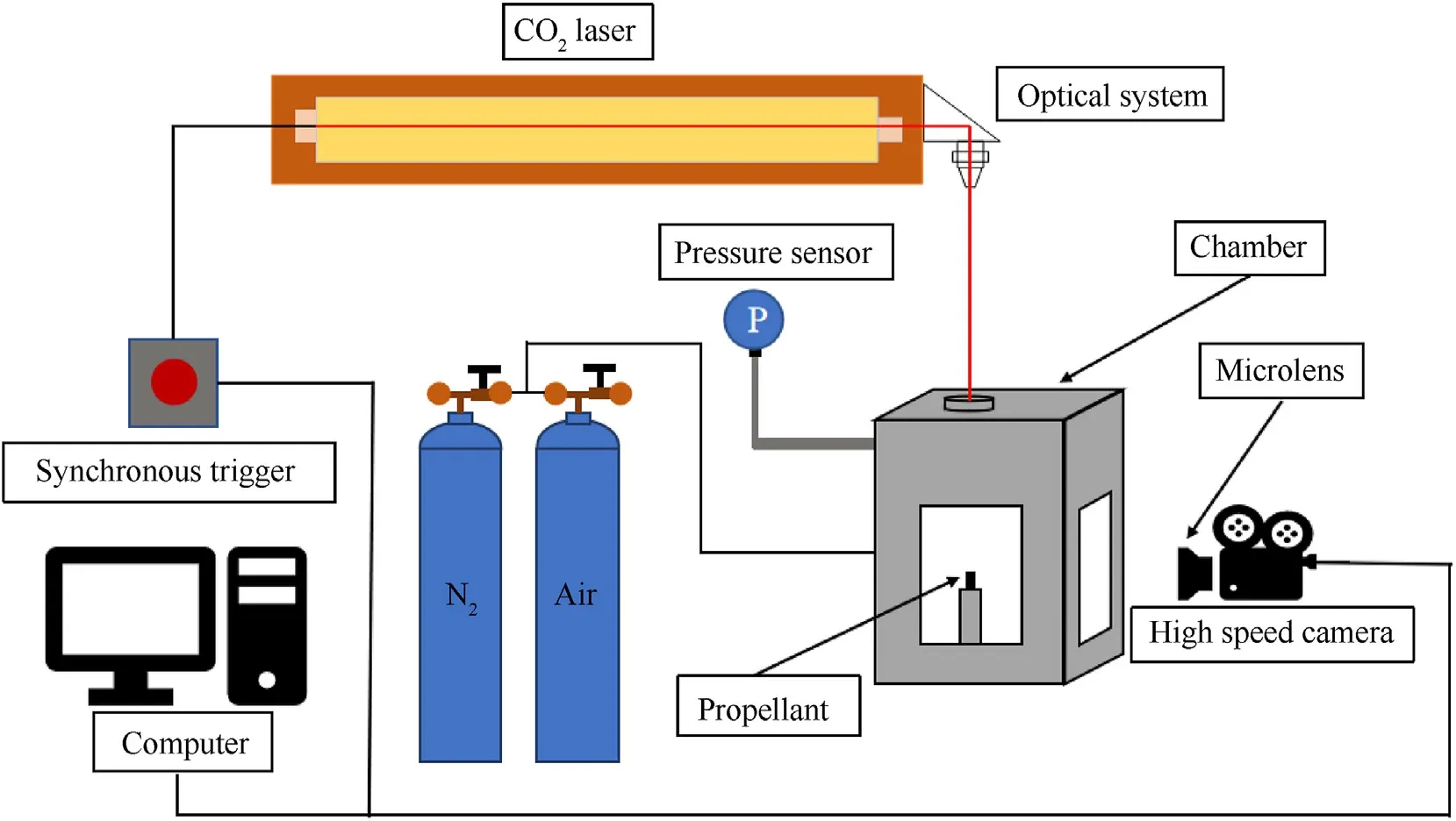
Fig.3.Schematic of the experimental system.
2.3.Experimental method
The experiment is carried out at room temperature (25°C)under the pressure of 0.5 MPa,1.0 MPa in nitrogen/air.The laser heat flux is set as 2.5 W/mm2.The laser loading time is 1 s.In the experiment,a high-speed camera (Chronos 1.4) is used,and the images are taken at a frame rate of 1000 fps.To reduce test error and enhance data reliability,each group is tested for five times and the average results are acquired.After adjusting the light path and the laser ignition control system,the specimen are fixed together using positioning bolt around the center hole under the combustion chamber,and the bolts are tightened to ensure the airtightness of the combustion chamber.The pressure in the combustion chamber is adjusted using the high-pressure cylinders,and the ambient pressure in the combustion chamber is detected by the pressure sensor to meet the experimental requirements.Images taken throughout the test process,including those of propellant ignition,combustion,and extinguishing are captured by a highspeed camera.After the flame is extinguished,gas in the combustion chamber is discharged.Finally,the bolts at the bottom of the combustion chamber are removed and the collecting plate is taken out.
2.4.Product analysis
The chemical and microscopic morphology information of CCPS is obtained from a Hitachi S-4800 scanning electron microscope(SEM) images along with X-ray energy dispersive spectrometer(EDS).The agglomeration size is measured by laser scattering particle analyzer(Mastersizer 2000).The sample quality is about 0.1 g for measuring the particle size distribution.The turbidity is kept between 10% and 20% in order to obtain accurate particle size distribution data.
3.Results and discussion
3.1.Al agglomeration
Generally,the process multiple aluminum particles evolving into a spherical agglomerate can be divided into three stages:accumulation,aggregation and agglomeration [24].Accumulation means that as the surface starts burning,aluminum particles are gradually exposed and melted on the burning surface,and then adjacent aluminum particles begin to pile up.Aggregation refers to the gathering of aluminum particles to form larger aggregates.At this time,the particles are not completely melted and turn into liquid.Remaining an irregular bone-like structure where solid and liquid contents coexist,the particles are intermediate products of accumulation and agglomeration.As the aggregates continue to melt,the skeleton structure of the aluminum clusters gradually collapses to form regular spherical aluminum droplets.After the entire agglomeration process is completed,the spherical droplets can be called agglomerates.
The combustion process of NEPE propellant in 1 MPa N2is photographed by a high-speed camera.The accumulationaggregation-agglomeration process can be clearly observed on the burning surface.In Fig.4(a),aluminum particles begin to accumulate and stick together as the burning surface progresses.As the temperature of the diffusion flame increases to 960 K,part of the aluminum particles begin to melt,and the accumulation gradually shrinks and particles become molten droplets.The large amount of gas generated during combustion pushes the droplets leave the combustion surface due to an upward lift.At this time,the droplets and combustion surface are connected by a piece of filigrees,as shown in Fig.4(b).Filigrees were first proposed by Price[19],which are formed by the adjacent aluminum particles sintering during the process of aluminum agglomeration on the surface of the propellant.Under the action of the adhesion between the filaments,the droplets are pulled back to the propellant surface(Fig.4(c)-Fig.4(e)).Subsequently,the aggregates continue to“swallow”the newly exposed aluminum particles on the propellant surface and the aluminum particles in the filigrees.The number of aluminum particles decreases,and the filaments are thinner.When t =46 ms,the droplets become spherical,marking the end of the agglomeration process,as shown in Fig.4(f).At this time,the diameter of the agglomerate is 441 μm,and the filigrees between the agglomerates and the burning surface are gradually broken(Fig.4(f)and Fig.4(g)).Then,the agglomerates are separated from the burning surface.

Fig.4.Agglomeration on the burning surface: (a) t =0 ms;(b) t =3 ms;(c) t =11 ms;(d) t =20 ms;(e) t =26 ms;(f) t =46 ms;(g) t =60 ms;(h) t =61 ms;(i) t =64 ms.
According to the experimental results of previous researchers,agglomeration usually occurs in metal-containing solid propellants.Fig.5 shows a schematic diagram of a typical agglomerate formation mechanism.

Fig.5.Schematic of the agglomeration mechanism.
For the NEPE solid propellant used in this experiment,the average diameter of RDX and Al is much smaller than the average diameter of AP.In the microstructure of the NEPE propellant,Al and RDX are distributed around AP particles,and BTTN and other additives are distributed in their gaps.RDX melts at 478 K,and chemical reactions rarely occur.The decomposition temperature of pure BTTN is 523-773 K[49],which is lower than the temperature of the propellant surface (900 K) and the melting point of aluminum particles(about 960 K).Therefore,RDX and BTTN in the solid phase zone near the combustion surface undergo phase changes and thermal decomposition to form a foam layer,which surrounds the aluminum particles,making it easier to approach each other and increasing the probability of agglomeration.
As aluminum particles are extremely easy to react with oxygen,the aluminum particles exposed on the burning surface quickly form a tough aluminum oxide coating on the surface,blocking the reaction between aluminum and oxygen [21].When the surface temperature of the propellant is lower than the melting point of alumina (2303 K),the aluminum particles are wrapped by the alumina shell and accumulated on the surface of the propellant[50],and the high-temperature RDX and BTTN form a molten layer on the combustion surface to serve as aluminum.The position where the particles are attached makes them neither easy to be ignited nor easily taken away by the gas[51].Therefore,the number of accumulated aluminum particles is increasing,which increases the diameter of the initial agglomerates.
The melting point of the aluminum particles is close to the temperature of the propellant surface.The aluminum particles inside the aluminum oxide shell are heated and melted into liquid aluminum.However,the thermal expansion coefficient of liquid aluminum is larger than that of alumina,which is accompanied by 6% volume expansion in this process,as the liquid aluminum heats up and expands,the outer oxide shell ruptures [52].And then the inner liquid aluminum is ejected from the cracks contacts the surrounding aluminum particles,quickly reacting with oxygen to form an aluminum oxide shell with a higher melting point.Individual aluminum particles gradually fuse together in this way[18].
As the burning progresses,the generation of combustible gas increases,resulting in the rise of temperature on the combustion surface and enhancement of the combustion wave.In previous studies,it was found that when the propellant was in stable combustion,the temperature of the diffusion flame could reach 2500 K[53],exceeding the melting point of alumina and causing it to melt.Under the action of surface tension,the agglomerates rapidly collapse and become spherical structures,indicating the end of the entire agglomeration process.At this time,the agglomerates are subjected to a vertical upward lift under the action of airflow,vertical downward gravity,and adhesion of the filaments.When the lift is higher than the force of gravity and adhesion combined,the filigrees break and the agglomerates are detached from the burning surface [28].
3.2.Second emergence of agglomerates
Throughout the burning process,the secondary agglomeration of aluminum agglomerates on the burning surface is captured.After the aluminum particles form agglomerates on the combustion surface,they are separated from the surface with fuel gas.When multiple agglomerates collide,they merge together and form larger agglomerates.This phenomenon is called the second emergence of agglomerates[54].
Fig.6 shows the whole process of the secondary agglomeration.Fig.6(a)-Fig.6(d)is the formation process of the first agglomerate.Whent=9 ms,the first molten agglomerate is separated from the combustion surface with a diameter of 361 μm.When t =11 ms,the second agglomerate is formed and separated from the combustion surface.After the second agglomerate collides with the first one behind,diffusion flames merging together can be observed.When the agglomerates collide with each other,their original spherical shapes turn into an irregular shape as shown in Fig.6(f).Under the action of surface tension,the merged molten aluminum agglomerates become spherical again.The new agglomerate has a significantly longer trailing flame,which is formed by the aluminum oxide smoke generated during the combustion of aluminum droplets.The trailing flame wraps the aluminum droplets and is consistent with the burning of single aluminum droplets [20].The agglomerates,after merging,return to the surface of the propellant.At this time,the diameter of the agglomerate increases to 445 μm.Subsequently,the agglomerate rolls to the right of the burning surface and continuously swallows newly generated aluminum particles on the surface of the propellant.The rolling and growth of the agglomerates can be observed from Fig.6(d)-Fig.6(i),with a total rolling distance of about 1100 μm and a duration of about 7 ms.When t =26 ms,the agglomerates are separated from the burning surface.Experiments show that rolling and growing of agglomerates on the combustion surface are commonly observed.

Fig.6.Secondary agglomeration process near the burning surface:(a)t =0 ms;(b)t =2 ms;(c)t =4 ms;(d)t =9 ms;(e)t =11 ms;(f)t =13 ms;(g)t =16 ms;(h)t =23 ms;(i)t =26 ms.
3.3.Analysis of the microscopic morphology of CCPs
A collecting plate is placed in the combustion chamber to collect CCPs,and then SU3500 SEM and EDS are used to analyze the microstructure of the CCPs.Through the SES images,it can be found that CCPs are mainly composed of agglomerates,SOPs and carbonized agglomerates (CAGs).The size of the agglomerate is usually very large,which can reach several hundred microns,and the agglomerated aluminum burns to form a large-sized oxide.The SOPs usually do not exceed the size of 1 μm.According to the combustion mechanism of aluminum in the propellant proposed by Price [27],the SOPs are mainly small-sized alumina particles formed by the oxidation of aluminum vapor.CAGs are more widely distributed in the combustion products,showing irregular shapes,usually larger than the size of the agglomerates.
3.3.1.SOPs
As shown in Fig.7(a),SOPs in the CCPs are highly dispersed.The surface of the SOP is smooth,appearing to be relatively completely spherical,and the size is usually no more than 1 μm.At the same time,smoke oxide clusters are observed next to the SOPs,which are aggregated by dispersed SOPs and the size can reach 3 μm,much larger than the initial diameter of SOP.The structure of a smoke oxide cluster is irregular,as shown in Fig.7(b).During the agglomeration of aluminum particles,when the temperature of the diffusion flame exceeds the melting point of alumina,the molten alumina will sinter and form larger alumina particles.However,in the part where the diffusion flame does not reach the melting point of alumina,the alumina particles will be condensed rather than being fused,forming smoke oxide clusters.In previous studies[55],it was found that the longer the residence time of aluminum particles in the high temperature zone,the higher the number of smoke alumina clusters formed in the combustion products.Therefore,the formation of smoke oxide clusters in the combustion products is mainly due to the high ambient temperature and the long residence time of SOPs in the combustion chamber.

Fig.7.SEM image of the (a) SOPs and (b) smoke oxide clusters.
3.3.2.Agglomerates
Fig.8(a)shows a typical agglomerate with a size of 354 μm.The agglomerate exhibits an imperfect spherical structure,which may be caused by the non-uniformity of the molten droplet diffusion flame.During the heating phase,the aluminum droplets are usually surrounded by a thin aluminum oxide shell.But as the temperature rises,the aluminum oxide shell usually ruptures and causes local ignition,leading to imperfect agglomerate shell.The surface of the agglomerate is analyzed by using EDS,as shown in Fig.8(b),and the content of the composition is shown in Table 2.The EDS results show that the agglomerate is mainly composed of O,Al,C,N,with the total content of Al and O reaching 77.19%,which obviously indicates that the shell is mainly made of alumina.When the agglomerate leaves the burning surface,it takes away part of the RDX&BTTN molten layer,envelops the molten droplets,and forms a thin film on the surface after cooling,making the content of C reach 18.92%.

Table 2Composition of CCPs.

Fig.8.(a) SEM and (b) EDS images of a typical agglomerate.
The SEM image of a broken agglomerate is shown in Fig.9(a).Since the expansion coefficient of liquid aluminum is greater than that of the alumina shell,as the diffusion flame is heated,thermal expansion of the liquid aluminum will usually cause the alumina shell to rupture.The internal structure of the agglomerate can be seen from the broken shell,and the interior of the agglomerate presents irregular lines.The EDS image of the inside of the agglomerate is shown in Fig.9(b),and the composition is shown in Table 2.The inside of the agglomerate is mainly composed of aluminum and oxygen.The content of Al reaches 66.36%,followed by O reaching 22.24%,proving that the molten droplet is mainly liquid aluminum.In the agglomeration process,when the flame temperature exceeds the melting point of alumina,the agglomerates are molten droplets that continuously swallow the aluminum particles on the burning surface and gradually form larger agglomerates.Therefore,during the agglomeration process,part of the molten alumina is wrapped in the new agglomerate,increasing the oxygen content.Fig.10 is a partially enlarged view of the shell of a crushed agglomerate.A clearer alumina shell can be observed,the thickness of which is about 1 μm,consistent with a previous study[26].At the same time,SOPs can be observed on the surface of the aluminum oxide shell,which wrap the shell tightly.It may be because before the liquid aluminum produces a complete alumina shell,aluminum vapor continuously burns through the gaps of the alumina shell,forming small-size SOPs and covering the shell.

Fig.9.(a) SEM and (b) EDS images of a broken agglomerate.

Fig.10.Partially enlarged view of a broken agglomerate shell.
In Fig.11(a),the surface of the larger agglomerate is covered with coral-like material.There are two possibilities about the source of the material.One is that it comes from the condensation product of filigrees.When the agglomerates are separated from the burning surface,the filigrees are broken and some of them are taken away.Then,the condensation product of filigrees forms the coral-like material covering the agglomerate surface.The second is that the agglomerates take away part of the RDX&BTTN molten layer when they leave the combustion surface,which eventually form the coral-like structure after cooling.The EDS image of the coral-like structure on the agglomerate surface is shown in Fig.11(b),and the composition is shown in Table 2.The coral-like structure is mainly composed of C,Al,and O,of which the content of C is the largest,close to 40%,proving that the structure is mainly formed by the CCPs of the molten layer of RDX&BTTN molten.The content of Al and O are 25.52% and 17.55%,respectively.It can be seen that the coral-like structure contains part of the filigrees’ CCPs.The upper right corner of the agglomerate is connected with two smaller broken agglomerates.It may be because the agglomerate is not in a completely molten drop state when it leaves the burning surface.When the agglomerate collides with two small-size agglomerates,local ignition occurs on the agglomerate surface,and the liquid aluminum quickly generates alumina with a higher melting point,connecting the agglomerates tightly.
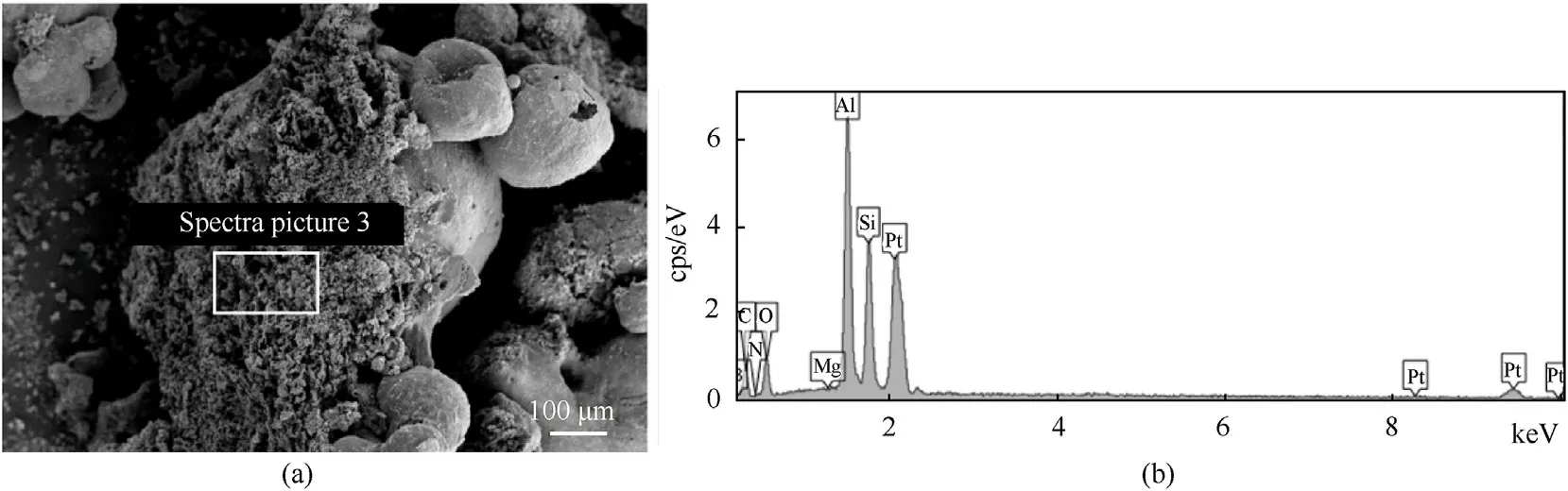
Fig.11.(a) SEM and (b) EDS images of an agglomerate covered by skeleton structure.
In addition,two agglomerates connected by a skeleton structure are found in the CCPs,as shown in Fig.12.The diameter of the left agglomerate is about 137 μm,and that of the right agglomerate is about 120 μm.The left one is more spherical than the right one,which may be because the alumina shell on the right agglomerate surface breaks when they collied.The inner liquid aluminum is sprayed onto the left agglomerate surface,reacting quickly with oxygen to form alumina and connect them together.However,the temperature of the diffusion flame does not reach the melting point of alumina,so there is no larger agglomerate formed in Fig.6(a).Due to the leakage of liquid aluminum inside the right agglomerate,the shell shrinks after cooling,resulting in irregular shapes of the agglomerates.

Fig.12.The SEM images of two agglomerates connected by a skeleton.
3.3.3.CAGs
Compared with spherical agglomerates,CAGs are more widely distributed in CCPs.Usually,the size of a CAG is relatively large and the shape is irregular.The diameter of the CAG in Fig.13 has reached 760 μm,while the size of spherical agglomerates is usually about 300 μm.The EDS image of a typical CAG is shown in Fig.13(b),and the composition is shown in Table 2.It can be seen that CAGs are mainly composed of O,C,and N.The formation of CAGs is usually caused by irregular movements of spherical agglomerates due to the effect of the combustion gas.First,the aluminum particles on the surface of the propellant are usually wrapped by the RDX&BTTN molten layer.When swallowed by large agglomerates,the molten layer is also drawn into the interior of the agglomerate.Then,when the agglomerates burn,they will spray and break,causing the molten layer to accumulate on the surface of the agglomerate,forming an irregular coral-like structure after cooling.Second,the combustion characteristics of the aluminum particles themselves make the structure irregular.As the thermal expansion of liquid aluminum wrapped in the aluminum oxide shell causes the agglomerates to break and spray,the aluminum oxide shell shrinks and deforms after cooling.In addition,when the agglomerates leave the combustion surface with the combustion gas,they usually collide with and squeeze other agglomerates,condensing them to irregular shapes after the heating process.

Fig.13.(a) SEM and (b) EDS images of a CAG.
3.4.Size distribution of agglomerates near the burning surface
The combustion process of the solid propellant is complicated.There is no mature theory that can quantitatively describe the influence of various factors on the particle size distribution of aluminum agglomerates,and the size of aluminum agglomerates is mainly measured by experiments[56].From an experimental point of view,it is difficult to decide whether a given particle is generated in an agglomeration process or not.Thus,a cut-off diameterDcutof 30 μm is adopted referring to the literature [9],to make a clear distinction of aluminum particles in their original and agglomerate states.A total number of at least 6000 agglomerates are counted to get the size distribution for each test.The mass average particle size D43is always used to evaluate the extent of agglomeration process[57,58],it is defined as
whereNrefers to the particle number andDis the diameter.
A laser particle size analyzer is used to measure the particle size of the aluminum agglomerates received under different experiment conditions.In the experiment,aluminum agglomerates are collected under experiment conditions of 0.5 MPa N2,1.0 MPa N2,and 0.5 MPa air.Two tests are carried out under each experiment condition,and the burning rate in each test is shown in Table 3.CCPs are collected for particle size analysis,and the analysis results are shown in Fig.14 and Table 4.The CCP size range from 29.6 to 600 μm.

Table 3Propellant burning rate under different experimental conditions (mm/s).

Table 4Particle size measurements of aluminum agglomerates under different experimental conditions (μm).

Fig.14.CCP size distribution under different experimental condition: (a) 0.5 MPa N2;(b) 1.0 MPa N2;(c) 0.5 MPa Air.
It can be seen that the agglomeration size distribution under different experimental conditions has the following characteristics:(1)As the pressure increases from 0.5 to 1.0 MPa in nitrogen gas,the number of large agglomerates decrease.As shown in Figs.14(a),17.3% of aluminum agglomerates have a diameter of over 100 μm,yet only 1.5% of agglomerates have a diameter of over 100 μm in Fig.14(b).This is because the combustion is more sufficient when the pressure is relatively high,resulting in a decrease in large agglomerates.And there is a peak around 60 μm in Fig.14(b),maybe because the higher burning rate of the propellant increases the probability of second emergence of agglomerates.At last,the mass average particle size D(4,3)decreases with the increase of pressure,indicating that the shape of the agglomerates is more regular.It is clear that the agglomeration size decreases with an increase of pressure.There are two reasons.First,with the increase of pressure,the heat conduction speeds up,and the thermal conductivity of surrounding gas increases,accelerating the heat conduction of the ambient air.Second,as the pressure increases,the thermal feedback rises,and the initial flame becomes closer to the propellant’s surface and heat transfer on the surface is more dramatic.These circumstances shorten the delay time of ignition,and the aluminum particles are easily ignited.Then,the particles turn into gas phase and rapidly leave the burning surface before coalescence occurs.Therefore,the agglomeration size decreases with the increase of pressure.This finding is consistent with the experimental results of Zhang[59]and Tejasvi[60].(2)By enhancing the oxygen content,the particle size of the aluminum agglomerates is reduced.Generally,increasing the oxygen content will increase the burning rate of the propellant.As shown in Table 3,the burning rates in 0.5 MPa air and 0.5 MPa N2are 5.41 mm/s and 2.90 mm/s,respectively.The mass average particle size of aluminum agglomerates in 0.5 MPa air is the smallest,which is 100.6 μm,while the mass average particle size in 0.5 MPa N2is 308.2 μm.When the propellant’s burning rate decreases,the residence time of aluminum particles on the burning surface is longer,so that the aluminum particles are more likely to agglomerate and form larger agglomerates.At the same time,the size of the aluminum agglomerates is more regular in 0.5 MPa air,and the cumulative percentage curve is smoother in Fig.14(c),indicating that the aluminum particles burn more thoroughly in 0.5 MPa air,and result in fewer large agglomerates.(3) Usually,the pressure and oxygen content could affect the burning rate of the solid propellant.For the NEPE propellant used in the experiment,the burning rate is maximum and the mass average particle size of the aluminum agglomerates is the smallest in 0.5 MPa air (Table 3).Therefore,it can be seen that the pressure and oxygen content of the ambient gas affects the particle size distribution of the aluminum agglomerates by influencing the combustion rate of the solid propellant.
4.Conclusions
This paper investigated the agglomeration of aluminum particles on the combustion surface of solid propellants,the microstructure of CCPs,and the particle size distribution by building a high-pressure sealed combustion chamber,and adopting sampling method,high-speed camera,SEM,EDS technology and laser particle size analyzer.The evolution process of multiple aluminum particles forming a single spherical agglomerate on the combustion surface can be divided into three stages:accumulation,aggregation and agglomeration.When the aluminum particles are gradually exposed,they aggregate on the burning surface and then form coral-like aggregates.As the temperature of the diffusion flame increases,the irregular aggregates quickly become spherical,ending the entire aggregation process.The secondary merging process of agglomerates is found on the burning surface.After leaving the surface,the agglomerates collide with adjacent ones.Molten droplets are fused into agglomerates with larger diameters under the heating of the diffusion flame and returned to the burning surface.The newly exposed aluminum particles are gradually swallowed and taken away.Through studying the microstructure of the collected residual combustion products by using a combination of scanning electron microscopy and energy dispersion,a variety of typical structures in the propellant combustion products are observed,including SOPs,agglomerates,and CAGs.Large smoke oxide clusters are found near the SOPs,aggregated by dispersed SOPs.The agglomerates in the combustion products are usually imperfect in shape.They collide with each other and are taken away from the burning surface,and leave the molten layer on the burning surface.As a result,the agglomerates in the condensedphase combustion products are usually broken and covered by coral skeletons,or connected by alumina.CAGs are usually larger in size and widely distributed,often due to the irregular movements of aluminum agglomerates.Measuring the particle size of CCPs under different experimental conditions,it can be found that increasing the pressure and oxygen content of the ambient gas could decrease the average size of aluminum agglomerates.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No.52006099),the Fundamental Research Funds of the Central Universities(Grant No.30920021102,No.309181B8812),and the Six Talent Peaks Project of Jiangsu Province of China (Grant No.2016-HKHT-017).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- Defence Technology的其它文章
- Deep learning-based method for detecting anomalies in electromagnetic environment situation
- Impact point prediction guidance of ballistic missile in high maneuver penetration condition
- Real-time localization for underwater equipment using an extremely low frequency electric field
- Numerical simulation of flow field characteristics and theimprovement of pressure oscillation of rotating detonation engine
- Comparative investigations of ternary thermite Al/Fe2O3/CuO and Al/Fe2O3/Bi2O3 from pyrolytic,kinetics and combustion behaviors
- Adaptive saturated tracking control for solid launch vehicles in ascending based on differential inclusion stabilization

